Bifunctional 1,2,4-Triazole/12-Tungstophosphoric Acid Composite Nanoparticles for Biodiesel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Heterocycle–Heteropoly Acid
2.2. Characterization
2.3. Transesterification of Rapeseed Oil
3. Results and Discussion
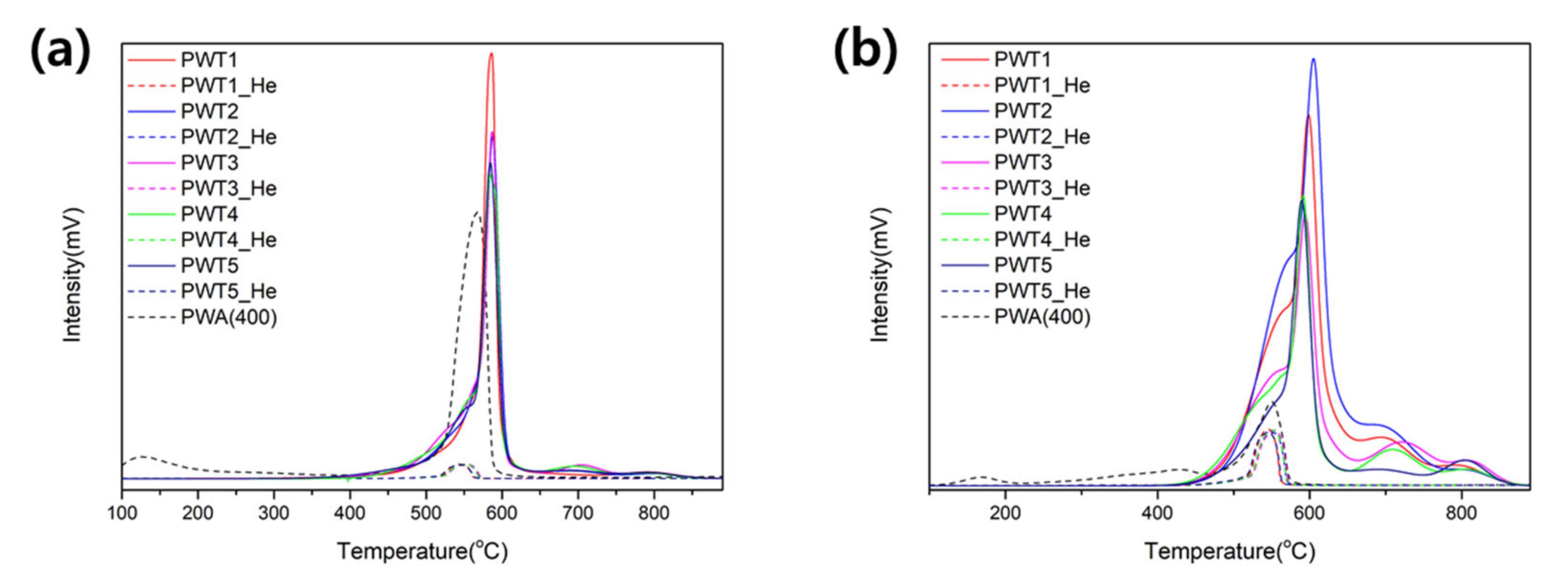
3.1. Synthesis and Characterization of the PWTx Bifunctional Catalysts
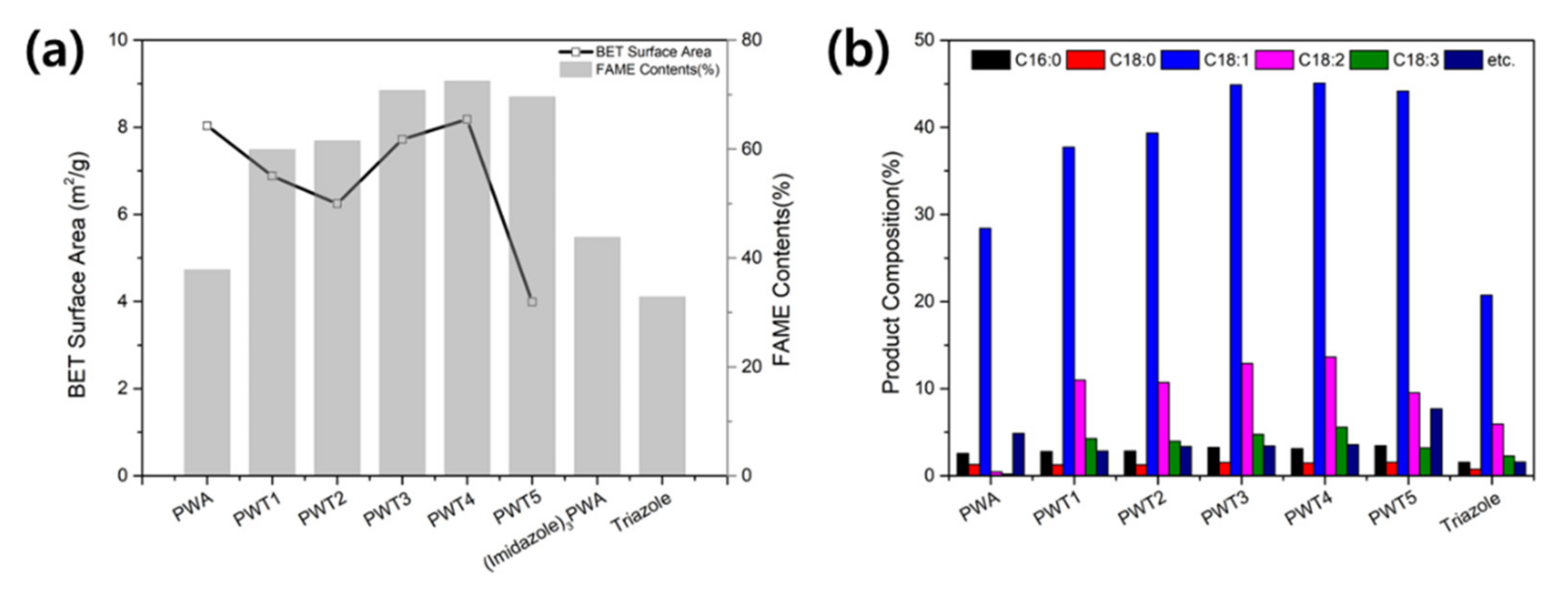
3.2. Transesterification of Rapeseed Oil with Methanol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, B.; Wu, C.; Chang, J. Dual drug release from electrospun poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles composite mats with distinct release profiles. Acta Biomater. 2012, 8, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Q.; Chen, P. Flexible Strain Sensor Based on Carbon Black/Silver Nanoparticles Composite for Human Motion Detection. Materials 2018, 11, 1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkateswarlu, S.; Lee, D.; Yoon, M. Bioinspired 2D-Carbon Flakes and Fe3O4 Nanoparticles Composite for Arsenite Removal. ACS Appl. Mater. Interfaces 2016, 8, 23876–23885. [Google Scholar] [CrossRef] [PubMed]
- Hana, D.; Lougou, B.G.; Xu, Y.; Shuai, Y.; Huang, X. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage. Appl. Energy 2020, 264, 114674. [Google Scholar] [CrossRef]
