Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal
Abstract
:1. Introduction
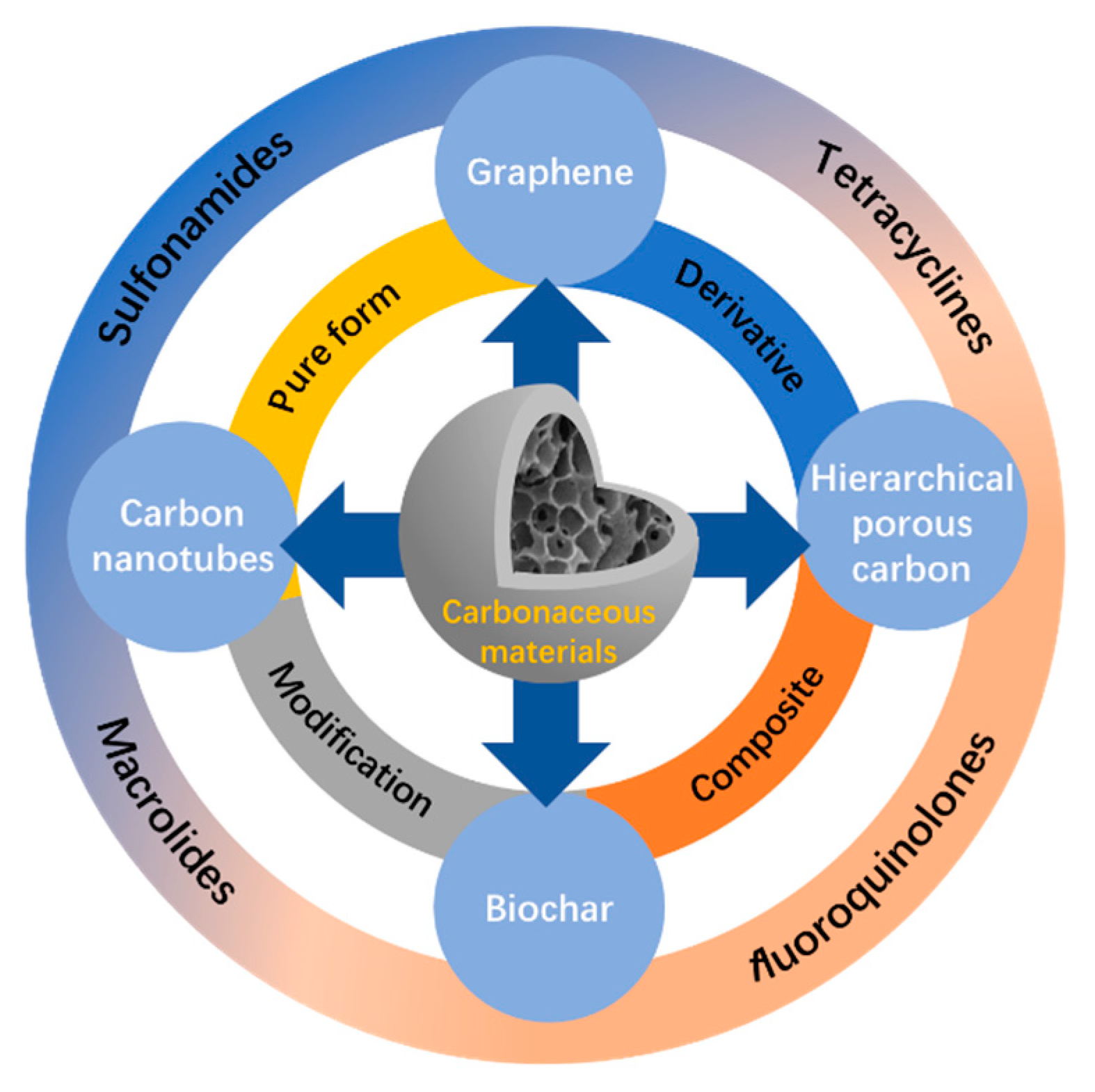
2. Characteristics of Carbonaceous Materials
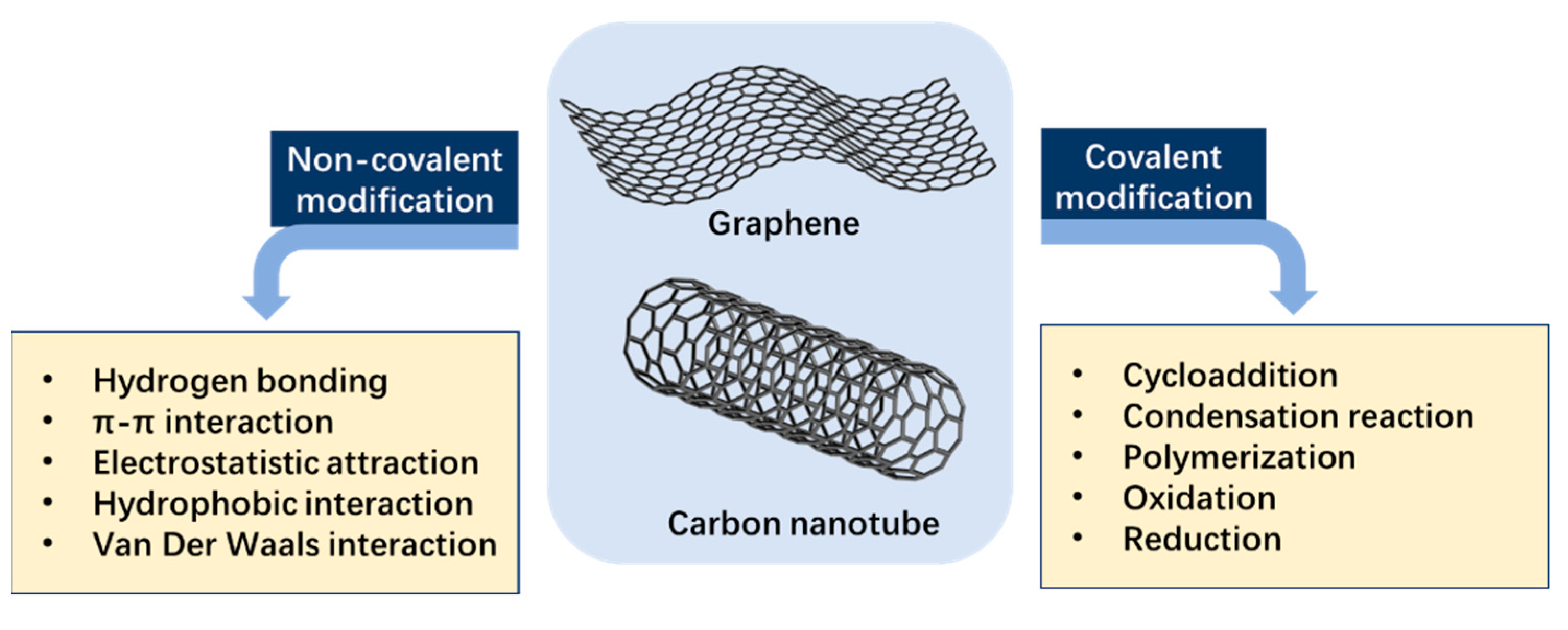
2.1. Graphene and Its Derivatives
2.2. Carbon Nanotube (CNT)
2.3. Biochar (BC)
2.4. Hierarchical Porous Carbon (HPC)
3. Adsorption Removal of Antibiotics by Carbon-Based Materials
3.1. Graphene-Based Materials Applied in Antibiotics Adsorption
| Antibiotic Class | Antibiotic Compounds | Adsorbent | Temperature (K) | pH | Equilibrium Time (Min) | Adsorption Capacity Qm (mg g−1) | Refs. |
|---|---|---|---|---|---|---|---|
| Tetracycline | Tetracycline | GO | 298 | 3.6 | 190 | 313.48 | [55] |
| Tetracycline | MAEGO | 303 | 4 | 1440 | 487.82 | [64] | |
| Tetracycline | GO@ATP | 308 | 5 | 120 | [66] | ||
| Tetracycline | DNGA | 298 | 4 | 20 | 607.1 | [67] | |
| Tetracycline | UCN-GH | 298 | 5 | 100 | [68] | ||
| Tetracycline | Alg-Cu@GO@MOF-525 | 318 | 7 | 900 | 533.2 | [69] | |
| Oxytetracycline | GO | 298 | 3.6 | 90 | 212.31 | [55] | |
| Oxytetracycline | GO | 293 | 5 | 60 | 130.4 | [61] | |
| Oxytetracycline | B-rGO | 293 | 5 | 60 | 83.9 | [61] | |
| Doxycycline | GO | 298 | 3.6 | 90 | 398.41 | [55] | |
| Doxycycline | GO@Fe3O4@β-cyclodextrin | 298 | 7 | 45 | 204.5 | [70] | |
| Sulfonamide | Sulfamethoxazole | GO | 298 | 5 | 1440 | 240 | [54] |
| Sulfamethoxazole | GO@β-cyclodextrin@ dopamine hydrochloride | 308 | 2 | 90 | 144 | [62] | |
| Sulfadiazine | GO@β-cyclodextrin@ dopamine hydrochloride | 308 | 2 | 90 | 152 | [62] | |
| Trimethoprim | GO | 298 | 8 | 60 | 204.08 | [56] | |
| Isoniazid | GO | 298 | 2 | 60 | 13.89 | [56] | |
| Quinolone | Ciprofloxacin | GO | 298 | 5 | 2880 | 379 | [54] |
| Norfloxacin | GO | 303 | 7 | 30 | 374.9 | [57] | |
| levofloxacin | MAEGO | 303 | 4 | 1440 | 330.71 | [64] | |
| β-lactams | Cephalexin | GO | 298 | 7 | 420 | 164.35 | [58] |
| Macrolide | Azithromycin | GO | 298 | 7 | 0.25 | 55.55 | [59] |
3.2. Carbon Nanotube-Based Materials Applied in Antibiotics Adsorption
| Antibiotic Class | Antibiotic Compounds | Adsorbent | Temperature (K) | pH | Equilibrium Time (Min) | Adsorption Capacity Qm (mg g−1) | Refs. |
|---|---|---|---|---|---|---|---|
| Tetracycline | Tetracycline | M-MWCNT | 308 | 4-7 | 240 | 494.91 | [76] |
| Tetracycline | Fe3O4/MWCNT-CdS | 298 | 5 | 60 | 116.27 | [78] | |
| Tetracycline | MWCNT/ZIF-8(Fe) | 298 | 6 | 360 | 589.42 | [82] | |
| Tetracycline hydrochloride | LDH@CNT | 298 | 8 | 480 | 756.2 | [80] | |
| Tetracycline hydrochloride | MWCNT/MIL-53(Fe) | 298 | 7 | 364.37 | [81] | ||
| Oxytetracycline hydrochloride | MWCNT/MIL-53(Fe) | 298 | 7 | 325.59 | [81] | ||
| Chlortetracycline hydrochloride | MWCNT/MIL-53(Fe) | 298 | 7 | 180.68 | [81] | ||
| Sulfonamide | Sulfamethoxazole | MWCNT | 298 | 3 | 720 | [71] | |
| Sulfamethazine | Fe3O4/MWCNTs | 298 | 7 | 1440 | [77] | ||
| Quinolone | Fluoroquinolone | O-MWCNT | 298 | 3 | 1440 | [73] | |
| Ciprofloxacin | CoFe2O4/CNTs | 298 | 6–7 | 300 | 63.32 | [79] | |
| Ciprofloxacin | CNTs/L-cys@GO/SA | 288 | 5.4 | 3600 | 200 | [83] | |
| Ciprofloxacin | 4.7%O-MWCNT | 298 | 4 | 60 | 177.8 | [84] | |
| β-lactams | Amoxicillin | MWCNT | 333 | 7 | 75 | 159.4 | [72] |
| Nitroimidazole | Metronidazole | SWCNT | 298 | 7 | 7200 | 101 | [75] |
| Dimetridazole | SWCNT | 298 | 7 | 7200 | 84 | [75] | |
| Cephalosporin | Cefixime | Fe3O4/MWCNT-CdS | 298 | 5 | 60 | 105.26 | [78] |
3.3. Biochar-Based Materials Applied in Antibiotics Adsorption
| Biomass | Engineering Method | Antibiotic | Pyrolysis Temp (°C) | Adsorption Temp (K) | pH | Equilibrium Time (Min) | Adsorption Capacity Qm (mg g−1) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Auricula dregs | Tetracycline | 700 | 298 | 7 | 60 | 11.9 | [85] | |
| Cow manure | Tetracycline | 700 | 298 | 6 | 1440 | 11.80 | [86] | |
| Camellia oleifera shells | H3PO4 | Tetracycline | 600 | 298 | 6 | 240 | 451.6 | [91] |
| Biogas residue | Citric acid | Tetracycline | 800 | 298 | 7 | 600 | 58.25 | [92] |
| Aerobic granular sludge | ZnCl2 | Tetracycline | 700 | 308 | 5 | 2880 | 93.44 | [93] |
| Flueggea suffruticosa | ZnCl2 | Tetracycline | 500 | 303 | 7 | 50 | 188.7 | [94] |
