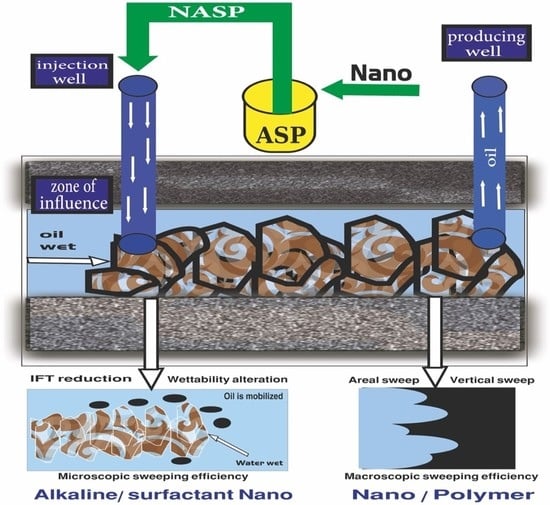
A Critical Overview of ASP and Future Perspectives of NASP in EOR of Hydrocarbon Reservoirs: Potential Application, Prospects, Challenges and Governing Mechanisms
Abstract
1. Introduction
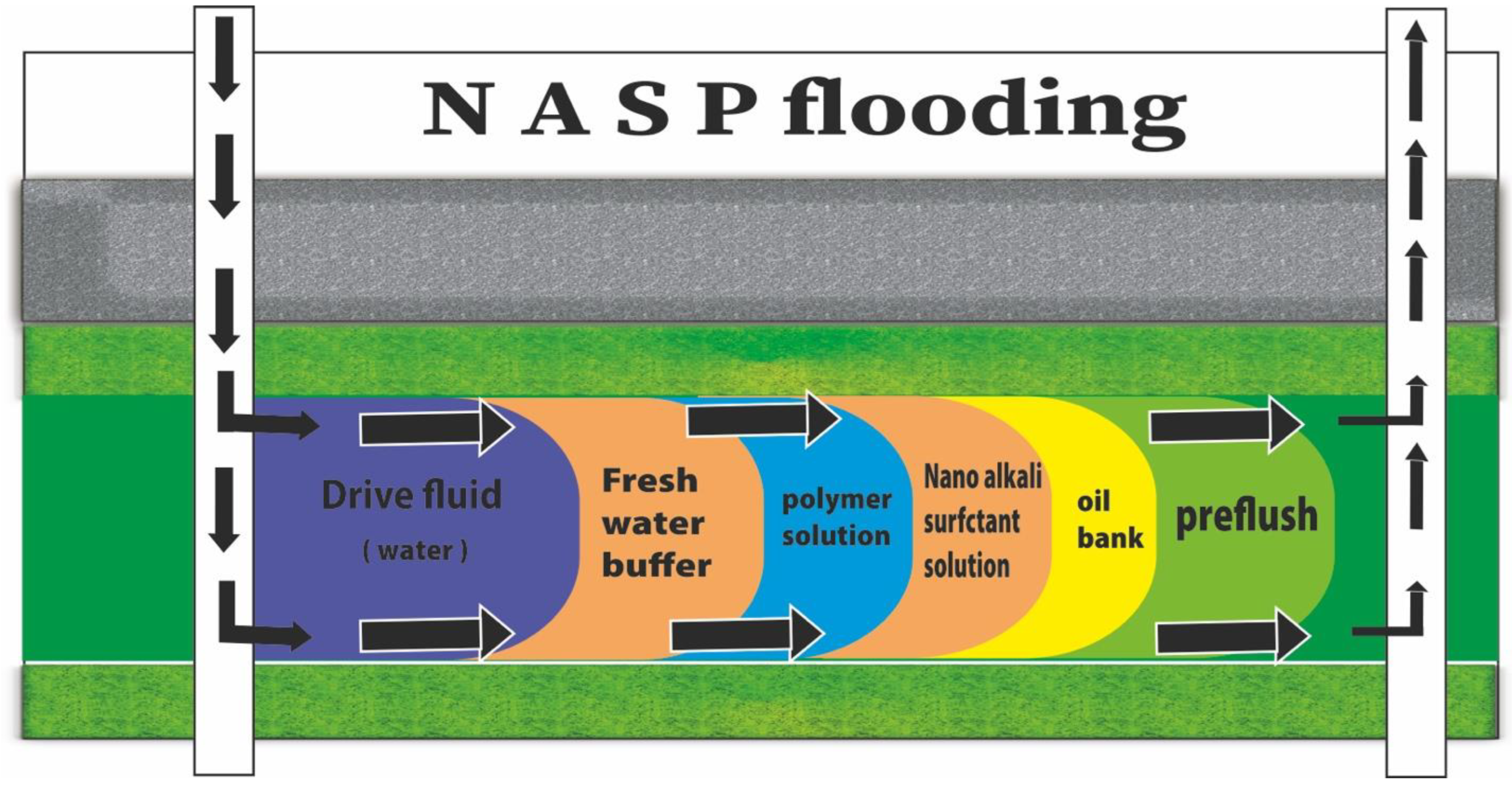
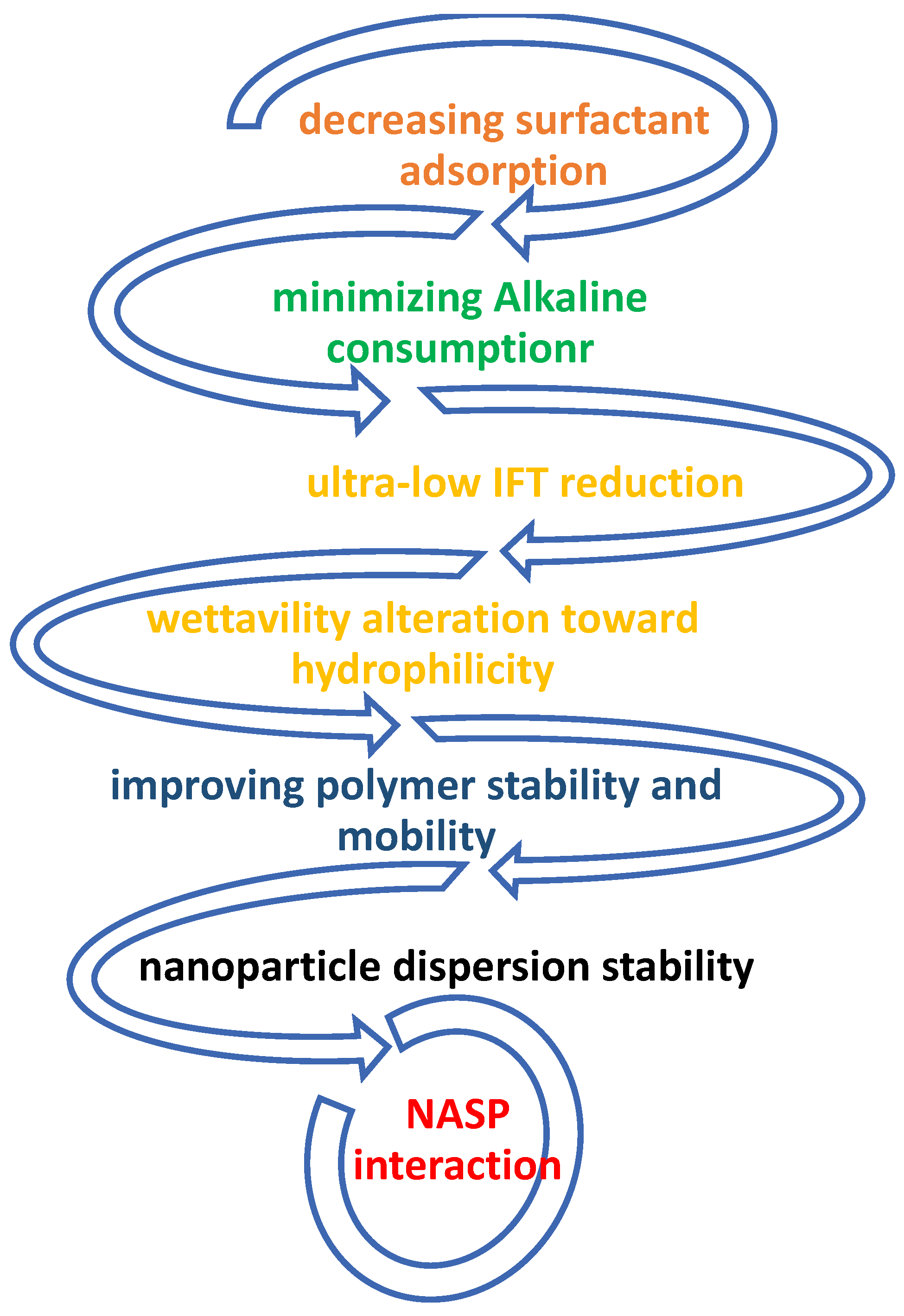
2. The Mechanisms and Role of NASP in CEOR
3. Natural Surfactants
4. Potential of NASP Synergism
5. NASP Prediction Technical Characteristics
- ▪
- The amount of surfactant is significantly lowered in NASP system;
- ▪
- Strong or a weak base alkali is used in the ASP synergy system;
- ▪
- NASP significantly increases oil recovery since it has physical and chemical (dual) effects;
- ▪
- It is forecasted that, when the four-element composites (N, A, S, and P) are used together, the IFT rapidly decreases to 0.001 or lower.
6. Screening the Reservoir Rock Properties
7. ASP/EOR Process Challenges
7.1. Operational Difficulties
7.1.1. Scaling Issues during ASP Flooding
7.1.2. Surfactant Precipitation
8. Prospects and Future Developments of ASP/CEOR
- ▪
- ASP limitations could be due to alkaline since alkaline reduces polymer viscosity. Thus, a big question is: can SP work more effectively than ASP?
- ▪
- Due to the carbonate rock complexity, most of the nano-EOR flooding has to be performed on sandstone rocks. Further studies should be implemented for understanding the effect of oil recovery on carbonate rocks;
- ▪
- More sophisticated and advanced tools should be used to accurately examine the role of NASP in changing the wettability and IFT;
- ▪
- Due to the lack of economic data in the research papers, more economic study should be implemented to evaluate the economic performance of NASP in accelerating oil recovery;
- ▪
- HS and HT could limit NASP to work effectively in maximizing oil recovery. This is why a more effective nano, surfactant and polymer should be developed to limit this issue.