- Ishmukhametov, I.; Batasheva, S.; Rozhina, E.; Akhatova, F.; Mingaleeva, R.; Rozhin, A.; Fakhrullin, R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers 2022, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Alcantara-Cobos, A.; Gutierrez-Segura, E.; Solache-Ríos, M.; Amaya-Chavez, A.; Solís-Casados, D.A. Tartrazine removal by ZnO nanoparticles and a zeolite-ZnO nanoparticlescomposite and the phytotoxicity of ZnO nanoparticles. Microporous Mesoporous Mater. 2020, 302, 110212. [Google Scholar] [CrossRef]
- Ananikov, V.P. Organic–Inorganic Hybrid Nanomaterials. Nanomaterials 2019, 9, 1197. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Singh, J.; Rawat, M.; Sharma, J.; Singh, P.P. Enhanced photocatalytic degradation of hazardous industrial pollutants with inorganic–organic TiO2–SnO2–GO hybrid nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 13389–13400. [Google Scholar] [CrossRef]
- Jamshidi, A.; Maleki, B.; Zonoz, F.M.; Tayebee, R. HPA-dendrimer functionalized magnetic nanoparticles (Fe3O4@DNH2-HPA) as a novel inorganic-organic hybrid and recyclable catalyst for the one-pot synthesis of highly substituted pyran derivatives. Mater. Chem. Phys. 2018, 209, 46–59. [Google Scholar] [CrossRef]
- Puglisi, A.; Annunziata, R.; Benaglia, M.; Cozzi, F.; Gervasini, A.; Bertacche, V.; Sala, M.C. Hybrid Inorganic-Organic Materials Carrying Tertiary Amine and Thiourea Residues Tethered on Mesoporous Silica Nanoparticles: Synthesis, Characterization, and Co-Operative Catalysis. Adv. Synth. Catal. 2009, 351, 219–229. [Google Scholar] [CrossRef]