| Walnut shell | FeCl3·6H2O, dicyandiamide | Tetracycline | 600 | 298 | 7.2 | 238.9 | [96] | |
| Water hyacinth | FeCl3·6H2O | Tetracycline | 700 | 318 | 200 | 202.62 | [101] | |
| Wheat Straw | Lignin | Tetracycline hydrochloride | 600 | 298 | 7 | 31.48 | [104] | |
| Flueggea suffruticosa | ZnCl2 | Chlortetracycline | 500 | 303 | 10 | 200.0 | [94] | |
| Flueggea suffruticosa | ZnCl2 | Oxytetracycline | 500 | 303 | 7 | 129.9 | [94] | |
| Coffee grounds | H3PO4 | Sulfadiazine | 700 | 298 | 180 | 139.2 | [90] | |
| Wheat stalk | K2FeO4 | Sulfadiazine | 700 | 298 | 6 | 540 | 47.85 | [100] |
| Garlic peel | Concentrated H2SO4 carbonization | Enrofloxacin | 298 | 7 | T1/2 = 34.13 | 142.3 | [88] | |
| Apple branches | FeCl3, humic acid | Enrofloxacin | 700 | 308 | 5 | 720 | 48.3 | [95] |
| Apple branches | FeCl3, humic acid | Moxifloxacin | 700 | 308 | 8 | 720 | 61.5 | [95] |
| Corn stalk | Ball-milling, urea | Norfloxacin | 600 | 298 | 5 | 11.48 | [89] | |
| Sludge | Bentonite | Norfloxacin | 550 | 298 | 6 | 1080 | 89.36 | [102] |
| Pomelo peel | MgFe2O4 | Levofloxacin | 700 | 298 | 5 | 240 | 115 | [97] |
| Vinasse | NiFe2O4 | Levofloxacin | 700 | 298 | 6 | 1080 | 172 | [98] |
| Rice husk | Montmorillonite, CO2 | Ciprofloxacin | 350 | 295 | 7 | 720 | 50.32 | [103] |
| Penicillin fermentation dregs | Acetic acid, K2FeO4 | Penicillin | 400 | 308 | 11 | 322.58 | [99] |
3.4. Hierarchical Porous Carbon-Based Materials Applied in Antibiotics Adsorption
| Hierarchical Porous Carbon Material | Carbon Precursor | Modification Method | Antibiotic | Ad Temp (K) | pH | Time (Min) | Ad Capacity Qm (mg g−1) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Macro-meso-micro hierarchical porous carbon | Wheat straw | KOH + KMnO4 activation | Tetracycline | 318 | 7 | 584.19 | [105] | |
| Fe-doped HPC | Eichhornia crassipes debris | HCl activation, Fe + amino acetic acid synergistic treatment | Tetracycline | 318 | 3–11 | 10 | 457.85 | [107] |
| N-doped bifunctional HPC | Glucose hydrochar | KHCO3 activation, nitrogen doping | Tetracycline | 303 | 4.85 | 629.76 | [45] | |
| Macro-meso-micro hierarchical porous carbon | Sodium lignin sulfonate | KOH activation | Tetracycline | 298 | 3 | 360 | 1297.0 | [109] |
| Micro/meso bimodal porous carbon | Soluble phenolic resin | Tetracycline | 298 | 7 | 701.31 | [111] | ||
| N-doped HPC | Soft-templated ZIF-8 for | Nitrogen doping | Tetracycline hydrochloride | 298 | 4.5 | 900 | 80.92 | [112] |
| Hierarchical micro/mesoporous carbon | Soybean | KOH activation | Chloramphenicol | 298 | 5 | 40 | 892.9 | [44] |
| Hierarchical micro/mesoporous carbon | Corncob | KOH activation | Chloramphenicol | 318 | 9 | 40 | 662.3 | [44] |
| Oxygen-enriched HPC | Sodium lignosulfonate | K2CO3 activation | Chloramphenicol | 303 | 4.86 | 720 | 534.0 | [108] |
| Macro-meso-micro hierarchical porous carbon | Sodium lignin sulfonate | KOH activation | Chloramphenicol | 298 | 3–11 | 360 | 1067.2 | [109] |
| Hierarchical micro/mesoporous carbon | Sodium carboxymethyl cellulose | KOH activation | Chloramphenicol | 298 | 2–6 | 60 | 769.95 | [110] |
| Hierarchical micro/mesoporous carbon | High-salted Spirulina residue | KHCO3 activation | Sulfathiazole | 298 | 7 | 240 | 218.4 | [106] |
4. Photocatalytic Degradation of Antibiotics by Carbon-Based Materials
4.1. Graphene-Based Materials Applied in Photocatalytic Degradation of Antibiotics
| Photocatalysts | Antibiotic | Dosage (g/L) | Detection Wavelength (nm) | Light Source | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|
| (Bi)BiOBr/rGO | Tetracycline | 1.0 | Visible light | >98% within 20 min | [123] | |
| rGO/Bi4O5Br2 | Tetracycline | 0.5 | 356 | Visible light | 95.2% within 60 min | [124] |
| α-Fe2O3/rGO | Tetracycline | 5.0 | Visible light | 99% within 140 min | [126] | |
| La2Zr2O7/rGO | Tetracycline | 1.0 | 357 | Visible light | 82.1% within 40 min | [128] |
| Graphene/TiO2/g–C3N4 | Tetracycline | 357 | Visible light | 83.5% within 80 min | [130] | |
| g-C3N4/MnO2/GO | Tetracycline | 0.5 | Visible light | 91.4% within 60 min | [131] | |
| 15%AgBr/5GO/Bi2WO6 | Tetracycline | 0.4 | 357 | Visible light | 73.3% within 15 min, up to 84% | [132] |
| Ag2O/Bi2WO6/rGO | Tetracycline | 1.0 | Visible light | 95.3% within 40 min | [134] | |