9. NASP Performance Anticipation in Changing the Wettability and IFT
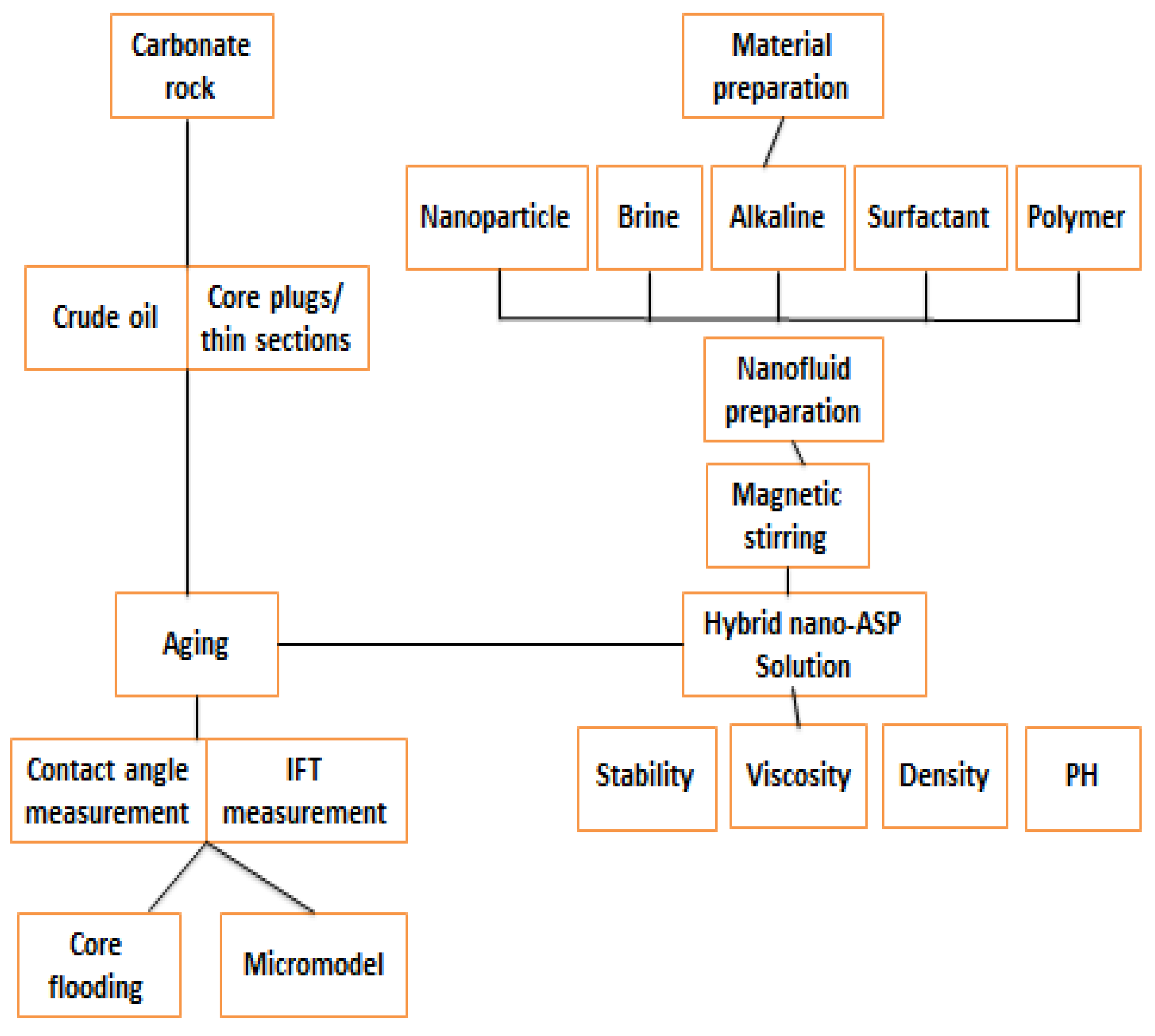
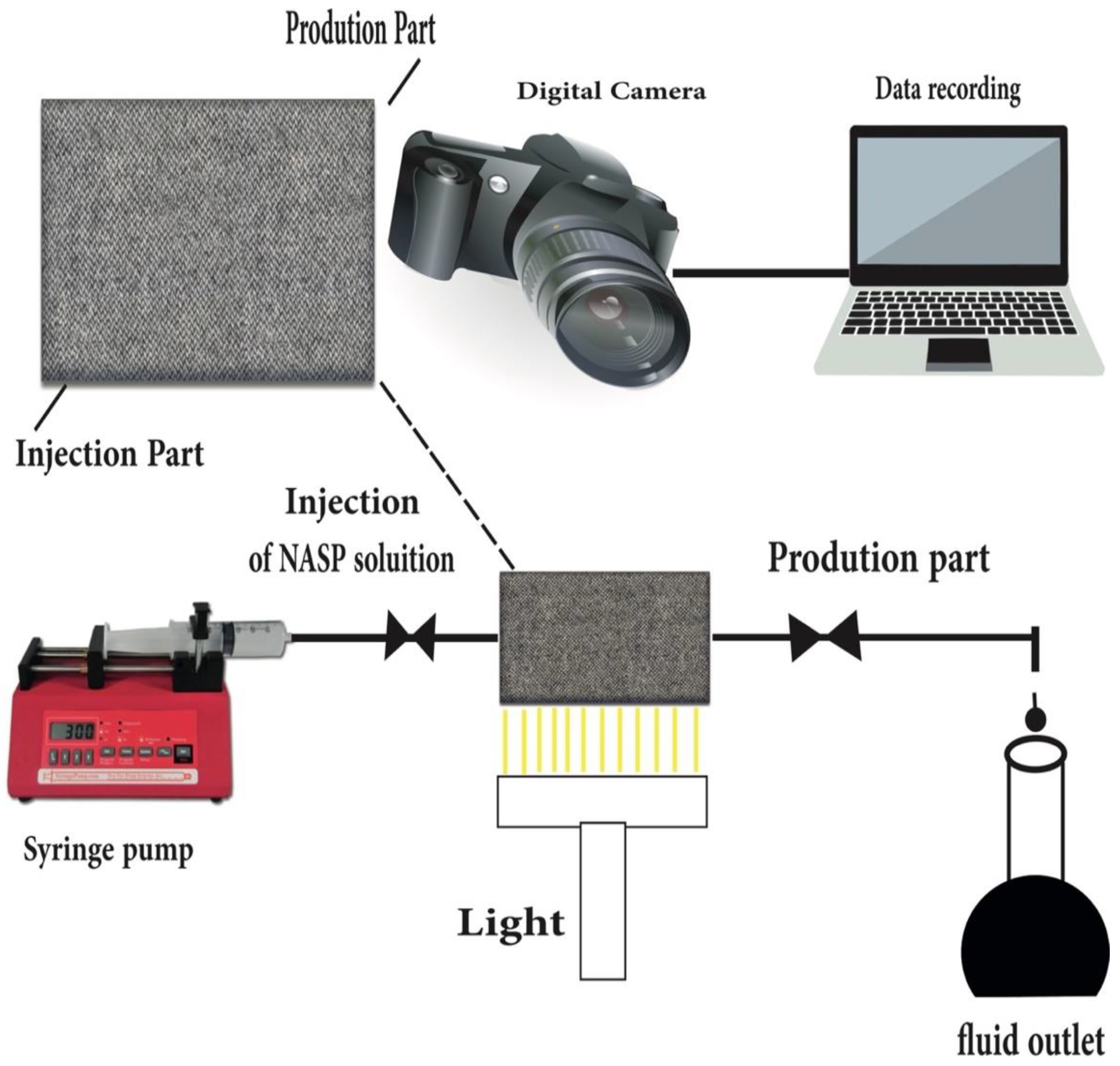
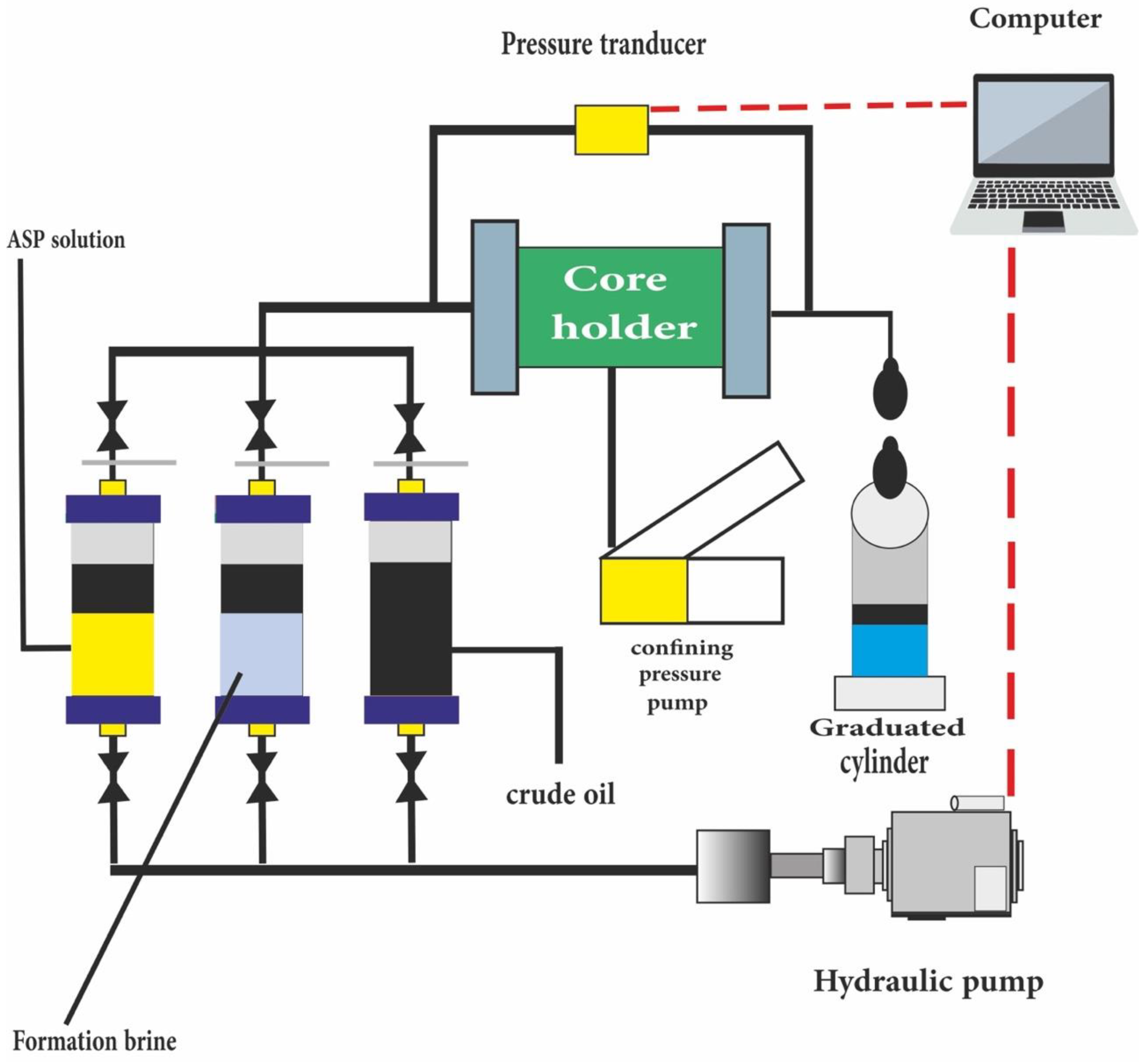
10. Core Flooding
11. Future Design, Materials and Features of NASP Process
11.1. Nano-EOR
11.2. Summary of Nano (NASP) EOR Flooding
- The capability of the nano-polymer suspensions for improving the oil recovery by the following mechanisms:
- Wettability alteration was explored using contact angle measurement; increasing temperature and adding salt to polymeric solutions caused a reduction in shear viscosity, and the addition of NPs to the solutions could relatively recover the viscosity;
- The presence of polymers in the nanofluids improved dispersion stability of NPs;
- The nano-polymer suspensions could improve the ability of the NPs for wettability alteration and faster equilibrium states obtained than the polymer-free nanofluids.
- The performance of the nano-surfactant solutions for improving the oil recovery by the following mechanisms:
- The adsorption process of these substances is one of the important methods to increase the oil recovery factor from oil reservoirs by wettability alteration;
- The results of the IFT experiments of these materials showed that surfactant nanofluid solutions could significantly reduce the IFT value between the oil and water system.
- Alkaline can activate the following mechanisms:
- Interfacial tension reduction;
- Wettability alteration;
- Control of adsorption of ions;
- Improving the emulsion stability;
- Inhibitor of clay swelling.