- Yan, S.; Jiang, C.; Guo, J.; Fan, Y.; Zhang, Y. Synthesis of Silver Nanoparticles Loaded onto Polymer-Inorganic Composite Materials and Their Regulated Catalytic Activity. Polymers 2019, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Edison, T.N.J.I.; Sethuraman, M.G. Electrocatalytic performance of carbon dots/palladium nanoparticles composite towards hydrogen evolution reaction in acid medium. Int. J. Hydrog. Energy 2020, 45, 28800–28811. [Google Scholar] [CrossRef]
- Likozar, B.; Levec, J. Transesterification of canola, palm, peanut, soybean and sunflower oil with methanol, ethanol, isopropanol, butanol and tert-butanol to biodiesel: Modelling of chemical equilibrium, reaction kinetics and mass transfer based on fatty acid composition. Appl. Energy 2014, 123, 108–120. [Google Scholar] [CrossRef]
- Furukawa, S.; Uehara, Y.; Yamasaki, H. Variables affecting the reactivity of acid-catalyzed transesterification of vegetable oil with methanol. Bioresour. Technol. 2010, 101, 3325–3332. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Katsoulidis, A.P.; Pomonis, P.J.; Kontominas, M.G. Transesterification of soybean frying oil to biodiesel using heterogeneous catalysts. Fuel Process. Technol. 2009, 90, 671–676. [Google Scholar] [CrossRef]
- Encinar, J.M.; Gonzalez, J.F.; Rodrıguez, J.J.; Tejedor, A. Biodiesel Fuels from Vegetable Oils: Transesterification of Cynara cardunculus L. Oils with Ethanol. Energy Fuels 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 2005, 28, 601–605. [Google Scholar] [CrossRef]