| BiVO4/FeVO4@rGO | Tetracycline | 0.6 | 356 | Visible light | 91.5% within 100 min | [136] |
| QDs-BiVO4/rGH | Tetracycline hydrochloride | 0.5 | 357 | Visible light | 73.2% within 120 min | [122] |
| CF/rGO | Oxytetracycline | 354 | Visible light | 84.7% | [125] | |
| GO/TiO2 | Amoxicillin | 0.6 | 230 | UV light | 99.84% within 60 min | [118] |
| rGO/Bi2WO6 | Norfloxacin | 0.5 | Visible light | 87.49% within 180 min | [120] | |
| BiVO4/GQDs/PCN | Norfloxacin | 1.0 | 273 | Visible light | 86.3% within 120 min | [133] |
| W-BiVO4-x/rGO | Ciprofloxacin | 1.0 | Visible light | 93.6% within 60 min | [121] | |
| NiAlCe LDH/rGO | Ciprofloxacin | 0.25 | 271 | Visible light | 94% within 180 min | [129] |
| ZnO/CdO/rGO | Ciprofloxacin | 0.5 | 270 | UV light | 99.28% within 75 min | [135] |
| CeO2/CdS/rGO | Ciprofloxacin | 0.5 | Sunlight | 90% within 120 min | [137] | |
| AgFeO2/GO3 | Lomefloxacin | 0.583 | 250–450 | Visible light | 88% within 75 min | [127] |
4.2. Carbon Nanotube-Based Materials Applied in Photocatalytic Degradation of Antibiotics
| Photocatalysts | Antibiotic | Dosage (g/L) | Detection Wavelength (nm) | Light Source | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Ag–AgBr/Bi2O2CO3/CNT | Tetracycline | 0.4 | Visible light | 100% within 40 min | [138] | |
| MWCNT/TiO2 | Tetracycline | 0.2 | 360 | UV light | 100% within 100 min | [139] |
| HPWx@Fe2O3-CNTs | Tetracycline | 0.25 | 356 | Visible light | 100% within 40 min | [149] |
| Fe-CNTs | Tetracycline hydrochloride | 0.05 | 358 | Visible light | 93.2% within 100 min | [145] |
| MWCNT/BiVO4 | Oxytetracycline | 0.25 | 360 | Visible light | 88.7% within 60 min | [141] |
| Fe-CNTs | Oxytetracycline | 0.05 | 355 | Visible light | 94.3% within 100 min | [145] |
| Fe-CNTs | Chlortetracycline | 0.05 | 370 | Visible light | 99.4% within 80 min | [145] |
| NiFe2O4/MWCNTs/BiOI | Doxycycline | 1.25 | 351 | UV light | 92.18% within 300 min | [150] |
| CNT@MIL-101(Fe) | Ciprofloxacin | 0.5 | Visible light | 90% within 45 min | [140] | |
| CuBi/MWCNTs | Ciprofloxacin | Visible light | 93% within 90 min | [144] | ||
| MWCNTs-{312}/{004}Bi5O7I | Ofloxacin | 1.0 | Visible light | 88.2% | [142] | |
| CuBi/MWCNTs | Ofloxacin | Visible light | 91% within 90 min | [144] | ||
| Bi2MoO6/Bi2WO6/MWCNTs | Ofloxacin | 2.0 | Visible light | 91.3% within | [146] | |
| CNTs/LaVO4 | Sulfamethazine | 0.3 | 255 | Visible light | Up to 95% within 90 min | [143] |
| Bi2MoO6/Bi2WO6/MWCNTs | Sulfadimidine | 2.0 | Visible light | 88.8% | [146] | |
| SWCNT/ZnO/Fe3O4 | Cefixime | 0.46 | 280 | UV-A light | 94.19% | [147] |
| Bi2WO6/CNT/TiO2 | Cephalexin | 0.75 | 262 | Sunlight | 98.7% within 70 min | [148] |
4.3. Biochar-Based Materials Applied in Photocatalytic Degradation of Antibiotics
4.4. Hierarchical Porous Carbon-Based Materials Applied in Photocatalytic Degradation of Antibiotics
5. Conclusions and Outlooks
- Hastening the formation of antibiotic-resistance genes and antibiotic-resistant bacteria is the main pathway for antibiotics to harm the ecosystem. However, using carbon-based materials to disrupt antibiotic resistant genes and alter bacterial resistance has been rarely reported. Further research should be conducted to determine the impact of carbon-based materials on antibiotic resistance.
- Some carbon-based materials have intrinsic toxicity, and others can produce toxic by-products when removing antibiotics, such as the leaching of metal ions. So, they may have a negative impact on the water environment. The long-term fate and environmental risks of carbon-based materials in the aqueous environment are still unclear, and further studies of their toxicity and biological responses are needed.
- Despite intense research, antibiotics’ adsorption and photodegradation mechanisms remain vaguely interpreted, as conclusions based on partial characterization analysis and traditional models are not accurate or comprehensive. Issues like the synergistic effect of the components in the carbon-based nanocomposites, the effect of the antibiotic species on the removal properties, and the applicability of the relevant models need to be further investigated.