12. Conclusions
- To sum up, CEOR was applied to greatly increase the ultimate oil recovery by wettability, IFT and mobility modification. This paper will add a new insight integrating nano-alkaline, polymer and surfactant flooding for the first time by addressing the main mechanism of each one. The main conclusions of this paper are as follows:
- ASP limitations could be due to alkaline since alkaline reduces polymer viscosity;
- Due to nano, surfactant, polymer, and alkaline synergy effects, most of the EOR mechanisms are greatly improved, leading to higher oil recovery as compared to using each component alone;
- The objective behind using NASP in hybrid is to modify wettability, IFT and mobility ratios, which are regarded as the main EOR mechanisms;
- NASP type and concentration play a major role in changing wettability and reducing IFT to a minimum level;
- For checking the mobility of chemical EOR, the micromodel is used to find the fluid flow distribution;
- Nanoparticle type and size play a major role in changing wettability and reducing IFT to the minimum level;
- Future recommendations by utilizing NASP will probably be a new finding to understand the details about the EOR system in both micro- and -macroscale settings;
- This review paper highlights the fact that natural surfactants are less costly, biodegradable, available, less toxic, more stable, and environmentally friendly, and it can reduce the IFT to an ultra-low value.
- NASP could effectively boost the oil recovery by more than 25% due to the synergism effect.
Author Contributions
Funding
Conflicts of Interest
References
- Litvinenko, V. The Role of Hydrocarbons in the Global Energy Agenda: The Focus on Liquefied Natural Gas. Resources 2020, 9, 59. [Google Scholar] [CrossRef]
- Sheng, J. Status of Alkaline Flooding Technology. J. Pet. Eng. Technol. 2015, 5, 44–50. [Google Scholar]
- Simon, R. Enhanced oil recovery: Definitions, fundamentals, applications, and research frontiers. Phys. Chem. Earth 1981, 13–14, 447–460. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, G.; Ge, J.; Jin, L. The Effect of Oil Viscosity, Permeability, and Residual Oil Saturation on the Performance of Alkaline Flooding in the Recovery of Heavy Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 702–710. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef]
- Hussein, I.A.; Feng, Y.; Bai, B. Chemical EOR. J. Chem. 2013, 2013, 871542. [Google Scholar] [CrossRef]
- Abbas, A.H.; Ajunwa, O.M.; Mazhit, B.; Martyushev, D.A.; Bou-Hamdan, K.F.; Alsaheb, R.A.A. Evaluation of OKRA (Abelmoschus esculentus) Macromolecular Solution for Enhanced Oil Recovery in Kazakhstan Carbonate Reservoir. Energies 2022, 15, 6827. [Google Scholar] [CrossRef]
- Mohajeri, M.; Reza Rasaei, M.; Hekmatzadeh, M. Experimental study on using SiO2 nanoparticles along with surfactant in an EOR process in micromodel. Pet. Res. 2019, 4, 59–70. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.; Yan, W.; Puerto, M.; Hirasaki, G.J.; Miller, C.A. Favorable Attributes of Alkaline-Surfactant-Polymer Flooding. SPE J. 2008, 13, 5–16. [Google Scholar] [CrossRef]
- Sheng, J.J. A Comprehensive Review of Alkaline-Surfactant-Polymer (ASP) Flooding. In Proceedings of the SPE Western Regional & AAPG Pacific Section Meeting 2013 Joint Technical Conference, Monterey, CA, USA, 19–25 April 2013. SPE-165358-MS. [Google Scholar]
- Burk, J.H. Comparison of Sodium Carbonate, Sodium Hydroxide, and Sodium Orthosilicate for EOR. SPE Reserv. Eng. 1987, 2, 9–16. [Google Scholar] [CrossRef]
- Hazarika, K.; Gogoi, S.B. Effect of alkali on alkali–surfactant flooding in an Upper Assam oil field. J. Pet. Explor. Prod. Technol. 2020, 10, 1591–1601. [Google Scholar] [CrossRef]
- El-Sayed, A.A.H.; Almalik, M.S. Effect of Horizontal-vertical Well Configuration On Oil Recovery By Alkaline Flooding. J. Can. Pet. Technol. 1995, 34, 19–24. [Google Scholar] [CrossRef]
- Rincón-García, F.; Ortiz-Moreno, H.; Marroquín, G.; Moreno-Montiel, N.; Sanchez, S.; Chacon, C.; Sánchez-Minero, F. Enhanced oil recovery by means of alkali injection. Behavior of the SARA fractions. Pet. Sci. Technol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Samanta, A.; Bera, A.; Mandal, A.; Ojha, K. Comparative Studies on Enhanced Oil Recovery by Alkali-Surfactant and Polymer Flooding. J. Pet. Sci. Eng. 2012, 2, 67–74. [Google Scholar] [CrossRef]
- Cooke, C.E., Jr.; Williams, R.E.; Kolodzie, P.A. Oil Recovery by Alkaline Waterflooding. J. Pet. Technol. 1974, 26, 1365–1374. [Google Scholar] [CrossRef]
- Leach, R.O.; Wagner, O.R.; Wood, H.W.; Harpke, C.F. A Laboratory and Field Study of Wettability Adjustment in Water Flooding. J. Pet. Technol. 1962, 14, 206–212. [Google Scholar] [CrossRef]
- Raimondi, P.; Gallagher, B.J.; Ehrlich, R.; Messmer, J.H.; Bennett, G.S. Alkaline Waterflooding: Design and Implementation of a Field Pilot. J. Pet. Technol. 1977, 29, 1359–1368. [Google Scholar] [CrossRef]
- Emery, L.W.; Mungan, N.; Nicholson, R.W. Caustic Slug Injection in the Singleton Field. J. Pet. Technol. 1970, 22, 1569–1576. [Google Scholar] [CrossRef]
- Graue, D.J.; Johnson, C.E., Jr. Field Trial of Caustic Flooding Process. J. Pet. Technol. 1974, 26, 1353–1358. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, G.; Ge, J.; Li, G.; Feng, A. Influence of Oil Viscosity on Alkaline Flooding for Enhanced Heavy Oil Recovery. J. Chem. 2013, 2013, 938237. [Google Scholar] [CrossRef]
- Alam, M.W.; Tiab, D. Mobility Control of Caustic Flood. Energy Sources 1988, 10, 1–19. [Google Scholar] [CrossRef]
- Kazempour, M.; Sundstrom, E.; Alvarado, V. Effect of Alkalinity on Oil Recovery During Polymer Floods in Sandstone. SPE Reserv. Eval. Eng. 2012, 15, 195–209. [Google Scholar] [CrossRef]
- Zhijian, Q.; Yigen, Z.; Xiansong, Z.; Jialin, D. A Successful ASP flooding Pilot in Gudong Oil Field. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. SPE-39613-MS. [Google Scholar]
- Strand, S.; Høgnesen, E.J.; Austad, T. Wettability alteration of carbonates—Effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 1–10. [Google Scholar] [CrossRef]
- Mohajeri, M.; Hemmati, M.; Shekarabi, A.S. An experimental study on using a nanosurfactant in an EOR process of heavy oil in a fractured micromodel. J. Pet. Sci. Eng. 2015, 126, 162–173. [Google Scholar] [CrossRef]
- Gupta, R.; Mohanty, K.K.K. Temperature Effects on Surfactant-Aided Imbibition Into Fractured Carbonates. SPE J. 2010, 15, 588–597. [Google Scholar] [CrossRef]
- Rilian, N.A.; Sumestry, M.; Wahyuningsih. Surfactant Stimulation to Increase Reserves in Carbonate Reservoir: “A Case Study in Semoga Field”. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010. SPE-130060-MS. [Google Scholar]
- Alvarez, J.O.; Neog, A.; Jais, A.; Schechter, D.S. Impact of Surfactants for Wettability Alteration in Stimulation Fluids and the Potential for Surfactant EOR in Unconventional Liquid Reservoirs. In Proceedings of the SPE Unconventional Resources Conference, The Woodlands, TX, USA, 1–3 April 2014. D011S002R003. [Google Scholar]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Pal, S.; Mushtaq, M.; Banat, F.; Al Sumaiti, A.M. Review of surfactant-assisted chemical enhanced oil recovery for carbonate reservoirs: Challenges and future perspectives. Pet. Sci. 2018, 15, 77–102. [Google Scholar] [CrossRef]
- Xie, X.; Weiss, W.W.; Tong, Z.J.; Morrow, N.R. Improved Oil Recovery from Carbonate Reservoirs by Chemical Stimulation. SPE J. 2005, 10, 276–285. [Google Scholar] [CrossRef]
- Vijapurapu, C.S.; Rao, D.N. Compositional effects of fluids on spreading, adhesion and wettability in porous media. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 335–342. [Google Scholar] [CrossRef]
- Adil, M.; Lee, K.; Mohd Zaid, H.; Ahmad, S.; Alnarabiji, M. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR). PLoS ONE 2018, 13, e0193518. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, G.; Ge, J.; Liao, K.; Pei, H.; Jiang, P.; Li, X. Study on organic alkali-surfactant-polymer flooding for enhanced ordinary heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 230–239. [Google Scholar] [CrossRef]
- Mohd Zaid, H.; Ahmad, S.; Yahya, N. The Effect of Zinc Oxide and Aluminum Oxide Nanoparticles on Interfacial Tension and Viscosity of Nanofluids for Enhanced Oil Recovery. Adv. Mater. Res. 2014, 1024, 56–59. [Google Scholar] [CrossRef]
- Onyekonwu, M.; Akaranta, O. Alkaline-Surfactant Flooding in Niger-Delta: An Experimental Approach. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 2–4 August 2016. SPE-184271-MS. [Google Scholar]
- Yang, J.; Jin, B.; Jiang, L.; Liu, F. An Improved Numerical Simulator for Surfactant/Polymer Flooding. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Nusa Dua, Indonesia, 20–22 October 2015. SPE-176206-MS. [Google Scholar]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Chen, T.; Song, Z.; Fan, Y.; Hu, C.; Qiu, L.; Tang, J. A Pilot Test of Polymer Flooding in an Elevated-Temperature Reservoir. SPE Reserv. Eval. Eng. 1998, 1, 24–29. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Lu, Z.; Feng, Y. Thermoviscosifying polymer used for enhanced oil recovery: Rheological behaviors and core flooding test. Polym. Bull. 2012, 70, 391–401. [Google Scholar] [CrossRef]
- Cui, X.-H.; Li, Z.-Q.; Cao, X.-I.; Song, X.-W.; Zhang, X. A Novel PPG Enhanced Surfactant-Polymer System for EOR. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011. SPE-143506-MS. [Google Scholar]