- Li, H.; Xie, W. Fatty Acid Methyl Ester Synthesis over Fe3+-Vanadyl Phosphate Catalysts. J. Am. Oil Chem. Soc. 2008, 85, 655–662. [Google Scholar] [CrossRef]
- Encinar, J.M.; Gonzalez, J.F.; Rodrıguez-Reinares, A. Biodiesel from Used Frying Oil. Variables Affecting the Yields and Characteristics of the Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Shi, W.; He, B.; Ding, J.; Li, J.; Yan, F.; Liang, X. Preparation and characterization of the organic–inorganic hybrid membrane for biodiesel production. Bioresour. Technol. 2010, 101, 1501–1505. [Google Scholar] [CrossRef]
- Nikseresht, A.; Daniyali, A.; Ali-Mohammadi, M.; Afzalinia, A.; Mirzaie, A. Ultrasound-assisted biodiesel production by a novel composite of Fe(III)-based MOF and phosphotangestic acid as efficient and reusable catalyst. Ultrason. Sonochem. 2017, 37, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Hamad, B.; Lopes de Souza, R.O.; Sapaly, G.; Carneiro Rocha, M.G.; Pries de Oliveira, P.G.; Gonzalez, W.A.; Andrade Sales, E.; Essayem, N. Transesterification of rapeseed oil with ethanol over heterogeneous heteropolyacids. Catal. Commun. 2008, 10, 92–97. [Google Scholar] [CrossRef]
- Rafiee, E.; Eavani, S. Heterogenization of heteropoly compounds: A review of their structure and synthesis. RSC Adv. 2016, 6, 46433–46466. [Google Scholar] [CrossRef]