- Currently, most studies on the removal of antibiotics by carbon-based materials are limited to batch experiments at the laboratory scale rather than the pilot scale, resulting in a gap between research and application. The experiments were usually conducted in mixed antibiotics or antibiotics-metals systems instead of a complex system with coexisted multi-pollutants. From an application point of view, it is vital to test the effectiveness of carbon-based materials for antibiotic removal in a system that resembles a natural water environment, and to investigate other pollutants’ influence on the removal process. More attention should be paid to interactive mechanisms among antibiotics, interfering substances, and carbon-based materials. Furthermore, for large-scale engineering applications, in addition to the removal properties of the materials, their mass production feasibility and economic efficiency should be considered, such as raw materials, production cost, production cycle, and yield.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; Angeles Ferro-Garcia, M.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerova, K.; Aamand, J.; Agathoss, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Huu Hao, N.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Manuel Peralta-Hernandez, J.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Zhao, Y.; Yao, Q.; Addo-Bankas, O.; Ji, B.; Yuan, Y.; Wei, T.; Esteve-Nunez, A. A review on antibiotics removal: Leveraging the combination of grey and green techniques. Sci. Total Environ. 2022, 838, 156427. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Basak, S.; Chakrabarti, S. Advancement towards Antibiotic Remediation: Heterostructure and Composite materials. Chemistryselect 2021, 6, 7323–7345. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: A review. Environ. Chem. Lett. 2022, 20, 1929–1963. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Hu, Y.H. Strategies of tuning catalysts for efficient photodegradation of antibiotics in water environments: A review. J. Mater. Chem. 2021, 9, 2592–2611. [Google Scholar] [CrossRef]
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of antibiotics and bacterial resistance genes in wastewater: Resistance mechanisms and antimicrobial resistance control approaches. World J. Microbiol. Biotechnol. 2022, 38, 152. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Pang, H.; Wang, J.-H.; Chi, Z.-Y.; Zhang, Q.; Kong, F.-T.; Xu, Y.-P.; Li, S.-Y.; Che, J. Occurrence of antibiotics in waters, removal by microalgae-based systems, and their toxicological effects: A review. Sci. Total Environ. 2022, 813, 151891. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Daramola, O.B.; Adetunji, A.T.; Ore, O.T.; Ayantunji, Y.J.; Omole, R.K.; Ajagbe, D.; Adekoya, S.O. Efficient removal of antibiotics from water resourcchenes is a public health priority: A critical assessment of the efficacy of some remediation strategies for antibiotics in water. Environ. Sci. Pollut. Res. 2022, 29, 56948–57020. [Google Scholar] [CrossRef]
- Juela, D.M. Promising adsorptive materials derived from agricultural and industrial wastes for antibiotic removal: A comprehensive review. Sep. Purif. Technol. 2022, 284, 120286. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, Y.; Zhang, C.; Xue, Y.; Chen, Y.; Teng, W.; Fan, J. Iron pyrophosphate doped carbon nanocomposite for tetracycline degradation by activation of peroxymonosulfate. New J. Chem. 2022, 46, 17985–17994. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible-light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–799. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous Carbons: Structure-Oriented Design and Versatile Applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- Xue, Y.; Teng, W.; Chen, Y.; Ma, Q.; Chen, X.; Sun, Y.; Fan, J.; Qiu, Y.; Fu, R. Amorphous Mn-La oxides immobilized on carbon sphere for efficient removal of As(V), Cd(II), and Pb(II): Co-adsorption and roles of Mn species. Chem. Eng. J. 2022, 429, 132262. [Google Scholar] [CrossRef]
- Viglasova, E.; Galambos, M.; Divis, D.; Dankova, Z.; Dano, M.; Krivosudsky, L.; Lengauer, C.L.; Matik, M.; Briancin, J.; Soja, G. Engineered biochar as a tool for nitrogen pollutants removal: Preparation, characterization and sorption study. Desalin. Water Treat. 2020, 191, 318–331. [Google Scholar] [CrossRef]
- Dano, M.; Viglasova, E.; Stamberg, K.; Galambos, M.; Galanda, D. Pertechnetate/Perrhenate Surface Complexation on Bamboo Engineered Biochar. Materials 2021, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U.; Inamuddin. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2017, 24, 27047–27069. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin. Carbon nanotube-based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Ouni, L.; Ramazani, A.; Fardood, S.T. An overview of carbon nanotubes role in heavy metals removal from wastewater. Front. Chem. Sci. Eng. 2019, 13, 274–295. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Weidner, E.; Karbassiyazdi, E.; Altaee, A.; Jesionowski, T.; Ciesielczyk, F. Hybrid Metal Oxide/Biochar Materials for Wastewater Treatment Technology: A Review. ACS Omega 2022, 7, 27062–27078. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Enaime, G.; Bacaoui, A.; Yaacoubi, A.; Luebken, M. Biochar for Wastewater Treatment-Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar—A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Zhang, H.; Shao, L.-M.; Lu, F.; He, P.-J. Preparation and Application of Hierarchical Porous Carbon Materials from Waste and Biomass: A Review. Waste Biomass Valorization 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Kang, W.; Lei, W.; Wang, X.; Lu, C.; Naebe, M. Structural design and mechanism analysis of hierarchical porous carbon fibers for advanced energy and environmental applications. J. Mater. Chem. 2021, 10, 10–49. [Google Scholar] [CrossRef]
- Zhang, M.; Igalavithana, A.D.; Xu, L.; Sarkar, B.; Hou, D.; Zhang, M.; Bhatnagar, A.; Cho, W.C.; Ok, Y.S. Engineered/designer hierarchical porous carbon materials for organic pollutant removal from water and wastewater: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2295–2328. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, Q.; Ma, Q.; Chen, Y.; Zhang, C.; Teng, W.; Fan, J.; Zhang, W.-X. Electrocatalytic Hydrogenation Boosts Reduction of Nitrate to Ammonia over Single-Atom Cu with Cu(I)-N3C1 Sites. Environ. Sci. Technol. 2022, 56, 14797–14807. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Nayanathara, R.M.O.; Song, X.; Shmulsky, R.; Hassan, E.B. Biobased hierarchically porous carbon featuring micron-sized honeycomb architecture for CO2 capture and water remediation. J. Environ. Chem. Eng. 2022, 10, 107460. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Wang, B.; Xie, J.; Ying, G.; Li, J.; Cheng, Z.; Li, J.; Chen, K. Highly efficient and rapid purification of organic dye wastewater using lignin-derived hierarchical porous carbon. J. Colloid Interface Sci. 2022, 625, 158–168. [Google Scholar] [CrossRef]
- Wang, G.; Yong, X.; Luo, L.; Yan, S.; Wong, J.W.C.; Zhou, J. Structure-performance correlation of high surface area and hierarchical porous biochars as chloramphenicol adsorbents. Sep. Purif. Technol. 2022, 296, 121374. [Google Scholar] [CrossRef]