- Mogbo, O. Polymer Flood Simulation in a Heavy Oil Field: Offshore Niger-Delta Experience. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011. [Google Scholar] [CrossRef]
- Gao, P.; Towler, B. Investigation of polymer and surfactant-polymer injections in South Slattery Minnelusa Reservoir, Wyoming. J. Pet. Explor. Prod. Technol. 2011, 1, 23–31. [Google Scholar] [CrossRef][Green Version]
- Zubari, H.K.; Sivakumar, V.C.B. Single Well Tests to determine the Efficiency of Alkaline-Surfactant Injection in a highly Oil-Wet Limestone Reservoir. In Proceedings of the Middle East Oil Show, Sanabis, Bahrain, 5–8 April 2003. SPE-81464-MS. [Google Scholar]
- Vavra, E.; Puerto, M.; Biswal, S.L.; Hirasaki, G.J. A systematic approach to alkaline-surfactant-foam flooding of heavy oil: Microfluidic assessment with a novel phase-behavior viscosity map. Sci. Rep. 2020, 10, 12930. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, M.; Yue, X.; Hou, J. Synergy of alkali and surfactant in emulsification of heavy oil in brine. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 273, 219–228. [Google Scholar] [CrossRef]
- Sheng, J.J. Chapter 8—Alkaline-Surfactant Flooding. In Enhanced Oil Recovery Field Case Studies; Sheng, J.J., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2013; pp. 179–188. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, G.; Ge, J.; Tang, M.; Zheng, Y. Comparative Effectiveness of Alkaline Flooding and Alkaline–Surfactant Flooding for Improved Heavy-Oil Recovery. Energy Fuels 2012, 26, 2911–2919. [Google Scholar] [CrossRef]
- Bryan, J.; Kantzas, A. Potential for Alkali-Surfactant Flooding in Heavy Oil Reservoirs Through Oil-in-Water Emulsification. J. Can. Pet. Technol. 2009, 48, 37–46. [Google Scholar] [CrossRef]
- Sheng, J. Alkaline-Polymer Flooding. In Enhanced oil Recovery Field Case Studies; Gulf Professional Publishing: Boston, MA, USA, 2013; pp. 169–178. [Google Scholar] [CrossRef]
- Pitts, M.J.; Wyatt, K.; Surkalo, H. Alkaline-Polymer Flooding of the David Pool, Lloydminster Alberta. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004. SPE-89386-MS. [Google Scholar]
- Pitts, M.J.; Dowling, P.; Wyatt, K.; Surkalo, H.; Adams, K.C. Alkaline-Surfactant-Polymer Flood of the Tanner Field. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. SPE-100004-MS. [Google Scholar]
- Chen, Z.; Zhao, X.; Wang, Z.; Fu, M. A comparative study of inorganic alkaline/polymer flooding and organic alkaline/polymer flooding for enhanced heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 150–157. [Google Scholar] [CrossRef]
- Doll, T.E. An Update of the Polymer-Augmented Alkaline Flood at the Isenhour Unit, Sublette County, Wyoming. SPE Reserv. Eng. 1988, 3, 604–608. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, M.; Shirif, E. Study of Alkaline/Polymer Flooding for Heavy-Oil Recovery Using Channeled Sandpacks. SPE Reserv. Eval. Eng. 2011, 14, 310–319. [Google Scholar] [CrossRef]
- Yang, D.-H.; Wang, J.-Q.; Jing, L.-X.; Feng, Q.-X.; Ma, X.-P. Case Study of Alkali—Polymer Flooding with Treated Produced Water. In Proceedings of the SPE EOR Conference at Oil & Gas West Asia, Muscat, Oman, 11–13 April 2010. SPE-129554-MS. [Google Scholar]
- Zhang, J.; Wang, K.; He, F.; Zhang, F. Ultimate Evaluation of the Alkali/Polymer Combination Flooding Pilot Test in XingLongTai Oil Field. In Proceedings of the SPE Asia Pacific Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 25–26 October 1999. SPE-57291-MS. [Google Scholar]
- Adams, W.T.; Schievelbein, V.H. Surfactant Flooding Carbonate Reservoirs. SPE Reserv. Eng. 1987, 2, 619–626. [Google Scholar] [CrossRef]
- Sun, C.; Guo, H.; Li, Y.; Song, K. Recent Advances of Surfactant-Polymer (SP) Flooding Enhanced Oil Recovery Field Tests in China. Geofluids 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Sangwai, J.S. Comparative effectiveness of production performance of Pickering emulsion stabilized by nanoparticle–surfactant–polymerover surfactant–polymer (SP) flooding for enhanced oil recoveryfor Brownfield reservoir. J. Pet. Sci. Eng. 2015, 129, 221–232. [Google Scholar] [CrossRef]
- Ge, M.-R.; Miao, S.-J.; Liu, J.-F.; Gang, H.-Z.; Yang, S.-Z.; Mu, B.-Z. Laboratory studies on a novel salt-tolerant and alkali-free flooding system composed of a biopolymer and a bio-based surfactant for oil recovery. J. Pet. Sci. Eng. 2021, 196, 107736. [Google Scholar] [CrossRef]
- Cheraghian, G. An Experimental Study of Surfactant Polymer for Enhanced Heavy Oil Recovery Using a Glass Micromodel by Adding Nanoclay. Pet. Sci. Technol. 2015, 33, 1410–1417. [Google Scholar] [CrossRef]
- Liu, W.; Luo, L.; Liao, G.; Zuo, L.; Wei, Y.; Jiang, W. Experimental study on the mechanism of enhancing oil recovery by polymer-surfactant binary flooding. Shiyou Kantan Yu Kaifa/Pet. Explor. Dev. 2017, 44, 600–607. [Google Scholar] [CrossRef]
- Olsen, D.K.; Hicks, M.D.; Hurd, B.G.; Sinnokrot, A.A.; Sweigart, C.N. Design of a Novel Flooding System for an Oil-Wet Central Texas Carbonate Reservoir. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–25 April 1990. SPE-20224-MS. [Google Scholar]
- Manji, K.H.; Stasiuk, B.W. Design Considerations For Dome’s David Alkali/Polymer Flood. J. Can. Pet. Technol. 1988, 27, 49–54. [Google Scholar] [CrossRef]
- Vargo, J.; Turner, J.; Bob, V.; Pitts, M.J.; Wyatt, K.; Surkalo, H.; Patterson, D. Alkaline-Surfactant-Polymer Flooding of the Cambridge Minnelusa Field. SPE Reserv. Eval. Eng. 2000, 3, 552–558. [Google Scholar] [CrossRef]
- Dang, C.T.; Nguyen, N.T.; Chen, Z.; Nguyen, H.X.; Bae, W.; Phung, T.H. A Comprehensive Evaluation of the Performances of Alkaline/Surfactant/Polymer in Conventional and Unconventional Reservoirs. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 22–24 October 2012. SPE-160444-MS. [Google Scholar]
- Feng, R.-S.; Guo, Y.-J.; Zhang, X.-M.; Hu, J.; Li, H.-B. Alkali/Surfactant/Polymer Flooding in the Daqing Oilfield Class II Reservoirs Using Associating Polymer. J. Chem. 2013, 2013, 275943. [Google Scholar] [CrossRef] [PubMed]
- Bealessio, B.A.; Blánquez Alonso, N.A.; Mendes, N.J.; Sande, A.V.; Hascakir, B. A review of enhanced oil recovery (EOR) methods applied in Kazakhstan. Petroleum 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Bormashenko, E.; Balter, S.; Bormashenko, Y.; Aurbach, D. Honeycomb structures obtained with breath figures self-assembly allow water/oil separation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 394–398. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.; Tiwari, P. Impact of Natural Surfactant (Reetha), Polymer (Xanthan Gum), and Silica Nanoparticles To Enhance Heavy Crude Oil Recovery. Energy Fuels 2019, 33, 4225–4236. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Arabsahebi, Y.; Shadizadeh, S.R.; Shokrollahzadeh Behbahani, S. Preliminary evaluation of mulberry leaf-derived surfactant on interfacial tension in an oil-aqueous system: EOR application. Fuel 2014, 117, 749–755. [Google Scholar] [CrossRef]
- Shadizadeh, S.; Kharrat, R. Experimental Investigation of Matricaria chamomilla Extract Effect on Oil-Water Interfacial Tension: Usable for Chemical Enhanced Oil Recovery. Pet. Sci. Technol. 2015, 33, 901–907. [Google Scholar] [CrossRef]
- Jalali, A.; Mohsenatabar Firozjaii, A.; Shadizadeh, S.R. Experimental investigation on new derived natural surfactant: Wettability alteration, IFT reduction, and core flooding in oil wet carbonate reservoir. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–11. [Google Scholar] [CrossRef]
- Saxena, N.; Saxena, A.; Mandal, A. Synthesis, characterization and enhanced oil recovery potential analysis through simulation of a natural anionic surfactant. J. Mol. Liq. 2019, 282, 545–556. [Google Scholar] [CrossRef]
- Deymeh, H.; Shadizadeh, S.R.; Motafakkerfard, R. Experimental investigation of Seidlitzia rosmarinus effect on oil–water interfacial tension: Usable for chemical enhanced oil recovery. Sci. Iran. 2012, 19, 1661–1664. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Mandal, A. Characterizations of Surfactant Synthesized from Jatropha Oil and its Application in Enhanced Oil Recovery. AIChE J. 2017, 63, 2731–2741. [Google Scholar] [CrossRef]
- Rahmati, M.; Mashayekhi, M.; Songolzadeh, R.; Daryasafar, A. Effect of Natural Leaf-derived Surfactants on Wettability Alteration and Interfacial Tension Reduction in Water-oil System: EOR Application. J. Jpn. Pet. Inst. 2015, 58, 245–251. [Google Scholar] [CrossRef]
- Khorram Ghahfarokhi, A.; Dadashi, A.; Daryasafar, A.; Moghadasi, J. Feasibility study of new natural leaf-derived surfactants on the IFT in an oil–aqueous system: Experimental investigation. J. Pet. Explor. Prod. Technol. 2015, 5, 375–382. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Taber, J.J. Technical Screening Guides for the Enhanced Recovery of Oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Francisco, CA, USA, 5–8 October 1983. SPE-12069-MS. [Google Scholar]
- Moe Soe Let, K.P.; Manichand, R.N.; Seright, R.S. Polymer Flooding a ~500-cp Oil. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. SPE-154567-MS. [Google Scholar]
- Vermolen, E.C.; van Haasterecht, M.J.; Masalmeh, S.K.; Faber, M.J.; Boersma, D.M.; Gruenenfelder, M. Pushing the Envelope for Polymer Flooding Towards High-temperature and High-salinity Reservoirs with Polyacrylamide Based Ter-polymers. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011. SPE-141497-MS. [Google Scholar]
- French, T. Evaluation of the Sho-Vel-Tum Alkali-Surfactant-Polymer (ASP) Oil Recovery Project-Stephens County, OK; National Petroleum Technology Office (NPTO): Tulsa, OK, USA, 1999.