- Han, X.-X.; Chen, K.-K.; Yan, W.; Hung, C.-T.; Liu, L.-L.; Wu, P.-H.; Lin, K.-C.; Liu, S.-B. Amino acid-functionalized heteropolyacids as efficient and recyclable catalysts for esterification of palmitic acid to biodiesel. Fuel 2016, 165, 115–122. [Google Scholar] [CrossRef]
- Su, F.; An, S.; Song, D.; Zhang, X.; Lu, B.; Guo, Y. Heteropoly acid and ZrO2 bifunctionalized organosilica hollow nanospheres for esterification and transesterification. J. Mater. Chem. A 2014, 2, 14127. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Duan, M.-H.; Yao, X.-H.; Fu, Y.-J.; Zu, Y.-G. Preparation of a novel cellulose-based immobilized heteropoly acid system and its application on the biodiesel production. Fuel 2016, 172, 293–300. [Google Scholar] [CrossRef]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.-T. Transesterification of crude Jatropha oil by activated carbon-supported heteropolyacid catalyst in an ultrasound-assisted reactor system. Renew. Energy 2014, 62, 10–17. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhu, W.; Cao, F. Zn1.2H0.6PW12O40 Nanotubes with Double Acid Sites as Heterogeneous Catalysts for the Production of Biodiesel from Waste Cooking Oil. ChemSusChem 2009, 2, 177–183. [Google Scholar] [CrossRef]