- Pi, Z.; Hou, K.; Yao, F.; He, L.; Chen, S.; Tao, Z.; Zhou, P.; Wang, D.; Li, X.; Yang, Q. In-situ regeneration of tetracycline-saturated hierarchical porous carbon by peroxydisulfate oxidation process: Performance, mechanism and application. Chem. Eng. J. 2022, 427, 131749. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Liu, Y.; Zhang, L.; Xing, B. Ultrathin porous carbon nanosheet as an efficient adsorbent for the removal of bisphenol A: The overlooked role of topological defects. Chemosphere 2022, 306, 135549. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, H.; Yan, J.; Shan, L.; Quan, G.; Pan, X.; Cui, L. Wheat straw derived biochar with hierarchically porous structure for bisphenol A removal: Preparation, characterization, and adsorption properties. Sep. Purif. Technol. 2022, 289, 120796. [Google Scholar] [CrossRef]
- Ma, C.-F.; Gao, Q.; Xia, K.-S.; Huang, Z.-Y.; Han, B.; Zhou, C.-G. Three-dimensionally porous graphene: A high-performance adsorbent for removal of albumin-bonded bilirubin. Colloids Surf. B-Biointerfaces 2017, 149, 146–153. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Mangla, D.; Annu; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Li, M.-F.; Liu, Y.-G.; Zeng, G.-M.; Liu, N.; Liu, S.-B. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Salihi, E.C.; Wang, J.; Kabacaoglu, G.; Kirkulak, S.; Siller, L. Graphene oxide as a new generation adsorbent for the removal of antibiotics from waters. Sep. Sci. Technol. 2021, 56, 453–461. [Google Scholar] [CrossRef]
- Moreira, V.R.; Rocha Lebron, Y.A.; da Silva, M.M.; de Souza Santos, L.V.; Jacob, R.S.; Barbosa de Vasconcelos, C.K.; Viana, M.M. Graphene oxide in the remediation of norfloxacin from aqueous matrix: Simultaneous adsorption and degradation process. Environ. Sci. Pollut. Res. 2020, 27, 34513–34528. [Google Scholar] [CrossRef]
- Wernke, G.; Shimabuku-Biadola, Q.L.; Dos Santos, T.R.T.; Silva, M.F.; Fagundes-Klen, M.R.; Bergamasco, R. Adsorption of cephalexin in aqueous media by graphene oxide: Kinetics, isotherm, and thermodynamics. Environ. Sci. Pollut. Res. 2020, 27, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Upoma, B.P.; Yasmin, S.; Shaikh, M.A.A.; Jahan, T.; Haque, M.A.; Moniruzzaman, M.; Kabir, M.H. A Fast Adsorption of Azithromycin on Waste-Product-Derived Graphene Oxide Induced by H-Bonding and Electrostatic Interactions. ACS Omega 2022, 7, 29655–29665. [Google Scholar] [CrossRef]
- Liu, F.-F.; Zhao, J.; Wang, S.; Xing, B. Adsorption of sulfonamides on reduced graphene oxides as affected by pH and dissolved organic matter. Environ. Pollut. 2016, 210, 85–93. [Google Scholar] [CrossRef]
- El Hadki, A.; Ulucan-Altuntas, K.; El Hadki, H.; Ustundag, C.B.; Kabbaj, O.K.; Dahchour, A.; Komiha, N.; Zrineh, A.; Debik, E. Removal of oxytetracycline by graphene oxide and Boron-doped reduced graphene oxide: A combined density function Theory, molecular dynamics simulation and experimental study. Flatchem 2021, 27, 100238. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, K.; Xu, X.; Liu, X.; Zhao, B.; Ding, H.; Yu, Z.; Deng, C. Preparation of beta-cyclodextrin/dopamine hydrochloride-graphene oxide and its adsorption properties for sulfonamide antibiotics. Environ. Sci. Pollut. Res. 2022, 29, 70192–70201. [Google Scholar] [CrossRef] [PubMed]
- Braki, Z.A.; Sohrabi, M.R.; Motiee, F. Efficient simultaneous removal of tetracycline and cefazolin from aqueous solution using a novel adsorbent based on zero-valent iron nanoparticles supported by montmorillonite and graphene oxide: Isotherms, kinetic, and thermodynamic studies. Int. J. Environ. Anal. Chem. 2022, 1–23. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Hu, S.; Zhang, Y.; Qian, C.; Li, A.; Zhang, S. Ultra-fast adsorption of four typical pollutants using magnetically separable ethanolamine-functionalized graphene. Sep. Purif. Technol. 2021, 271, 118862. [Google Scholar] [CrossRef]
- Shen, J.-W.; Li, J.; Dai, J.; Zhou, M.; Ren, H.; Zhang, L.; Hu, Q.; Kong, Z.; Liang, L. Molecular dynamics study on the adsorption and release of doxorubicin by chitosan-decorated graphene. Carbohydr. Polym. 2020, 248, 116809. [Google Scholar] [CrossRef] [PubMed]
- Xiaosan, S.; Boyang, S.; Yiru, W.; Jie, Z.; Sanfan, W.; Nan, W. Adsorption performance of GO-doped activated ATP composites towards tetracycline. RSC Adv. 2022, 12, 19917–19928. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Liu, Y.; Fang, T.; Zhang, L.; Xing, B. Ring defects-rich and pyridinic N-doped graphene aerogel as floating adsorbent for efficient removal of tetracycline: Evidence from NEXAFS measurements and theoretical calculations. J. Hazard. Mater. 2022, 435, 128940. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, G.; Zhang, Y.; Li, D.; Ling, C.; Wang, Q.; Liu, G. Superhigh co-adsorption of tetracycline and copper by the ultrathin g-C3N4 modified graphene oxide hydrogels. J. Hazard. Mater. 2022, 424, 127362. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Du, Q.; Pi, X.; Wang, Y.; Sun, Y.; Wang, M.; Zhang, Y.; Chen, K.; Zhu, J. Effective Removal of Tetracycline from Water Using Copper Alginate @ Graphene Oxide with in-situ Grown MOF-525 Composite: Synthesis, Characterization and Adsorption Mechanisms. Nanomaterials 2022, 12, 2897. [Google Scholar] [CrossRef]
- Ghafoori, M.; Cheraghi, M.; Sadr, M.K.; Lorestani, B.; Sobhanardakani, S. Magnetite graphene oxide modified with beta-cyclodextrin as an effective adsorbent for the removal of methotrexate and doxorubicin hydrochloride from water. Environ. Sci. Pollut. Res. 2022, 29, 35012–35024. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Mostafapour, F.; Bazrafshan, E.; Saleh, T.A. Studies on the adsorption of amoxicillin on multi-wall carbon nanotubes. Water Sci. Technol. 2017, 75, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, R.; Zhong, C. Adsorption of fluoroquinolone by carbon nanotubes: A combined experimental and density functional theory study. Chem. Pap. 2020, 74, 3847–3856. [Google Scholar] [CrossRef]
- Veclani, D.; Melchior, A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298, 111977. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Leyva-Ramos, R.; Rodriguez-Ramos, I.; Mendoza-Mendoza, E.; Moral-Rodriguez, A.E. Adsorption capacity of different types of carbon nanotubes towards metronidazole and dimetridazole antibiotics from aqueous solutions: Effect of morphology and surface chemistry. Environ. Sci. Pollut. Res. 2020, 27, 17123–17137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tian, Y.; Chu, X.; Cui, L.; Zhang, H.; Li, M.; Zhao, P. Preparation and characteristics of a magnetic carbon nanotube adsorbent: Its efficient adsorption and recoverable performances. Sep. Purif. Technol. 2021, 257, 117917. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhu, X.; Wang, J. Adsorptive removal of plasticizer (dimethyl phthalate) and antibiotic (sulfamethazine) from municipal wastewater by magnetic carbon nanotubes. J. Mol. Liq. 2020, 319, 114267. [Google Scholar] [CrossRef]
- Sereshti, H.; Beyrak-Abadi, E.; Bidhendi, M.E.; Ahmad, I.; Shahabuddin, S.; Nodeh, H.R.; Sridewi, N.; Ibrahim, W.N.W. Sulfide-Doped Magnetic Carbon Nanotubes Developed as Adsorbent for Uptake of Tetracycline and Cefixime from Wastewater. Nanomaterials 2022, 12, 3576. [Google Scholar] [CrossRef]
- Yao, J.; Deng, Y.; Pan, S.; Korna, R.; Wen, J.; Yuan, N.; Wang, K.; Li, H.; Yang, Y. The difference in the adsorption mechanisms of magnetic ferrites modified carbon nanotubes. J. Hazard. Mater. 2021, 415, 125551. [Google Scholar] [CrossRef]