- Raney, K.; Ayirala, S.; Chin, R.; Verbeek, P. Surface and Subsurface Requirements for Successful Implementation of Offshore Chemical Enhanced Oil Recovery. SPE Prod. Oper. 2012, 27, 294–305. [Google Scholar] [CrossRef]
- He, L.; Gang, C.; Guochen, S. Technical Breakthrough in PCPs’ Scaling Issue of ASP Flooding in Daqing Oilfield. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. [Google Scholar]
- Arensdorf, J.; Hoster, D.; McDougall, D.; Yuan, M. Static and dynamic testing of silicate scale inhibitors. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010. [Google Scholar]
- Song, R.; Xu, D.; Zuo, X.; Ma, Z.; Qing, H.; Liu, Y. A case study of ASP multi-well groups. In Proceedings of the SPE EOR Conference at Oil & Gas West Asia, Muscat, Oman, 11–13 April 2010. [Google Scholar]
- Li, Y.; Wang, F.; Wu, J.; Yang, Z.; Chen, G.; Zong, L.; Peng, S. Current status and prospects of ASP flooding in Daqing Oil Fields. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Chang, H.L.; Zhang, Z.Q.; Wang, Q.M.; Xu, Z.S.; Guo, Z.D.; Sun, H.Q.; Cao, X.L.; Qiao, Q. Advances in Polymer Flooding and Alkaline/Surfactant/Polymer Processes as Developed and Applied in the People’s Republic of China. J. Pet. Technol. 2006, 58, 84–89. [Google Scholar] [CrossRef]
- Pu, H. An update and perspective on field-scale chemical floods in Daqing oilfield, China. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Exhibition, Bahrain, 15–18 March 2009. [Google Scholar]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry, L., Ed.; Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Rezaei, A.; Riazi, M.; Escrochi, M.; Elhaei, R. Integrating surfactant, alkali and nano-fluid flooding for enhanced oil recovery: A mechanistic experimental study of novel chemical combinations. J. Mol. Liquids 2020, 308, 113106. [Google Scholar] [CrossRef]
- Rajabi, M.; Moradi, R.; Mehrizadeh, M. Experimental investigation of chemical solutions effects on wettability alteration and interfacial tension reduction using nano-alkaline–surfactant fluid: An EOR application in carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2021, 11, 1925–1941. [Google Scholar] [CrossRef]
- Salih, N.M.; Kamal, I.M.; Préat, A. Classical observations and stable isotopes for identification the diagenesis of Jeribe formation at Jambour oil Fields-Kurdistan Region-Iraq. Pet. Sci. Technol. 2019, 37, 1548–1556. [Google Scholar] [CrossRef]
- Salih, N.; Mansurbeg, H.; Kolo, K.; Gerdes, A.; Préat, A. In situ U-Pb dating of hydrothermal diagenesis in tectonically controlled fracturing in the Upper Cretaceous Bekhme Formation, Kurdistan Region-Iraq. Int. Geol. Rev. 2020, 62, 2261–2279. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2014, 5, 181–199. [Google Scholar] [CrossRef]
- Abhishek, R.; Hamouda, A.A.; Murzin, I. Adsorption of silica nanoparticles and its synergistic effect on fluid/rock interactions during low salinity flooding in sandstones. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 397–406. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Jiang, S. Review on enhanced oil recovery by nanofluids. Oil Gas Sci. Technol. 2018, 73, 37. [Google Scholar] [CrossRef]
- Moradi, B.; Pourafshary, P.; Jalali Farahani, F.; Mohammadi, M.; Emadi, M.A. Application of SiO2 Nano Particles to Improve the Performance of Water Alternating Gas EOR Process. In Proceedings of the SPE Oil & Gas India Conference and Exhibition, Mumbai, India, 24–26 November 2015. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Zhu, J.; Cao, F.; Liu, Y.; Li, X.; Willson, R.; Yang, Z.; Chu, C.-W.; Ren, Z. Nanofluid of graphene-based amphiphilic Janus nanosheets for tertiary or enhanced oil recovery: High performance at low concentration. Proc. Natl. Acad. Sci. USA 2016, 113, 201608135. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Chen, G.; Tailin, L.; Ren, D.; Ma, J.; Sheng, Y.; Karwani, S. Wettability of Hybrid Nanofluid-Treated Sandstone/Heavy Oil/Brine Systems: Implications for Enhanced Heavy Oil Recovery Potential. Energy Fuels 2018, 32, 11118–11135. [Google Scholar] [CrossRef]
- Roustaei, A.; Moghadasi, J.; Iran, A.; Bagherzadeh, H.; Shahrabadi, A. An Experimental Investigation of Polysilicon Nanoparticles’ Recovery Efficiencies through Changes in Interfacial Tension and Wettability Alteration. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. SPE-156976-MS. [Google Scholar]
- Dahkaee, K.P.; Sadeghi, M.T.; Fakhroueian, Z.; Esmaeilzadeh, P. Effect of NiO/SiO2 nanofluids on the ultra interfacial tension reduction between heavy oil and aqueous solution and their use for wettability alteration of carbonate rocks. J. Pet. Sci. Eng. 2019, 176, 11–26. [Google Scholar] [CrossRef]
- Onyekonwu, M.O.; Ogolo, N. Investigating the Use of Nanoparticles in Enhancing Oil Recovery. In Proceedings of the Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 6–8 August 2010; Volume 2. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, K.; Zydney, A.L. Effect of ionic strength on membrane fouling during ultrafiltration of plasmid DNA. Sep. Purif. Technol. 2017, 176, 287–293. [Google Scholar] [CrossRef]
- Bayat, A.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of Metal Oxide Nanoparticles on Enhanced Oil Recovery from Limestone Media at Several Temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Mohammadi, M.; Moghadasi, J.; Naseri, S. An Experimental Investigation of Wettability Alteration in Carbonate Reservoir Using#3-Al2O3 Nanoparticles. Iran. J. Oil Gas Sci. Technol. 2014, 3, 18–26. [Google Scholar]
- Naik, S.; You, Z.; Bedrikovetsky, P. Rate enhancement in unconventional gas reservoirs by wettability alteration. J. Nat. Gas Sci. Eng. 2015, 26, 1573–1584. [Google Scholar] [CrossRef]
- Ogolo, N.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced Oil Recovery Using Nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar] [CrossRef]
- Azarshin, S.; Moghadasi, J.; Arab Aboosadi, Z. Surface functionalization of silica nanoparticles to improve the performance of water flooding in oil wet reservoirs. Energy Explor. Exploit. 2017, 35, 014459871771628. [Google Scholar] [CrossRef]
- Nazari Moghaddam, R.; Bahramian, A.; Fakhroueian, Z.; Arya, S. A Comparative Study of Using Nanoparticles for Enhanced Oil Recovery: Wettability Alteration of Carbonate Rocks. Energy Fuels 2015, 29, 150223100050005. [Google Scholar] [CrossRef]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef]
- El-Diasty, A.I.; Aly, A.M. Understanding the Mechanism of Nanoparticles Applications in Enhanced Oil Recovery. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–16 September 2015. D021S009R004. [Google Scholar]
- Ju, B.; Fan, T. Experimental study and mathematical model of nanoparticle transport in porous media. Powder Technol. 2009, 192, 195–202. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Sharifi, M.; Riazi, M.; Rezvani, H.; Tabaei, M. Potential effects of metal oxide/SiO2 nanocomposites in EOR processes at different pressures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 372–384. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Engeset, B.; Suwarno, S.; Torsæter, O. Improved Oil Recovery by Nanofluids Flooding: An Experimental Study. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2012. SPE-163335-MS. [Google Scholar]
- Ehtesabi, H.; Ahadian, M.; Taghikhani, V. Enhanced Heavy Oil Recovery Using TiO 2 Nanoparticles: Investigation of Deposition during Transport in Core Plug. Energy Fuels 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Nikolov, A.; Wasan, D. Enhanced Oil Recovery (EOR) Using Nanoparticle Dispersions: Underlying Mechanism and Imbibition Experiments. Energy Fuels 2014, 28, 3002–3009. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V.; Ghazanfari, M.H. Enhanced Heavy Oil Recovery in Sandstone Cores Using TiO2 Nanofluids. Energy Fuels 2014, 28, 423–430. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohammadi, S.; Ghazanfari, M.H.; Kharrat, R.; Masihi, M. Monitoring wettability alteration by silica nanoparticles during water flooding to heavy oils in five-spot systems: A pore-level investigation. Exp. Therm. Fluid Sci. 2012, 40, 168–176. [Google Scholar] [CrossRef]
- Hu, Z.; Azmi, S.M.; Raza, G.; Glover, P.W.J.; Wen, D. Nanoparticle-Assisted Water-Flooding in Berea Sandstones. Energy Fuels 2016, 30, 2791–2804. [Google Scholar] [CrossRef]
- Nwidee, L.; Al-Anssari, S.; Barifcani, A.; Sarmadivaleh, M.; Lebedev, M.; Iglauer, S. Nanoparticles Influence on Wetting Behaviour of Fractured Limestone Formation. J. Pet. Sci. Eng. 2016, 149, 782–788. [Google Scholar] [CrossRef]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Jiang, R.; Li, K.; Horne, R. A Mechanism Study of Wettability and Interfacial Tension for EOR Using Silica Nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, CA, USA, 9–11 October 2017. D031S046R003. [Google Scholar]
- Mohd, S.; Moslan, M.; Rosli, W.; Wan Sulaiman, W.R.; Ismail, A.; Jaafar, M.Z. Applications of Aluminium Oxide and Zirconium Oxide Nanoparticles in Altering Dolomite Rock Wettability using Different Dispersing Medium. Chem. Eng. Trans. 2017, 56, 1339–1344. [Google Scholar] [CrossRef]
- Cheraghian, G.; Sajad, K.; Nassar, N.; Alexander, S.; Barron, A. Silica Nanoparticle Enhancement in the Efficiency of Surfactant Flooding of Heavy Oil in a Glass Micromodel. Ind. Eng. Chem. Res. 2017, 56, 8528–8534. [Google Scholar] [CrossRef]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Manteghian, M. Investigation of synergistic effects between silica nanoparticles, biosurfactant and salinity in simultaneous flooding for enhanced oil recovery. RSC Adv. 2019, 9, 20281–20294. [Google Scholar] [CrossRef]
- Nwidee, L.N.; Al-Anssari, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. Nanofluids for Enhanced Oil Recovery Processes: Wettability Alteration Using Zirconium Oxide. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 22–25 March 2016. D011S002R003. [Google Scholar]
- Haeri, F.; Rao, D.N. Precise Wettability Characterization of Carbonate Rocks To Evaluate Oil Recovery Using Surfactant-Based Nanofluids. Energy Fuels 2019, 33, 8289–8301. [Google Scholar] [CrossRef]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability Alteration of Sandstone Cores by Alumina-Based Nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Hosseinpour, N.; Bahramian, A.; Fakhroueian, Z.; Arya, S. Effect of ZrO2 nanoparticles on the interfacial behavior of surfactant solutions at air–water and n-heptane–water interfaces. Fluid Phase Equilibria 2014, 361, 289–295. [Google Scholar] [CrossRef]