- Suzuki, K.; Sugawa, M.; Kikukawa, Y.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Strategic Design and Refinement of Lewis Acid−Base Catalysis by Rare-Earth-Metal-Containing Polyoxometalates. Inorg. Chem. 2012, 51, 6953–6961. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeon, Y.K.; Park, J.-I.; Shul, Y.-G. Heterocycle-modified 12-tungstophosphoric acid as heterogeneouscatalyst for epoxidation of propylene with hydrogen peroxide. J. Mol. Catal. A Chem. 2013, 378, 232–237. [Google Scholar] [CrossRef]
- Baranov, A.A.; Domashnev, D.I.; Leonova, L.S.; Belmesov, A.A.; Antonenko, A.O.; Nefedov, D.Y.; Shmygleva, L.V.; Dobrovolsky, Y.A. Effect of solution pH on morphology and electrochemical properties of cesium salts of phosphotungstic acid. Mater. Sci. Eng. B 2020, 256, 114544. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Boke, C.P.; Karaman, O.; Medetalibeyoglu, H.; Karaman, C.; Atar, N.; Yol, M.L. A new approach for electrochemical detection of organochlorine compound lindane: Development of molecular imprinting polymer with polyoxometalate/carbon nitride nanotubes composite and validation. Microchem. J. 2020, 157, 105012. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. In-situ polymerization of polyaniline modified by phosphotungstic acid on the surface of hollow carbon for two-way efficient reduction of nitrate in water. Chem. Eng. J. 2022, 430, 133175. [Google Scholar] [CrossRef]
- Ning, Y.; Niu, S.; Han, K.; Lu, C. Catalytic capability of phosphotungstic acid supported on bamboo activated carbon in esterification for biodiesel production with density functional theory. Biomass Bioenergy 2020, 143, 105873. [Google Scholar] [CrossRef]