- Xia, C.; Huang, H.; Liang, D.; Xie, Y.; Kong, F.; Yang, Q.; Fu, J.; Dou, Z.; Zhang, Q.; Meng, Z. Adsorption of tetracycline hydrochloride on layered double hydroxide loaded carbon nanotubes and site energy distribution analysis. Chem. Eng. J. 2022, 443, 136398. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, T.; Jia, Y.; Xu, N.; Yang, S.; Zhang, C.; Gao, S.; Shen, W.; Wang, Z. Adsorption performance and optimization by response surface methodology on tetracycline using Fe-doped ZIF-8-loaded multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jiang, Z.; Cao, J.; Yu, F. Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 2020, 242, 125188. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Dai, Y.; Li, J.; Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 2020, 238, 124432. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Adsorption and removal of seven antibiotic compounds present in water with the use of biochar derived from the pyrolysis of organic waste feedstocks. J. Environ. Chem. Eng. 2021, 9, 105868. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, W.; Huang, K.; Shao, H.; Qu, C.; Liu, J. One-step synthesis of garlic peel derived biochar by concentrated sulfuric acid: Enhanced adsorption capacities for Enrofloxacin and interfacial interaction mechanisms. Chemosphere 2022, 290, 133263. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Liu, Y.; Tang, W.; Geng, S.; Chen, J. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar. Chemosphere 2022, 303, 135264. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wang, Y.; Li, R.-X.; Cao, H.-L.; Li, Y.-F.; Lu, J. Impacts of temperatures and phosphoric-acid modification to the physicochemical properties of biochar for excellent sulfadiazine adsorption. Biochar 2022, 4, 14. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Cheng, H.; Cheng, J.; Du, K.; Hu, Y.; Chen, Y. High mesoporosity phosphorus-containing biochar fabricated from Camellia oleifera shells: Impressive tetracycline adsorption performance and promotion of pyrophosphate-like surface functional groups (C-O-P bond). Bioresour. Technol. 2021, 329, 124922. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, J.; Cui, Q.; Zhang, W.; Zhu, X. A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ. Res. 2022, 207, 112175. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, Y.; Zhang, Y.; Liu, S.; Wang, C.; Chen, W.; Liu, C.; Chen, Z.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lou, X.; Hu, Q.; Sun, T. Adsorption of antibiotics from water by using Chinese herbal medicine residues derived biochar: Preparation and properties studies. J. Mol. Liq. 2021, 325, 114967. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Chu, M.; Gao, H.; Zhang, D. Biochar composite derived from cellulase hydrolysis apple branch for quinolone antibiotics enhanced removal: Precursor pyrolysis performance, functional group introduction and adsorption mechanisms. Environ. Pollut. 2022, 313, 120104. [Google Scholar] [CrossRef]
- Li, X.; Shi, J. Simultaneous adsorption of tetracycline, ammonium and phosphate from wastewater by iron and nitrogen modified biochar: Kinetics, isotherm, thermodynamic and mechanism. Chemosphere 2022, 293, 133574. [Google Scholar] [CrossRef]
- Yao, B.; Luo, Z.; Du, S.; Yang, J.; Zhi, D.; Zhou, Y. Sustainable biochar/MgFe(2)O4 adsorbent for levofloxacin removal: Adsorption performances and mechanisms. Bioresour. Technol. 2021, 340, 125698. [Google Scholar] [CrossRef]
- Xu, Z.; Xiang, Y.; Zhou, H.; Yang, J.; He, Y.; Zhu, Z.; Zhou, Y. Manganese ferrite modified biochar from vinasse for enhanced adsorption of levofloxacin: Effects and mechanisms. Environ. Pollut. 2021, 272, 115968. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Magnetic porous biochar with nanostructure surface derived from penicillin fermentation dregs pyrolysis with K2FeO4 activation: Characterization and application in penicillin adsorption. Bioresour. Technol. 2021, 327, 124818. [Google Scholar] [CrossRef]
- Yan, J.; Zuo, X.; Yang, S.; Chen, R.; Cai, T.; Ding, D. Evaluation of potassium ferrate activated biochar for the simultaneous adsorption of copper and sulfadiazine: Competitive versus synergistic. J. Hazard. Mater. 2022, 424, 127435. [Google Scholar] [CrossRef]
- Qu, J.; Wang, S.; Jin, L.; Liu, Y.; Yin, R.; Jiang, Z.; Tao, Y.; Huang, J.; Zhang, Y. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr(VI) and tetracycline adsorption from water. Bioresour. Technol. 2021, 340, 125692. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Meng, Z.; Song, E.; Sun, X.; Hu, X.; Li, W.; Liu, Z.; Gao, S.; Song, B. Co-adsorption capabilities and mechanisms of bentonite enhanced sludge biochar for de-risking norfloxacin and Cu2+contaminated water. Chemosphere 2022, 299, 134414. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.; Rehman, M.Z.U.; Yousaf, B.; Ahmed, R.; Miana, M.M.; Ashrafa, A.; Munirf, M.A.M.; Rashida, M.S.; Naeem, A. Carbon dioxide activated biochar-clay mineral composite efficiently removes ciprofloxacin from contaminated water—Reveals an incubation study. J. Clean. Prod. 2022, 332, 130079. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Luo, J.; Li, Y.; Guo, T.; Gao, B. Performance of lignin impregnated biochar on tetracycline hydrochloride adsorption: Governing factors and mechanisms. Environ. Res. 2022, 215, 114339. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H.; Guan, D.-X. Improved adsorption properties of tetracycline on KOH/KMnO4 modified biochar derived from wheat straw. Chemosphere 2022, 296, 133981. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Zhang, S.; Chen, Y.-D.; Wang, R.; Ho, S.-H. Tailoring a novel hierarchical cheese-like porous biochar from algae residue to boost sulfathiazole removal. Environ. Sci. Ecotechnol. 2022, 10, 100168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Shi, J.; Luo, X. Rapid and efficient adsorption of tetracycline from aqueous solution in a wide pH range by using iron and aminoacetic acid sequentially modified hierarchical porous biochar. Bioresour. Technol. 2022, 346, 126672. [Google Scholar] [CrossRef]
- Chen, A.; Pang, J.; Wei, X.; Chen, B.; Xie, Y. Fast one-step preparation of porous carbon with hierarchical oxygen-enriched structure from waste lignin for chloramphenicol removal. Environ. Sci. Pollut. Res. 2021, 28, 27398–27410. [Google Scholar] [CrossRef]
- Xie, A.; Dai, J.; Chen, X.; Ma, P.; He, J.; Li, C.; Zhou, Z.; Yan, Y. Ultrahigh adsorption of typical antibiotics onto novel hierarchical porous carbons derived from renewable lignin via halloysite nanotubes-template and in-situ activation. Chem. Eng. J. 2016, 304, 609–620. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Z.; Xie, A.; Dai, J.; Cui, J.; Lang, J.; Wei, M.; Dai, X.; Li, C.; Yan, Y. Preparation of hierarchical porous carbons from sodium carboxymethyl cellulose via halloysite template strategy coupled with KOH-activation for efficient removal of chloramphenicol. J. Taiwan Inst. Chem. Eng. 2017, 80, 424–433. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, L.; Qi, C.; Zhang, M. Highly Effective Removal of Tetracycline from Water by Hierarchical Porous Carbon: Batch and Column Adsorption. Ind. Eng. Chem. Res. 2019, 58, 20036–20046. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, P.; Wang, Z.; Zuo, C.; Chen, W.; Ao, T. Facile fabrication of N-doped hierarchical porous carbons derived from soft-templated ZIF-8 for enhanced adsorptive removal of tetracycline hydrochloride from water. J. Hazard. Mater. 2022, 423, 127103. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Chen, W.; Wang, B.; Sun, T.; Wu, B.; Wang, Y. Photocatalytic Degradation of Some Typical Antibiotics: Recent Advances and Future Outlooks. Int. J. Mol. Sci. 2022, 23, 8130. [Google Scholar] [CrossRef] [PubMed]
- Kuvarega, A.T.; Mamba, B.B. TiO2-based Photocatalysis: Toward Visible Light-Responsive Photocatalysts Through Doping and Fabrication of Carbon-Based Nanocomposites. Crit. Rev. Solid State Mater. Sci. 2017, 42, 295–346. [Google Scholar] [CrossRef]
- Dai, L.; Sun, F.; Fan, Q.; Li, H.; Yang, K.; Guo, T.; Zheng, L.; Fu, P. Carbon-based titanium dioxide materials for hydrogen production in water-methanol reforming: A review. J. Environ. Chem. Eng. 2022, 10, 107326. [Google Scholar] [CrossRef]