- Suleimanov, B.; Ismayilov, F.; Veliyev, E. Nanofluid for enhanced oil recovery. J. Pet. Sci. Eng. 2011, 78, 431–437. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Allam, N.K. Unveiling the Synergistic Effect of ZnO Nanoparticles and Surfactant Colloids for Enhanced Oil Recovery. Colloid Interface Sci. Commun. 2019, 29, 33–39. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Zhong, X.; Sun, W.; Pu, H.; Zhao, J.X. Surfactant-Augmented Functional Silica Nanoparticle Based Nanofluid for Enhanced Oil Recovery at High Temperature and Salinity. ACS Appl. Mater. Interfaces 2019, 11, 45763–45775. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Son, H.; Kim, H.; Kim, J.W. Nanofluid Enhanced Oil Recovery Using Hydrophobically Associative Zwitterionic Polymer-Coated Silica Nanoparticles. Energy Fuels 2017, 31, 7777–7782. [Google Scholar] [CrossRef]
- Qi, L.; Song, C.; Wang, T.; Li, Q.; Hirasaki, G.J.; Verduzco, R. Polymer-Coated Nanoparticles for Reversible Emulsification and Recovery of Heavy Oil. Langmuir 2018, 34, 6522–6528. [Google Scholar] [CrossRef]
- Cheraghian, G. Effect of Nanoclay on Heavy Oil Recovery During Polymer Flooding. Pet. Sci. Technol. 2015, 33, 999. [Google Scholar] [CrossRef]
- Sharma, T.; Sangwai, J.S. Silica nanofluids in polyacrylamide with and without surfactant: Viscosity, surface tension, and interfacial tension with liquid paraffin. J. Pet. Sci. Eng. 2017, 152, 575–585. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Silica Nanoparticle Assisted Polymer Flooding of Heavy Crude Oil: Emulsification, Rheology, and Wettability Alteration Characteristics. Ind. Eng. Chem. Res. 2018, 57, 6364–6376. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Pet. Sci. Eng. 2013, 11, 128–138. [Google Scholar] [CrossRef]



| CEOR Type | Ref. | Chemical Name | Conc. | Porosity % | Permeability (md) | Lithology | |
|---|---|---|---|---|---|---|---|
| Alkaline | [12] | Na2CO3 | 1.0 | NA. | 405–608 | Sandstone | |
| NaOH | 1.0 | ||||||
| Na4Si04 | 0.5 | ||||||
| [13] | Na2CO3 | 0.85 | 25 | 70 | Sandstone | ||
| [2] | NaOH | 0.5 | NA. | 1580 | Sandstone | ||
| [14] | NaOH | 0.5 | 33.59 | 6000 | Sandstone | ||
| [15] | NaOH | 0.2 | NA | NA | Carbonate | ||
| [2] | Na2CO3 | NA | 35.5 | 3200 | Sandstone | ||
| [16] | NaOH | 0.5 | 38.7 | NA | Sandstone | ||
| [17] | NaOH | 0.2 | NA | NA | Sandstone | ||
| [18] | NaOH | NA | 15 | 119 | Sandstone | ||
| [19] | NaOH | 4.85 | 20.6 | 25 | Sandstone | ||
| [20] | NaOH | 0.15 | 16 | NA | Sandstone | ||
| [21] | NaOH | 0.2 | 30 | 495–320 | Sandstone | ||
| [22] | NaOH | 0.5 | NA | 2110 | Sandstone | ||
| [23] | NaOH | 1.0 | NA | NA | sandstone | ||
| [24] | NaOH | 2.0 | 19.7 | 93.64 | Sandstone Sandstone | ||
| Na2CO3 | 2.0 | 19.6 | 176.25 | ||||
| [25] | Na2C03 | 1.20 | 35 | 3850 | Sandstone | ||
| Surfactant | [26] | Cationic C12TAB | NA | 45–50 | 2–5 | Carbonate | |
| [27] | SDS | 1000 | 52.2 | 250 | Carbonate | ||
| [25] | OPIO and CY1 | NA | 35 | 3850 | Sandstone | ||
| [28] | Anionic surfactant | NA | 29.06 | 19.72 | Carbonate | ||
| [29] | Nonionic surfactants | NA | NA | 20.89 | Carbonate | ||
| [30] | Anionic surfactant | NA | 3–5 | NA | Carbonate | ||
| [31] | nonionic ethoxy alcohol | 3000–4000 | 3–5 | NA | Carbonate | ||
| [32] | Anionic (GAC) surfactants | NA | NA | NA | Carbonate | ||
| [33] | Nonionic (POA) | 750 | 15.4 | 56 | Carbonate | ||
| [34] | Nonionic ethoxy alcohol | 50–3500 | NA | NA | Carbonate | ||
| [16] | SDS | 1000 | 38.6 | 212 | Sandstone | ||
| [35] | SDBS | 250 | 37 | 284 | Sandstone | ||
| [36] | SLPS | 1000 | 38 | NA | Sandstone | ||
| [37] | SDS | 3000 | NA | NA | Glass bed | ||
| [38] | XD | 1000 | NA | NA | Sandstone | ||
| [39] | SDBS | 2000 | 25 | NA | Sandstone | ||
| [13] | SDS | NA | 23–29 | 50–94 | Sandstone | ||
| Polymer | [12] | PAM | 3200 | NA | 405 to 608 | Sandstone | |
| Xanthan gum | 1540 | NA | 405 to 608 | Sandstone | |||
| [36] | (HPAM) | 1000 | 37.5 | 3780 | Sandstone | ||
| [40] | (Gum Arabic/Poly acrid | NA | NA | NA | Sandstone | ||
| [41] | HPAM | 1100 | 21.6 | 420 | Sandstone | ||
| [42] | TVP | 2000 | NA | NA | Sandstone | ||
| [43] | HPAM | 1800 | NA | NA | Sandstone | ||
| [44] | PAM | 500 | >39 | 100–60 | Sandstone | ||
| [16] | PHPAM | 1500 | 37.3 | 218 | Sandstone | ||
| [45] | HPAM | 1200 | 15.2 | 23.34 | Sandstone | ||
| Alkaline Surfactant | [46] | Xylene + NaOH | 5000 + 10,000 | NA | NA | Carbonate | |
| [47] | IOS + Na2CO3 | (200–10,000) + 5000 | 37 | 2400 | Micromodel | ||
| [48] | Na2CO3 + alkyl ether sulfates | 1500 + 50 | NA | NA | NA | ||
| [38] | Na2CO3 + XD | 10,000 + 1000 | NA | NA | Sandstone | ||
| Na2CO3 + SDS | 10,000 + 10,000 | NA | NA | Sandstone | |||
| [49] | (IOS) + NaOH + Na4Si04 | NA | NA | NA | Sandstone | ||
| [50] | NaOH + SLPS | 3000 + 300 | 44.7 | 2131 | Sandstone | ||
| NaOH + SLPS | 8000 + 1000 | 43.50 | 1994 | ||||
| NaOH + SLPS | 10,000 + 1000 | 44.21 | 2016 | ||||
| [13] | SDS + Na2CO3 | 1000 + 8500 | 25 | 70 | Sandstone | ||
| [51] | Na2CO3 + sodium alkane sulfonate | (1000–15,000) + 1000 | 41–45 | 790–19,220 | Sandstone | ||
| Alkaline polymer | [52] | Na2CO3 + anionic PAM | NA | 15.5 | 21 | Sandstone | |
| [53] | Na2CO3 + Pusher 1000E | 8000 + 600 | 29 | 1400 | Sandstone | ||
| [54] | NaOH + Alcoflood 1275A | 10,000 + 1000 | 20 | 200 | Sandstone | ||
| [55] | NaOH + HPAM | 10,000 + 1000 | 38.92 | 2350 | Sandstone | ||
| Ethylenediamine + HPAM | 10,000 + 1000 | 39.41 | 2230 | ||||
| Na2CO3 + HPAM | 10,000 + 1000 | 40.33 | 2420 | ||||
| [56] | Na2CO3 + anionic PAM | NA | 15.5 | 21 | Carbonate | ||
| [57] | (NaOH + Na2CO3) + HPAM | (4000 + 2000) + 250 | 34.43 | 4800 | Sandstone | ||
| [57] | HPAM + (NaOH + Na2CO3) | 1000 + (1000 + 2000) | 35.25 | 6400 | Sandstone | ||
| [52] | Na2CO3 + Alcoflood 1175 | 10,000 + 800 | 29 | 1400 | Sandstone | ||
| [58] | Na2CO3 + PAM | 10,000 + 1500 | 31 | 840.9 | Sandstone | ||
| [25] | Na2C03 + OP-10 | 1200 + 10,000 | 35 | 3850 | Sandstone | ||
| [59] | Na2CO3 + HPAM | 20,000 + 1000 | 27.6 | 2063 | Carbonate | ||
| Surfactant polymer | [60] | alkyl ether sulfates + Witco petroleum sulfonate | 1000 + 1000 | 12 | 5.9 | Carbonate | |
| [61] | Amphoteric + PAM | 2500 + 1400 | 29.1 | 3442 | Sandstone | ||
| [62] | PAM + SDS | 1000 + 2200 | 21 | 66 | Sandstone | ||
| [16] | SDS + PHPAM | 1000 + 2000 | 36.8 | 1224 | Sandstone | ||
| [63] | bio-surfactant and biopolymer | 1001 + 5000 | 17 | 400 | Sandstone | ||
| [61] | PS + PAM | 2000 + 2000 | 21 | 115 | Sandstone | ||
| [39] | SDBS + HPAM | 2000 + 2000 | 25 | NA | Sandstone | ||
| [64] | PAM + SDS | 2800 + 1000 | NA | NA | Glass micromodel | ||
| PAM + SDS | 2800 + 2000 | ||||||
| PAM + SDS | 2800 + 3000 | ||||||
| [61] | (Amphoteric +anionic) + PAM | 1200 + 1500 | 15 | 110 | Sandstone | ||
| [36] | HPAM + SDS | 1000 + 1000 | 38 | 1410 | Sandstone | ||
| [45] | anionic surfactant + HPAM | 1200 + 1200 | 15.2 | 23.34 | Sandstone | ||
| [43] | SLPS + HPAM | 4000 + 1800 | NA | 1500 | Sandstone | ||
| [65] | KPS + HPAM | 3000 + 115 | 14.7 | 5.08 | Sandstone | ||
| [43] | SLPS + (HPAM) | 4000 + 1800 | NA | 1500 | Sandstone | ||
| Alkaline surfactant polymer (ASP) | [60] | NaOH + SDS + PHPAM | 5000 + 1000 + 2500 | NA | NA | Sandstone | |
| NaOH + SDS + PHPAM | 5000 + 2000 + 2500 | ||||||
| NaOH + SDS + PHPAM | 5000 + 3000 + 2500 | ||||||
| [66] | Amphoteric Petrostep B-100 + Pusher 700E + Na2CO3 | 4000 + 1200 + 20,000 | 8–43 | 1–600 | Carbonate | ||
| [67] | Na2CO3 + SDS+ PAM | 10,000 + 1000 + 800 | NA | NA | Sandstone | ||
| [16] | NaOH + SDS + PHPAM | 5000 + 1000 + 1500 | 38.7 | NA | Sandstone | ||
| NaOH + SDS + PHPAM | 7000 + 1000 + 1500 | ||||||
| NaOH + SDS + PHPAM | 10,000 + 1000 + 1500 | ||||||
| [16] | NaOH + SDS + PHPAM | 5000 + 1000 + 1500 | 38.7 | NA | Sandstone | ||
| NaOH + SDS + PHPAM | 5000 + 1000 + 2000 | ||||||
| NaOH + SDS + PHPAM | 5000 + 1000 + 2500 | ||||||
| [68] | Na2CO3 + Petrostep B-100 + Alcoflood1175A | 12500 + 1000 + 1475 | 18 | 845 | Carbonate | ||
| [69] | Diethylene glycol butyl ether + alcoflood-2545 + NaBO2 | 3000 + 10,000 + 10,000 | 17.7 | 239 | Sandstone | ||
| [70] | HAPAM + NaOH + heavy alkylbenzene sulfonate | 1000 + 1200 + 3000 | NA | 252 | Sandstone | ||
| [71] | Na2CO3 + (anionic BES and lignosulfonate PS) + PAM | 12,000 + 3000 + 1700 | NA | NA | Sandstone | ||
| CEOR Type | Ref. | Work Type | Oil Improvement % | Remark | |||
| Alkaline | [12] | Experimental | 17.2 | Na2CO3 is more effective for oil increment | |||
| 9.42 | |||||||
| 8.91 | |||||||
| [13] | Experimental | 4.4 | Due to soap formation, Interfacial tension between oleic and aqueous phase reduced | ||||
| [2] | Experimental | 12.4 | Low salinity leads to O/W emulsions if the salinity is above 0.7 W/O emulsions happen | ||||
| [14] | Experimental | 13.33 | IFT reduction due to soap formation improves oil recovery | ||||
| [15] | Experimental | NA | Alkaline flooding is more applicable for medium crude oil as compared to light crude oil due to a higher ratio of soap formation in medium crude oil | ||||
| [2] | Experimental | 14 | Alkaline is also applicable and can accelerate oil recovery in horizontal wells | ||||
| [16] | Experimental | 13.88 | Strong base (NaOH) alkaline injection enhanced oil recovery | ||||
| [17] | Experimental | NA | Higher oil recovery due to in situ. Emulsion formation | ||||
| [18] | Experimental | 2 | additional oil guaranteed by changing the wettability | ||||
| [19] | Field | 6–8 | the amount of IFT reduction determines the success of alkali job | ||||
| [20] | Field | 2 | Changing in rock surface wettability directly affects oil recovery | ||||
| [21] | Field | 5 to 7 | Formation of the emulsion by alkaline improves volumetric sweep efficiency | ||||
| [22] | Experimental | 12.9 | The oil displacement experiment proved that oil recovery is enhanced by using alkaline injection | ||||
| [23] | Experimental | <1 | Orthosilicate was very successful at stopping water channeling and increasing oil recovery | ||||
| [24] | Experimental | 2.52 3.67 | NaOH is more effective than Na2CO3 | ||||