- Fournier, M.; Feumi-Jantou, C.; Rabia, C.; Herve, G.; Launay, S. Polyoxometalates Catalyst Materials: X-Ray Thermal Stability Study of Phosphorus-containing Heteropolyacids. J. Mater. Chem. 1992, 2, 971–978. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- La, K.W.; Jung, J.C.; Kim, H.; Baeck, S.-H.; Song, I.K. Effect of acid–base properties of H3PW12O40/CexTi1−xO2 catalysts on the direct synthesis of dimethyl carbonate from methanol and carbon dioxide: A TPD study of H3PW12O40/CexTi1−xO2 catalysts. J. Mol. Catal. A Chem. 2007, 269, 41–45. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, Y.; Wang, C.; Hu, C.; Wu, Y.; Wang, E. Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A 2003, 256, 203–212. [Google Scholar] [CrossRef]
- Tomishige, K.; Sakaihori, T.; Ikeda, Y.; Fujimoto, K. A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia. Catal. Lett. 1999, 58, 225–229. [Google Scholar] [CrossRef]
- Alonso, D.M.; Granados, M.L.; Mariscal, R.; Douhal, A. Polarity of the acid chain of esters and transesterification activity of acid catalysts. J. Catal. 2009, 262, 18–26. [Google Scholar] [CrossRef]
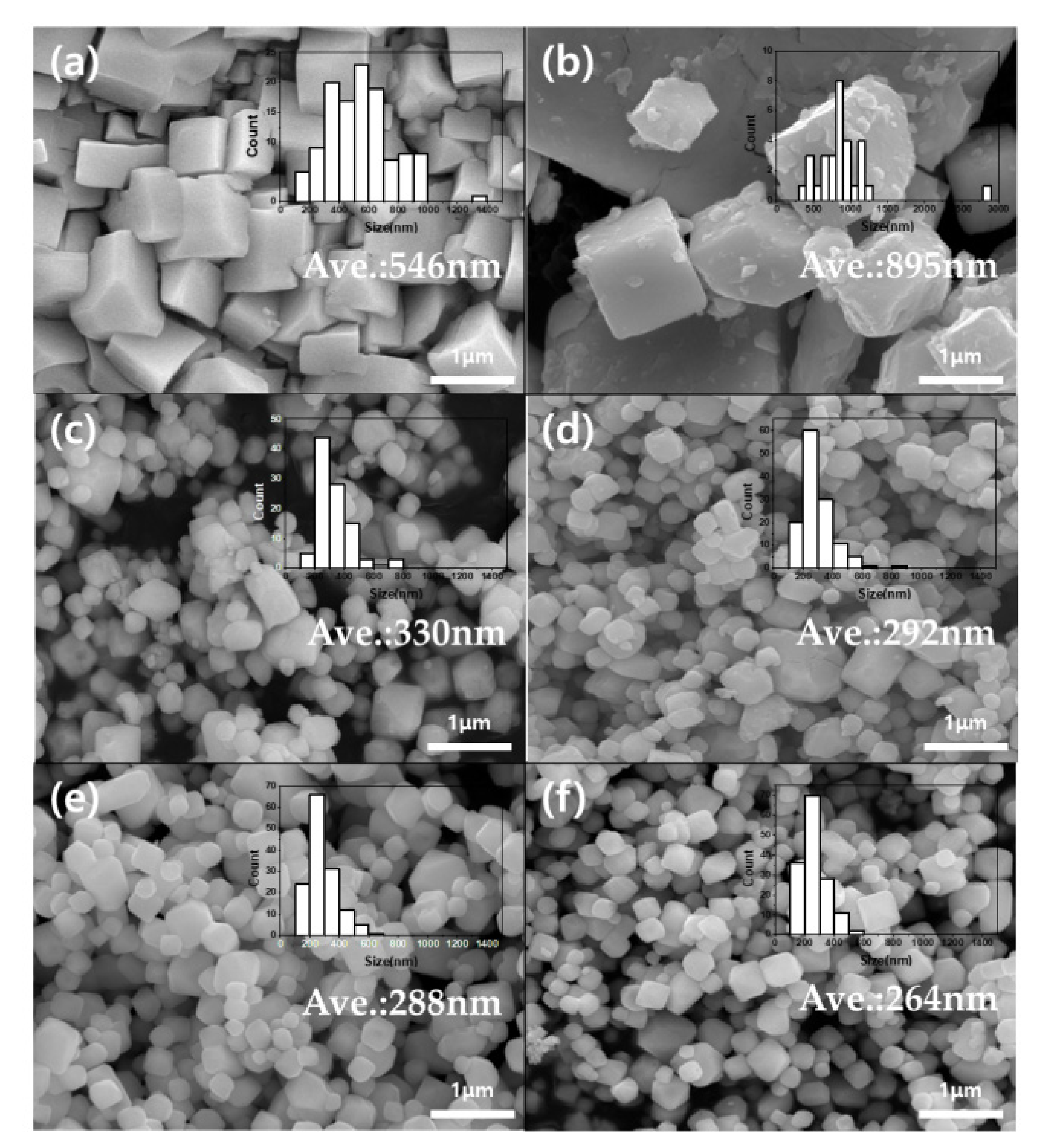
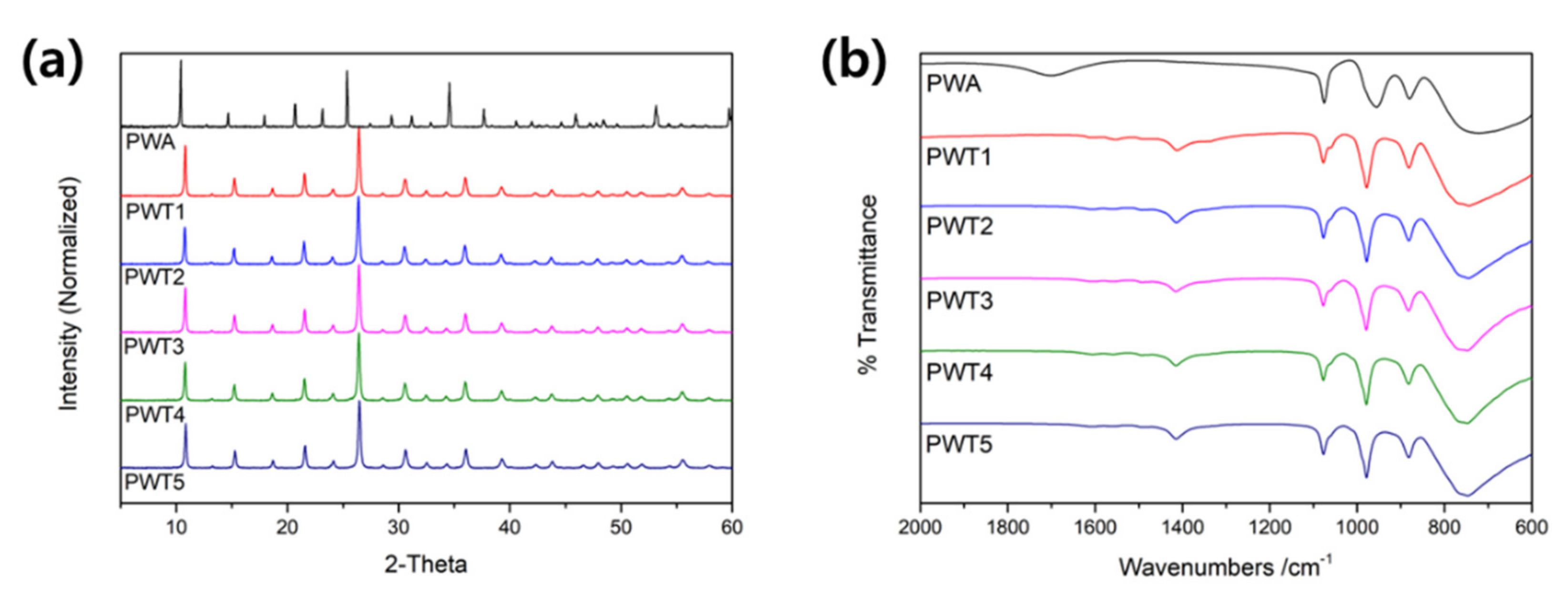
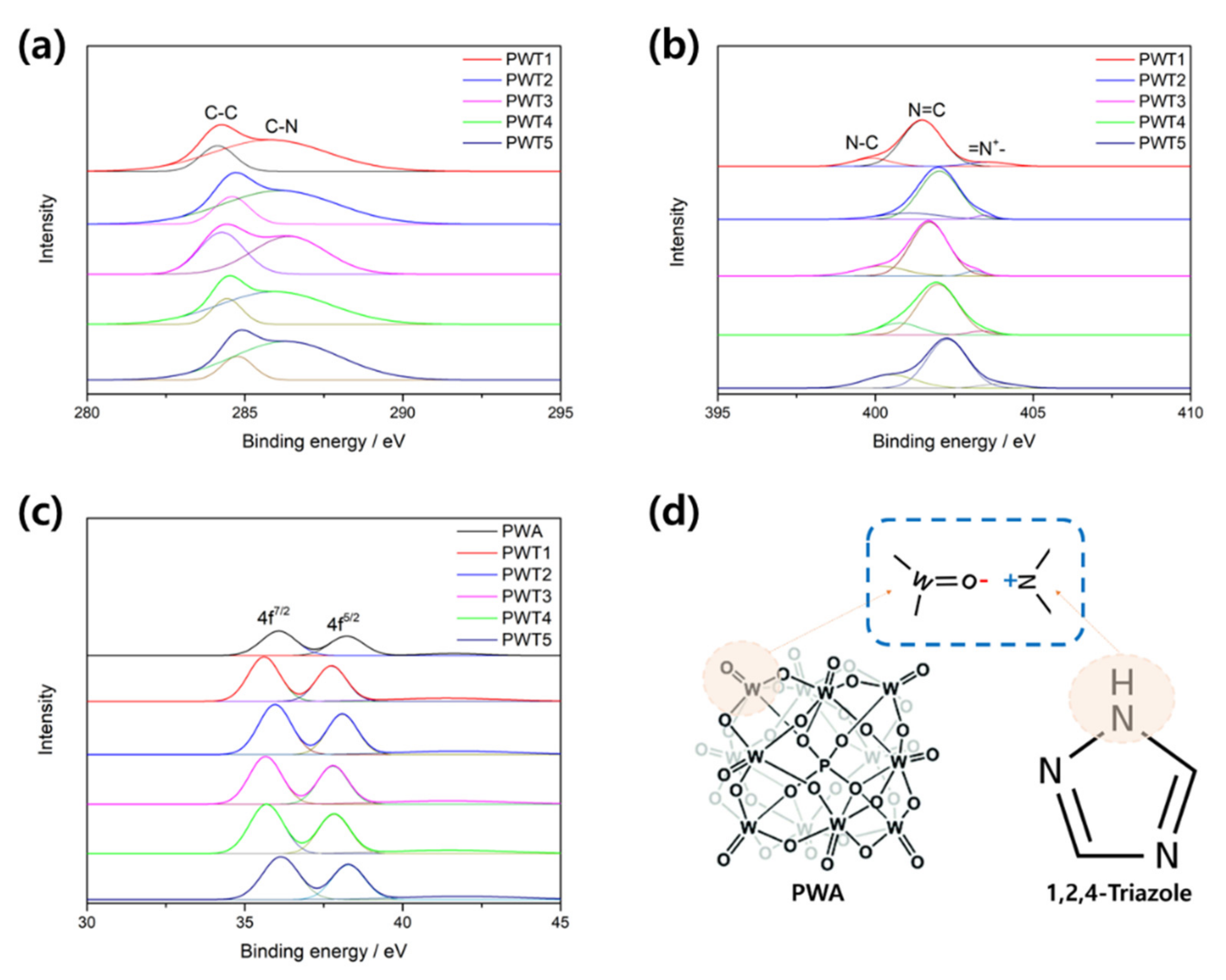
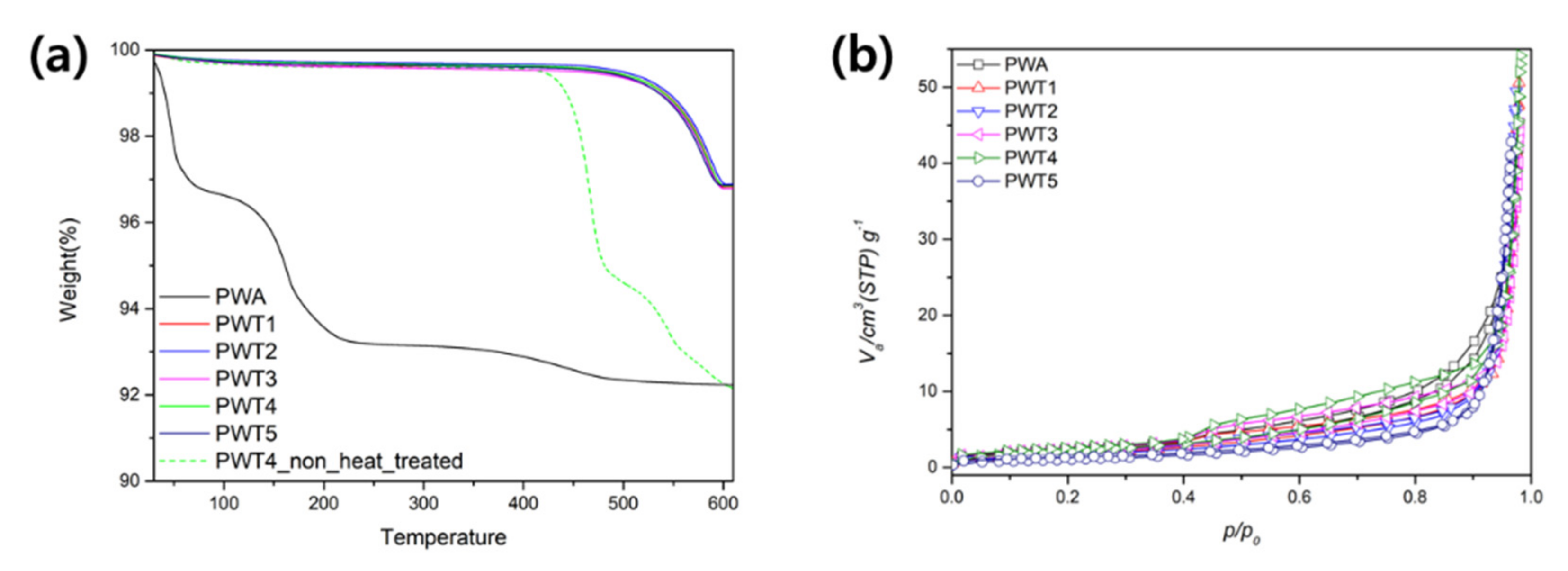
| Triazole/PWA Molar Ratio | Carbon (wt%) | Nitrogen (wt%) | pH | |
|---|---|---|---|---|