- Jayaraman, T.; Murthy, A.P.; Elakkiya, V.; Chandrasekaran, S.; Nithyadharseni, P.; Khan, Z.; Senthil, R.A.; Shanker, R.; Raghavender, M.; Kuppusami, P.; et al. Recent development on carbon based heterostructures for their applications in energy and environment: A review. J. Ind. Eng. Chem. 2018, 64, 16–59. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, J.; Ji, B.; Ma, L.; Yang, Z. Novel formation of large area N-TiO2/graphene layered materials and enhanced photocatalytic degradation of antibiotics. J. Environ. Chem. Eng. 2020, 8, 102206. [Google Scholar] [CrossRef]
- Balarak, D.; Mengelizadeh, N.; Rajiv, P.; Chandrika, K. Photocatalytic degradation of amoxicillin from aqueous solutions by titanium dioxide nanoparticles loaded on graphene oxide. Environ. Sci. Pollut. Res. 2021, 28, 49743–49754. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B-Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Hu, X.; Fan, J. rGO/Bi2WO6 composite as a highly efficient and stable visible-light photocatalyst for norfloxacin degradation in aqueous environment. J. Colloid Interface Sci. 2021, 589, 336–346. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Zhou, F.; Zhao, Q.; Wang, K.; Chen, X.; Gao, Q. Insight into a novel microwave-assisted W doped BiVO4 self-assembled sphere with rich oxygen vacancies oriented on rGO (W-BiVO4-x/rGO) photocatalyst for efficient contaminants removal. Sep. Purif. Technol. 2021, 277, 119610. [Google Scholar] [CrossRef]
- Ma, C.; Seo, W.C.; Lee, J.; Kim, Y.; Jung, H.; Yang, W. Construction of quantum dots self-decorated BiVO4/reduced graphene hydrogel composite photocatalyst with improved photocatalytic performance for antibiotics degradation. Chemosphere 2021, 275, 130052. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Q.; Chen, P.; Zheng, H.; Shi, J.; Shu, H.; Liu, Y. Photocatalytic degradation of tetracycline by using a regenerable (Bi) BiOBr/rGO composite. J. Clean. Prod. 2022, 339, 130771. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Ha, E.; Zhang, H.; Li, C. Reduced graphene oxide/Bi4O5Br2 nanocomposite with synergetic effects on improving adsorption and photocatalytic activity for the degradation of antibiotics. Chemosphere 2021, 265, 129013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zang, L.; Wang, L.; Tian, Y.; Yang, Z.; Yue, Y.; Sun, L. Magnetic cobalt ferrite/reduced graphene oxide (CF/rGO) porous balls for efficient photocatalytic degradation of oxytetracycline. J. Environ. Chem. Eng. 2022, 10, 108259. [Google Scholar] [CrossRef]
- Shibu, M.C.; Benoy, M.D.; Shanavas, S.; Abu Haija, M.; Duraimurugan, J.; Kumar, G.S.; Ahamad, T.; Maadeswaran, P.; Van Le, Q. White LED active—Fe2O3/rGO photocatalytic nanocomposite for an effective degradation of tetracycline and ibuprofen molecules. Environ. Res. 2022, 212, 113301. [Google Scholar] [CrossRef]
- Yashas, S.R.; Shivaraju, H.P.; McKay, G.; Shahmoradi, B.; Maleki, A.; Yetilmezsoy, K. Designing bi-functional silver delafossite bridged graphene oxide interfaces: Insights into synthesis, characterization, photocatalysis and bactericidal efficiency. Chem. Eng. J. 2021, 426, 131729. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Huang, L.; Liu, X.; Han, Y.; Wang, L. La2Zr2O7/rGO synthesized by one-step sol-gel method for photocatalytic degradation of tetracycline under visible-light. Chem. Eng. J. 2020, 384, 123380. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, J.; Yao, J.; Zhao, Y.; Meng, Q.; He, G.; Chen, H. Synthesis of Ce-doped NiAl LDH/RGO composite as an efficient photocatalyst for photocatalytic degradation of ciprofloxacin. J. Environ. Chem. Eng. 2021, 9, 105405. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Zhang, J.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z?scheme photocatalyst of graphene layers anchored TiO2/g?C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Z.; Tan, S.; Yu, G.; Chen, H.; Zhou, L.; Yu, L.; Su, Y.; Zhang, Y.; Deng, F.; et al. Construction of Z-scheme g-CN4/MnO2/GO ternary photocatalyst with enhanced photodegradation ability of tetracycline hydrochloride under visible light radiation. Environ. Res. 2021, 200, 111427. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Li, X.; Wu, Y.; Chen, Z.; Huang, X.; Wang, D.; Yang, Q.; Liu, J.; Tian, S.; Chen, X.; et al. AgBr nanoparticles decorated 2D/2D GO/Bi2WO6 photocatalyst with enhanced photocatalytic performance for the removal of tetracycline hydrochloride. Chem. Eng. J. 2021, 410, 128283. [Google Scholar] [CrossRef]
- Wang, M.; Yu, H.; Wang, P.; Chi, Z.; Zhang, Z.; Dong, B.; Dong, H.; Yu, K.; Yu, H. Promoted photocatalytic degradation and detoxication performance for norfloxacin on Z-scheme phosphate-doped BiVO4/graphene quantum dots/P-doped g-C3N4. Sep. Purif. Technol. 2021, 274, 118692. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Z.; Tong, Z. Engineering Z-scheme silver oxide/bismuth tungstate heterostructure incorporated reduced graphene oxide with superior visible-light photocatalytic activity. J. Colloid Interface Sci. 2021, 596, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. ZnO-CdO nanocomposites incorporated with graphene oxide nanosheets for efficient photocatalytic degradation of bisphenol A, thymol blue and ciprofloxacin. J. Hazard. Mater. 2022, 424, 127332. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhu, Z.; Hu, C.; Zhong, S.; Zhang, L.; Liu, B.; Wang, W. One-step preparation (3D/2D/2D) BiVO4/FeVO4@rGO heterojunction composite photocatalyst for the removal of tetracycline and hexavalent chromium ions in water. Chem. Eng. J. 2020, 390, 124522. [Google Scholar] [CrossRef]
- Yao, J.; Gao, Z.; Meng, Q.; He, G.; Chen, H. One-step synthesis of reduced graphene oxide based ceric dioxide modified with cadmium sulfide (CeO2/CdS/RGO) heterojunction with enhanced sunlight-driven photocatalytic activity. J. Colloid Interface Sci. 2021, 594, 621–634. [Google Scholar] [CrossRef]
- Zuo, H.; Wu, C.; Du, H.; Shi, H.; Fu, Y.; Zhang, T.; Yan, Q. Construction of Z-scheme Ag-AgBr/Bi2O2CO3/CNT heterojunctions with remarkable photocatalytic performance using carbon nanotubes as efficient electronic mediators. Chemosphere 2022, 302, 134927. [Google Scholar] [CrossRef]
- Ahmadi, M.; Motlagh, H.R.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef]
- Yan, D.; Hu, H.; Gao, N.; Ye, J.; Ou, H. Fabrication of carbon nanotube functionalized MIL-101(Fe) for enhanced visible-light photocatalysis of ciprofloxacin in aqueous solution. Appl. Surf. Sci. 2019, 498, 143836. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, X.; Xu, Y.; Lai, W.; Yan, K.; Huang, L.; Ling, J.; Zheng, L. Photocatalytic performance of multi-walled carbon nanotube/BiVO4 synthesized by electro-spinning process and its degradation mechanisms on oxytetracycline. Chem. Eng. J. 2019, 373, 880–890. [Google Scholar] [CrossRef]
- Gao, P.; Huang, S.; Tao, K.; Li, Z.; Feng, L.; Liu, Y.; Zhang, L. Synthesis of adjustable {312}/{004} facet heterojunction MWCNTs/Bi5O7I photocatalyst for ofloxacin degradation: Novel insights into the charge carriers transport. J. Hazard. Mater. 2022, 437, 129374. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Alalm, M.G.; Fujii, M. Effective photocatalytic degradation of sulfamethazine by CNTs/LaVO4 in suspension and dip coating modes. Sep. Purif. Technol. 2020, 235, 116138. [Google Scholar] [CrossRef]
- Khazaee, Z.; Mahjoub, A.R.; Khavar, A.H.C. One-pot synthesis of CuBi bimetallic alloy nanosheets-supported functionalized multiwalled carbon nanotubes as efficient photocatalyst for oxidation of fluoroquinolones. Appl. Catal. B-Environ. 2021, 297, 120480. [Google Scholar] [CrossRef]