| [25] | Field | 9.13 | Ultra-low IFT after alkaline flooding | ||||
| Surfactant | [26] | Experimental | 20 | changing rock surface wettability due to the sulfate that is present in the injection fluid | |||
| [27] | Experimental | 9 | Oil recovery affected by the type and concentration of the surfactant used in the formation | ||||
| [25] | Field | 11.64 | Higher amount of IFT reduction leads to more oil recovery | ||||
| [28] | Experimental | 30 | Optimum surfactant concentration is related with brine salinity | ||||
| [29] | Field | NA. | About 58,000 bbl of oil is produced after using Nonionic surfactants over only three months | ||||
| [30] | Experimental | NA | The performance of anionic surfactants was more effective than nonionic surfactants | ||||
| [31] | Experimental | 15.0 | to optimize surfactant performance injection rate, conc. and volume are the important parameters | ||||
| [32] | Experimental | NA | IFT significantly diminished | ||||
| [33] | Experimental | 10.4 | Nonionic surfactant outperformed cationic surfactant | ||||
| [34] | Experimental | NA | surfactants decreased IFT and changed the contact angle | ||||
| [16] | Experimental | 17.96 | IFT decreased marginally | ||||
| [35] | Experimental | 2.1 | Due to surfactant degradation, the oil recovery was low | ||||
| [36] | Experimental | 4 | Increase in capillary number yields more oil recovery | ||||
| [37] | Experimental | NA | IFT reduced from 19.59 to 2.82 mN/m | ||||
| [38] | Experimental | 11.5 | At lower concentration, a novel XD yielded a good oil recovery that can be compared with SDS | ||||
| [39] | Experimental | 4.6 | Compared to SP flooding, oil recovery by using surfactant flooding was reduced | ||||
| [13] | Experimental | 7.1 | Adsorption phenomena indicated that SDS was a suitable choice for sandstone formation | ||||
| Polymer | [12] | Experimental | 11 10.5 | Polymer type selection is critical | |||
| [36] | Experimental | 10.7 | Increasing the viscosity of water by HPAM improves vertical sweep efficiency | ||||
| [40] | Experimental | 5.2 | Core flood test indicates that this type of polymer is less effective than other polymer types due to less oil improvement | ||||
| [41] | Field | 9.8 | Higher molecular weight improves thermal stability | ||||
| [42] | Experimental | 13.5 | Temperature affects polymer performance | ||||
| [43] | Experimental | 6.3 | PPG is more effective in higher and lower permeability zones compared to conventional polymer (HPAM) | ||||
| 13.4 | |||||||
| [44] | Field | 7 | Earlier injection of polymer is more profitable | ||||
| [16] | Experimental | 16.12 | High viscosity of polymer leads to an increase in macroscopic displacement | ||||
| [45] | Experimental | 8.80 | From the results, it can be indicated that polymer is used mostly to reach to the unrecoverable oil zones | ||||
| Alkaline surfactant | [46] | Field | 10–15 | Alkaline-surfactant flooding offers a potential scheme to recover part of the high residual oil that was not recovered by waterfront | |||
| [47] | Experimental | NA | Emulsification of heavy oil by AS was effective | ||||
| [48] | Experimental | NA | For mobilizing heavy oil, AS flooding is a very suitable choice | ||||
| [38] | Experimental | 14.58 | In situ soap by alkali and surfactant reduces IFT significantly | ||||
| 10.42 | |||||||
| [49] | Field | NA | Alkaline is not able to mobilize oil alone, when surfactant added IFT reaches minimum value and oil easily mobilized | ||||
| [50] | Experimental | 12.10 | Surfactant and soap (in situ) surfactant formation efficiently reduces IFT | ||||
| 15.80 | |||||||
| 18.63 | |||||||
| [13] | Experimental | 18 | As shown in adsorption phenomena, alkali plays a major role in reducing surfactant adsorption | ||||
| [51] | Experimental | 10.5 | Surfactant reduces the alkaline consumption | ||||
| Alkaline polymer | [52] | Field | NA | AP synergy effect was efficient for improving EOR mechanisms | |||
| [53] | Field | 21.1 | AP was sufficient to improve oil recovery | ||||
| [54] | Field | 17 | Binary system of A and K performance is more significant than using each of the chemicals alone | ||||
| [55] | Experimental | 21.02 | In AP flooding, alkaline selection plays a critical role in oil recovery improvement | ||||
| 25.21 | |||||||
| 18.12 | |||||||
| [56] | Field | 26.4 | Soap formation by Na2CO3 and viscosity improvement by anionic polymer yielded higher recovery | ||||
| [57] | Experimental | 18.58 | Conc. of polymer influences oil recovery | ||||
| 27.60 | |||||||
| [57] | Experimental | 16.56 | Conc. of Alkaline influences oil recovery | ||||
| 27.39 | |||||||
| [52] | Field | 21.1 | 67% OOIP was recovered by AP | ||||
| [58] | Experimental | 22.8 | Polymer solution should be injected at a good speed | ||||
| [25] | Field | 18.12 | alkali cannot to the oil region without polymer | ||||
| [59] | Experimental | 1.98 | Mobility control improved | ||||
| Surfactant Polymer (SP) | [60] | Experimental | 12.0 | Using two surfactants was more effective | |||
| [61] | Field | 16.3 | Using surfactant with polymer yield extra oil recovery | ||||
| [62] | Experimental | 17.25 | Temperature and initial oil saturation affects oil recovery | ||||
| [16] | Experimental | 20.99 | Better mobility control is obtained by using polymer with surfactant | ||||
| [63] | Experimental | 15.94 | The binary system demonstrated high interfacial activity with IFT min below 0.01 mN/m | ||||
| [61] | Field | 13.8 | Synergism of polymer and surfactant further improves oil recovery | ||||
| [39] | Experimental | 20 | Oil recovery after using dual chemicals (S and P) was higher than the total oil that is produced by using S and P alone | ||||
| [64] | Experimental | 41 | CMC of SDS is 0.21 means that higher concentrations of CMC have a marginally effect on oil recovery | ||||
| 41.4 | |||||||
| 42 | |||||||
| [61] | Field | 14.5 | Polymer and surfactant synergism developed by choosing the optimum conc. of each | ||||
| [36] | Experimental | 13.7 | Without polymer injection surfactant cannot go through unsweep zones | ||||
| [45] | Experimental | 11.29 | Anionic surfactant for sandstone reservoir is very effective | ||||
| [43] | Experimental | 13.6 | Polymer and surfactant synergistic yields higher oil displacement | ||||
| [65] | Experimental | 23.96 | Polymer controls mobility control and surfactant reduces IFT | ||||
| [43] | Experimental | 22.4 | SLPS improves displacement efficiency and (HPAM + PPG) improves sweep efficiency | ||||
| Alkaline surfactant Polymer (ASP) | [60] | Experimental | 23.69 | Increase in surfactant conc. leads to oil recovery enhancement | |||
| 27.18 | |||||||
| 28.72 | |||||||
| [66] | Experimental | 45 | An alkaline surfactant polymer formulation was substantially better in recovering oil than surfactant or polymer surfactant | ||||
| [67] | Experimental | 7.4 | A, S and P synergism yielded higher oil recovery. Alkaline reduces surfactant adsorption. Surfactant reduces alkaline consumption and polymer increases the viscosity of water. These three functions play a great role in recovery enhancement | ||||
| [16] | Experimental | 23.69 | Effect of different alkaline concentration in ASP slug yields different oil recovery, indicating that optimum concentration of alkaline should be guaranteed | ||||
| 24.08 | |||||||
| 24.91 | |||||||
| [16] | Experimental | 23.69 | Optimum concentration of polymer is required during ASP injection for higher oil recovery | ||||
| 23.5 | |||||||
| 24.2 | |||||||
| [68] | Field | 28.1 | ASP synergy effect makes the process efficient | ||||
| [69] | Field | 10–28 | Pore scale displacement efficiency improved due to synergy of three chemicals | ||||
| [70] | Field | >25 | NaOH and heavy alkylbenzene sulfonate reduces IFT dramatically and polymer pushes the heavy oil | ||||
| [71] | Experimental | 15.5 | Present the co-surfactant in the ASP slug is critical in releasing the trapped oil in the porous part of the reservoir rock | ||||
| Ref. | Name | CMC | Type | IFT From-to mN/m | Contact Angle From-To | Oil Recovery Improvement % | Properties |
|---|---|---|---|---|---|---|---|
| [73] | Reetha Extract | 2.3 | Natural non-ionic | 18.6 to 7.02 | NA | 6.8 | Applicability of new surfactant and increase oil recovery from 18.5 to 25.3 |
| [74] | Mulberry leaves extract | 2.6 | Natural cationic | 44 to 17.9 | 62.5° to 42.5 | 7 | Suitable for carbonate rock |
| [75] | Matricaria chamomilla extract | 0.05 | Natural Nonionic | 30.63 to 12.53 | NA | NA | Good IFT reduction ability |
| [76] | Cordia Myxa plant | 0.06 | Natural | 33 to 16.24 | NA | 27 | Good adsorption |
| [77] | Mahua oil | NA | NA | 10−2 | NA | 20 | Applicable for sandstone reservoir |
| [78] | Seidlitzia rosmarinus extract | 0.08 | Cationic | 32 to 9 | NA | NA | The reduced IFT is not as low for EOR application |
| [79] | Jatropha oil-based | NA | Nonionic | 0.917 | NA | 25 | Good surface activity |
| [80] | Henna extract | 0.02 | Cationic | 43.9 to 3.05 | 66 to 37 | 7 | Good wetting ability |
| [81] | Olive leaf extract | 1.95 | Natural cationic | 36.5 to 14 | NA | NA | Good adsorption |
| [81] | Spistan leaf Extract | 2.1 | Natural cationic | 36.5 to 20.15 | NA | NA | good associative and interfacial properties |
| [81] | Prosopi leaf Extract | 2.3 | Natural cationic | 36.5 to 15.1 | NA | NA | Good adsorption |
| CEOR Type | Oil Recovery % | Basic Principle |
|---|---|---|
| Nano | 5–23 | Improvement in sweep and displacement |
| Alkaline | 2–5 | Improvement in displacement |
| Polymer | 2–10 | Improvement in sweep |
| Surfactant | 5–15 | Improvement in displacement |
| SP | 5–20 | Improvement in sweep and displacement |
| AP | 5–18 | Improvement in sweep and displacement |
| ASP | 5–25 | Improvement in sweep and displacement |
| Nano-polymer | 4–20 | Improvement in sweep and displacement |
| Nano-surfactant | 5–20 | Improvement in sweep and displacement |
| NSP | 8–22 | Improvement in sweep and displacement |
| NASP | >25 (predicted by our study) | Improvement in sweep and displacement |
| Nanoparticle | Nano-Composites | EOR Mechanisms |
|---|---|---|
| SnO2, ZrO2, Carbon nanoparticles | CTAB + Al2O3 | Wettability alteration by disjoining pressure |
| SiO2 | NiO + SiO2 | Change wettability of oil by disjoining pressure |