| PWT1 | 1 | 2.36 | 4.04 | 1.8 |
| PWT2 | 3 | 2.41 | 3.98 | 3.4 |
| PWT3 | 4 | 2.40 | 4.06 | 4.7 |
| PWT4 | 6 | 2.44 | 4.06 | 4.9 |
| PWT5 | 10 | 2.38 | 4.08 | 5.0 |
| Surface Area (m2/g) | Pore Volume (cm3/g) | Ave. Pore Diameter (nm) | |
|---|---|---|---|
| PWA | 8.0337 | 1.8458 | 34.657 |
| PWT1 | 6.8811 | 1.581 | 45.511 |
| PWT2 | 6.2458 | 1.435 | 48.98 |
| PWT3 | 7.7215 | 1.774 | 36.201 |
| PWT4 | 8.181 | 1.8796 | 40.952 |
| PWT5 | 3.9909 | 0.9169 | 66.4 |
| Acidity Capacity (mmol/g) | Basicity Capacity (mmol/g) | |
|---|---|---|
| PWT1 | 28.27 | 17.33 |
| PWT2 | 28.69 | 20.53 |
| PWT3 | 29.41 | 13.37 |
| PWT4 | 29.07 | 11.61 |
| PWT5 | 28.02 | 10.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Lee, C.; Kim, H.; Jeon, Y.; Shul, Y.-G.; Park, J. Bifunctional 1,2,4-Triazole/12-Tungstophosphoric Acid Composite Nanoparticles for Biodiesel Production. Nanomaterials 2022, 12, 4022. https://doi.org/10.3390/nano12224022
Lee G, Lee C, Kim H, Jeon Y, Shul Y-G, Park J. Bifunctional 1,2,4-Triazole/12-Tungstophosphoric Acid Composite Nanoparticles for Biodiesel Production. Nanomaterials. 2022; 12(22):4022. https://doi.org/10.3390/nano12224022
Chicago/Turabian StyleLee, Gicheon, Chanmin Lee, Hyungjin Kim, Yukwon Jeon, Yong-Gun Shul, and Jinwon Park. 2022. "Bifunctional 1,2,4-Triazole/12-Tungstophosphoric Acid Composite Nanoparticles for Biodiesel Production" Nanomaterials 12, no. 22: 4022. https://doi.org/10.3390/nano12224022