| ZnO | SDS + ZrO2 | Decreasing the contact to water wet by disjoining pressure |
| Al2O3 | SiO2 + PAM | Wettability alteration by disjoining pressure |
| Fe, SiO2, GO, TiO2 | ZrO2, NiO | Reduce interfacial tension |
| Al2O3, CuO, Fe2O3 | SDS + Al2O3 | Reduce the viscosity of crude |
| MgO | CuO/TiO2 + PAM | Optimized permeability |
| Ref. | Nano Type | Lith. | Contact Angle | IFT | Oil Improvement % | Remark | ||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||
| [98] | SiO2 | Qz | 131 | 38.82 | 19.2 | 17.5 | 2 | Different nanoparticles’ type and size have different performances |
| TiO2 | 131 | 21.64 | 19.2 | NA | 11 | |||
| Al2O3 | 131 | 28.6 | 19.2 | 12.8 | 8 | |||
| [99] | SiO2 | Sst | 51 | 30.5 | 21 | 20.3 | 10.1 | Due to NP adsorption, the wettability altered from oil to water wet |
| [100] | SiO2 | Sst | NA. | NA | NA. | NA. | 4.29 | Wedge film creation by nanoparticles |
| [101] | SiO2 | Sst | 122 | 16 | 13.62 | 10.69 | 23.5 | Increase in capillary number due to SIO2 |
| [102] | graphene nanosheets | Sst | NA. | NA. | NA. | NA. | 6.7–15.2 | Size of nanoparticle plays a great role in EOR |
| [103] | SiO2/TiO2 | Sst | 154 | 23 | NA. | NA. | NA. | Spherical shape of nanoparticle improves uniformity |
| Al2O3/TiO2 | 154 | 24 | ||||||
| [104] | HLP | Sst | 135.5 | 95 | 26.3 | 1.75 | 32.2 | NPS yields higher oil recovery without creating any damage to the formation |
| NWP | 135.5 | 82 | 26.3 | 2.55 | 28.57 | |||
| [105] | NiO/SiO2 NCs | Carb. | 174 | 32 | 28 | 1.84 | NA | NiO/SiO2 Nanocomposite responsible for altering the wettability in carbonate rock reservoir |
| [106] | LHPN | Sst | 35 | <10 | NA. | NA. | 1.92 | Capillary number improvement leads to oil enhancement |
| NWPN | 35 | 0 | 29.23 | |||||
| HLPN | 35 | NA. | 29.01 | |||||
| [107] | SiO2 | Sst | 12 | 40 | 17.5 | 7 | 28 | Sweep efficiency improved by IFT reduction |
| [108] | TiO2 | Carb. | 55.3 | 61.9 | 17.5 | 12.5 | 6.6 | Temperature affects oil recovery |
| SiO2 | 54.8 | 57.7 | 16.7 | 11.1 | 2.9 | |||
| [109] | γ-Al2O3 | Carb. | 119.8 | 40 | NA. | NA. | 11.25 | γ-Al2O3 plays the main role in altering the wettability from oil to water wet |
| [110] | Al2O3 | Sst | 53.68 | 28.6 | NA. | NA. | NA. | As a result of nanoparticle deposition, rock surface altered to water wet |
| TiO2 | 53.68 | 21.6 | ||||||
| SiO2 | 53.68 | 38.8 | ||||||
| [111] | Al2O3 | Sst | NA. | NA. | NA. | NA. | 12.5 | For guaranteeing optimum oil recovery design, engineers should select the effective nanoparticle type and size |
| MgO | 1.7 | |||||||
| Ni2O3 | 2 | |||||||
| ZnO | 3.3 | |||||||
| Fe2O3 | 9.2 | |||||||
| [112] | SiO2 | Micrm. | 134.4 | 54.52 | 37.5 | 22.1 | 10 | Amine-functionalized silica nanoparticles are more effective than typical nanoparticles |
| 134.4 | 23.71 | 37.5 | 13 | 28 | ||||
| [113] | SiO2 | Carb. | 140.2 | 68.5 | NA. | NA. | 7.7 | Disjoining pressure of SiO2 was the main mechanism to remove the oil from the surface |
| [114] | SiO2 | Sst | 135.5 | 66 | 26.5 | 1.95 | 25.43 | SiO2 is more effective for light oil reservoir |
| 130 | 101 | 28.3 | 7.3 | 14.55 | ||||
| [115] | SiO2 | Sst | NA. | NA. | NA. | NA. | 5–35 | Arrangement of silicon nanoparticle improves IFT |
| [116] | LHPN | Sst | 87 | 28 | 28 | 7 | 21 | Wettability is altered when polysilicon is adsorbed on the sandstone pore wall |
| [117] | TiO2/SiO2 NCs | Carb. | 138 | 48 | 39 | 13.2 | 26 | Trapped oil is mobilized by the nanocomposite |
| [118] | LHP | Sst | NA. | NA. | 14.7 | 9.3 | 2 | Nanofluid was more effective for secondary recovery |
| [119] | TiO2 | Sst | NA. | NA. | 23 | 18 | 14 | Low concentration of TiO2 improved the oil recovery |
| [117] | Fe2O3/SiO2 NC | Sst | 138 | 52 | 39 | 17.5 | 31 | Nanocomposite was able to alter wettability of the rock surface dramatically |
| [120] | SiO2 | Sst | 74 | 1.2 | 16 | 1.4 | 33 | SiO2 can desorb the oil from the rock |
| [121] | TiO2 | Sst | 125 | 90 | NA. | NA. | 31 | Higher disjoining pressure as a result of using higher concentration |
| [122] | SiO2 | Micrm. | 100 | 0 | NA. | NA. | 8.7 (0.1 wt%) | Contact angle and IFT are dependent on the weight % of nanoparticle |
| 26 (0.3 wt%) | ||||||||
| [123] | TiO2 | Sst | 18 | 8 | 47.5 | 44.5 | 9.5–13.3 | Decrease in capillary force |
| [124] | ZrO2 and NiO | Carb. | 152 | 44 | NA. | NA. | NA. | Additional IFT reduction after using nanoparticle |
| [125] | Al2O3 | Sst | 131 | 92 | 38.5 | 2.25 | 20.2 | Dispersant agent (propanol) was used for the first time and was effective in IFT reduction |
| Fe2O3 | 132.5 | 101 | 38.5 | 2.75 | 17.3 | |||
| SiO2 | 134 | 82 | 38.5 | 1.45 | 22.5 | |||
| [126] | SiO2 | Carb. | NA. | NA. | NA. | NA. | 8.7 | By using nanoparticles, rheological properties of the displacing phase improved |
| Nano-Surfactant | ||||||||
| [127] | SDS + Al2O3 | Carb. | 92 | 75 | 9.88 | 2.75 | NA | Anionic surfactant is less effective than cationic surfactant for carbonate reservoir |
| SDS + ZrO2 | 92 | 84 | 9.88 | 2.78 | ||||
| [128] | NaCl + CAPB + SiO2 | Carb. | 156.2° | 75.1° | 39.63 | 1.10 | 12.2 | Decrease of IFT from 39.63 to 1.10 mN/m leads to oil improvement |
| [129] | SDS + SiO2 | Micrm. | 73 | 11 | NA | NA | 13 | Extra heavy oil recovery as compared to SDS alone |
| [130] | 3.22 ZrO2 + 0.50 g of CTAB | Carb. | 180 | 32 | NA | NA | 10 | Positive outcome is observed by surfactant and nanoparticle synergism |
| [131] | rhamnolipid BS-spherical + silica | Carb. | 112 | 8 | NA | 1.85 | 26.1 | Spherical shape nanoparticle is more effective than other shape nanoparticle due to uniformity |
| rhamnolipid BS-sponge + silica | 120 | 17 | NA | 1.94 | 25.1 | |||
| [132] | ZrO2 + SDS | Carb. | 152 | 44 | NA | NA | 8 | From the tests, it was obvious that ZrO2 is very effective in changing the wettability from oil wet limestone to water wet |
| [133] | SiO2 + ALFOTERRA | Carb. | 167 | 146 | 23.2 | 7.2 | 10 | Using nano was effective in additional oil recovery in ambient and HPHT conditions |
| [134] | Anionic surfactant + Al2O3 | Carb. | 142 | 0 | NA. | NA. | NA | At relatively low concentrations, Al2O3 can improve anionic surfactant to alter the oil wet to water wet more effectively |
| [129] | A2O3/SiO2 + SDS, CTAB | Carb. | 73 | 11 | NA | NA | 15 | Small size and high surface area of nanoparticles were very effective |
| [127] | CTAB + Al2O3 | Carb. | 70 | 52 | 8.46 | 1.65 | NA | Smaller particle size of Al2O3 leads to higher surface energy, resulting in bigger repulsion force |
| CTAB + ZrO2 | 70 | 60 | 8.46 | 1.85 | ||||
| [135] | SDS + ZrO2 | Carb. | NA | NA | 48 | 10 | NA | At and below CMS nanoparticles have a great role in IFT reduction |
| [136] | non-ferrous metal + anionic surfactant | Sst | 23 | 19 | 31.4 | 9.2 | 12–17 | Nanoparticles decrease surfactant adsorption |
| [27] | ZrO2 + SDS | Carb. | 101 | 30 | 16 | 3.1 | 25 | Cationic surfactant was more effective at altering the wettability |
| ZrO2 + CTAB | 101 | 16 | 18.4 | 5.4 | 32.5 | |||
| [64] | Cationic anionic + silica | NA | 59 | 46 | 45 | 43 | 45 | Nanoparticle size 5–75 was effective at reducing the IFT |
| [137] | SDS + ZnO | Sst | NA | NA | 32.5 | 7.1 | 19 | Sodium dodecyl sulphate gives better stability of ZnO |
| [35] | ZnO + SDBS | Sst | 44.45 | 42.47 | 10.86 | 10.2 | 8.5–10.2 | Decreasing in NP size leads to contact angle reduction |
| [138] | SiO2 + Soloterra964 | Sst | 43.4 | 103.2 | 13.78 | 0.78 | 17.23 | Nanosurfactant was a suitable EOR agent |
| [127] | TX-100 + Al2O3 | Carb. | 85 | 62 | 9.13 | 2.55 | NA. | For carbonate, nonionic surfactant is more effective in altering the wettability as compared to ionic surfactant |
| TX-100 + ZrO2 | 85 | 71 | 9.13 | 2.64 | NA. | |||
| Nano-Polymer | ||||||||
| [139] | SiO2 + 2-Poly(MPC) | Sst | NA. | NA. | 47 | 35 | 5.2 | Using copolymer with nano silica yielded higher oil recovery |
| [140] | Silica + DMAEMA | Sst | 85 | 62.2 | 27 | 14 | 9.9 | Nanoparticles reduce polymer adsorption |
| [141] | Nanoclay + HPAM | Sst | NA. | NA. | NA. | NA. | 5 | Improvement in viscosity after using Nanoclay/HPAM |
| [142] | SiO2 + PAM | Sst | NA. | NA. | 27 | 10.2 | 24.7 | Due to disjoining pressure, oil wet is changed to water wet |
| [143] | SiO2 + Xanthan gum | Sst | 86 | 20 | 17.8 | 6.4 | 7.81 | More oil is produced from unswept areas leading to improving residual oil recovery |
| [144] | SiO2 + PVP | Sst | 54 | 22 | 19.2 | 7.9 | 0–6.1 | Oil recovery increases with increasing the concentration of nanoparticles due to improved adsorption ratio |
| [98] | Al2O3 + PVP | Sst | 54 | 21 | NA. | NA. | 7–24 | IFT and contact angle improved synergistically, in a nanocomposite form as compared to individual nanoparticle |
| Nano-SP | ||||||||
| [62] | SiO2+ PAM + SDS | Sst | NA. | NA. | NA. | 0.13 | 17.49 | Pressure drop increased to 0.38 MPa |
| [62] | Clay + PAM + SDS | Sst | NA. | NA. | NA. | 0.238 | 18.28 | Higher viscosity as compared to conventional SP |
| [64] | Nanoclay + 2800 PAM + 0.2 SDS | Micrm. | NA. | NA. | NA. | NA. | 6.8 | The injection of nano to SP leads to a more uniform flow pattern in a micromodel, which yields a more stable front |
| Nanoclay + 3000 PAM + 0.2 SDS | 8 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarbast, R.; Salih, N.; Préat, A. A Critical Overview of ASP and Future Perspectives of NASP in EOR of Hydrocarbon Reservoirs: Potential Application, Prospects, Challenges and Governing Mechanisms. Nanomaterials 2022, 12, 4007. https://doi.org/10.3390/nano12224007
Sarbast R, Salih N, Préat A. A Critical Overview of ASP and Future Perspectives of NASP in EOR of Hydrocarbon Reservoirs: Potential Application, Prospects, Challenges and Governing Mechanisms. Nanomaterials. 2022; 12(22):4007. https://doi.org/10.3390/nano12224007
Chicago/Turabian StyleSarbast, Rasan, Namam Salih, and Alain Préat. 2022. "A Critical Overview of ASP and Future Perspectives of NASP in EOR of Hydrocarbon Reservoirs: Potential Application, Prospects, Challenges and Governing Mechanisms" Nanomaterials 12, no. 22: 4007. https://doi.org/10.3390/nano12224007
APA StyleSarbast, R., Salih, N., & Préat, A. (2022). A Critical Overview of ASP and Future Perspectives of NASP in EOR of Hydrocarbon Reservoirs: Potential Application, Prospects, Challenges and Governing Mechanisms. Nanomaterials, 12(22), 4007. https://doi.org/10.3390/nano12224007