- Zhang, S.; Rong, F.; Huang, S.; Zhao, S.; Wang, M.; He, L.; Zhang, Z.; Du, M. Atomic Fe sites embedded within carbon nanotubes for the efficient photodegradation of multiple tetracyclines. Sep. Purif. Technol. 2022, 287, 120530. [Google Scholar] [CrossRef]
- Wang, W.; Tian, J.; Zhu, Z.; Zhu, C.; Liu, B.; Hu, C. Insight into quinolones and sulfonamides degradation, intermediate product identification and decomposition pathways with the assistance of Bi2MoO6/Bi2WO6/MWCNTs photocatalyst. Process Saf. Environ. Prot. 2021, 147, 527–546. [Google Scholar] [CrossRef]
- Erim, B.; Cigeroglu, Z.; Sahin, S.; Vasseghian, Y. Photocatalytic degradation of cefixime in aqueous solutions using functionalized SWCNT/ZnO/Fe3O4 under UV-A irradiation. Chemosphere 2022, 291, 132929. [Google Scholar] [CrossRef] [PubMed]
- Rabanimehr, F.; Farhadian, M.; Nazar, A.R.S.; Moghadam, M. Fabrication of Z-scheme Bi2WO6/CNT/TiO2 heterostructure with enhanced cephalexin photodegradation: Optimization and reaction mechanism. J. Mol. Liq. 2021, 339, 116728. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, S.; Yang, Y.; Liu, S.; Cao, Q.; Wang, Y.; Wagberg, T.; Hu, G. Artificial chloroplast-like phosphotungstic acid—Iron oxide microbox heterojunctions penetrated by carbon nanotubes for solar photocatalytic degradation of tetracycline antibiotics in wastewater. Adv. Compos. Hybrid Mater. 2022, 5, 3158–3175. [Google Scholar] [CrossRef]
- Yan, X.; Qian, J.; Pei, X.; Zhou, L.; Ma, R.; Zhang, M.; Du, Y.; Bai, L. Enhanced photodegradation of doxycycline (DOX) in the sustainable NiFe2O4/MWCNTs/BiOI system under UV light irradiation. Environ. Res. 2021, 199, 111264. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, Q.; Gao, Y.; Jian, H.; Wang, C.; Sun, H. Effects of biochar-dissolved organic matter on the photodegradation of sulfamethoxazole and chloramphenicol in biochar solutions as revealed by oxygen reduction performances and free radicals. Sci. Total Environ. 2021, 781, 146807. [Google Scholar] [CrossRef]
- Xiao, Y.; Lyu, H.; Tang, J.; Wang, K.; Sun, H. Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms. Chem. Eng. J. 2020, 384, 123311. [Google Scholar] [CrossRef]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Chen, T.; Sun, J.; Ma, X.; Wang, Y.; Wang, J.; Xie, Z. Preparation of TiO2-modified Biochar and its Characteristics of Photo-catalysis Degradation for Enrofloxacin. Sci. Rep. 2020, 10, 6588. [Google Scholar] [CrossRef] [Green Version]
- Louros, V.L.; Silva, V.; Silva, C.P.; Calisto, V.; Otero, M.; Esteves, V.I.; Freitas, R.; Lima, D.L.D. Sulfadiazine’s photodegradation using a novel magnetic and reusable carbon based photocatalyst: Photocatalytic efficiency and toxic impacts to marine bivalves. J. Environ. Manag. 2022, 313, 115030. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, X.; Xie, X.; Li, S.; Zhang, X.; Wang, Z. Visible-LED-light-driven photocatalytic degradation of ofloxacin and ciprofloxacin by magnetic biochar modified flower-like Bi2WO6: The synergistic effects, mechanism insights and degradation pathways. Sci. Total Environ. 2021, 764, 142879. [Google Scholar] [CrossRef]
- Chen, Z.; He, Z.; Zhou, M.; Xie, M.; He, T.; Zhao, Y.; Chen, X.; Wu, Y.; Xu, Z. In-situ synthesis of biochar modified PbMoO4: An efficient visible light-driven photocatalyst for tetracycline removal. Chemosphere 2021, 284, 131260. [Google Scholar] [CrossRef]
- Huang, H.-B.; Zhang, N.; Yu, K.; Zhang, Y.-Q.; Cao, H.-L.; Lu, J.; Cao, R. One-Step Carbothermal Synthesis of Robust CdS@BPC Photocatalysts in the Presence of Biomass Porous Carbons. ACS Sustain. Chem. Eng. 2019, 7, 16835–16842. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Zhao, J. pi-pi stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-light-driven photocatalytic degradation of dye and antibiotics by activated biochar composited with K+ doped g-C3N4: Effects, mechanisms, actual wastewater treatment and disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@biochar nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar] [CrossRef] [PubMed]
- Azalok, K.A.; Oladipo, A.A.; Gazi, M. Hybrid MnFe-LDO-biochar nanopowders for degradation of metronidazole via UV-light-driven photocatalysis: Characterization and mechanism studies. Chemosphere 2021, 268, 128844. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, P.; Zhang, L.; Chen, M.; Tang, J.; Qin, C.; Lee, S.L.J.; Lin, S. Facile fabrication of ZnO decorated ZnFe-layered double hydroxides @ biochar nanocomposites for synergistic photodegradation of tetracycline under visible light. Chem. Eng. J. 2022, 434, 134772. [Google Scholar] [CrossRef]
- Peng, H.; Li, Y.; Wen, J.; Zheng, X. Synthesis of ZnFe2O4/B,N-codoped biochar via microwave-assisted pyrolysis for enhancing adsorption-photocatalytic elimination of tetracycline hydrochloride. Ind. Crops Prod. 2021, 172, 114066. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Xie, T.; Zhang, Y.; Wang, Y.; Yang, H. Photocatalytic removal of sulfamethoxazole using yeast biomass-derived NixP/biocarbon composites in the presence of dye sensitizer. J. Environ. Chem. Eng. 2022, 10, 107426. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, T.; Liu, S.; Liu, Y.; Chen, H.; Li, Z.; Du, J.; Lei, Z.; Peng, H. N-doped magnetic three-dimensional carbon microspheres@TiO2 with a porous architecture for enhanced degradation of tetracycline and methyl orange via adsorption/photocatalysis synergy. Chem. Eng. J. 2021, 411, 128615. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Pan, J.; Xue, Z.; Li, J.; Wang, G. Metal-organic complex-derived 3D porous carbon-supported g-C3N4/TiO2 as photocatalysts for the efficient degradation of antibiotic. Crystengcomm 2021, 23, 4717–4723. [Google Scholar] [CrossRef]

| Biomass | Pyrolysis Temp (°C) | Photocatalysts | Antibiotic | Dosage (g/L) | Light Source | Degradation Efficiency | Refs. |
|---|---|---|---|---|---|---|---|
| Poplar sawdust | 500 | PbMoO4/BC | Tetracycline | 3.0 | Visible light | 61.0% within 120 min | [157] |
| Potato stems and leaves | 600 | CdS/BPC | Tetracycline | 0.1 | Visible light | 84.6% within 120 min | [158] |
| Enteromorpha | 700 | g-C3N4/BC | Tetracycline | 0.2 | Visible light | 88% within 60 min | [159] |
| Caragana korshinskii | 650 | K-g-C3N4/BC | Tetracycline | 1.0 | Visible light | 90.94% within 180 min | [160] |
| Rice husks | 400 | ZnO/ZnFe-LDH/BC | Tetracycline | 0.2 | Visible light | 87.7% within 240 min | [163] |
| Sugarcane bagasse | 600 | ZnFe/BN-BC | Tetracycline hydrochloride | 0.33 | Sunlight | 98.19% within 120 min | [164] |
| Rice straw | 700 | Pure BC | Sulfamethoxazole | 0.5 | Visible light | 96.28% within 720 min | [151] |
| Reed straw | 500 | Zn-TiO2/pBC | Sulfamethoxazole | 1.25 | Visible light | 81.21% within 180 min | [153] |
| Baker’s yeast | 900 | NixP/BC | Sulfamethoxazole | 0.4 | Visible light | 98.71% within 120 min | [165] |
| Primary paper mill sludge | 800 | Mag-TiO2/KBC | Sulfadiazine | 0.1 | Sunlight | t1/2= 5.6 ± 0.4 h | [155] |
| Rice straw | 700 | Pure BC | Chloramphenicol | 0.5 | Visible light | 95.23% within 720 min | [151] |
| Caragana korshinskii | 650 | K-g-C3N4/BC | Chloramphenicol | 1.0 | Visible light | 82.42% within 180 min | [160] |
| Poplar woodchips | 300 | Ball-milled BC | Enrofloxacin | 0.2 | Visible light | Up to 82.5% within 150 min | [152] |
| Corn stalk | 500 | TiO2/KBC | Enrofloxacin | 2.5 | UV light | 85.25% within 60 min | [154] |
| Reed straw | 500 | Bi2WO6/Fe3O4/BC | Ofloxacin | 0.4 | Visible light | 83.1% within 60 min | [156] |
| Reed straw | 500 | Bi2WO6/Fe3O4/BC | Ciprofloxacin | 0.4 | Visible light | 91.5% within 60 min | [156] |
| Caragana korshinskii | 650 | K-g-C3N4/BC | Norfloxacin | 1.0 | Visible light | 83.62% within 180 min | [160] |
| husks and paper sludge | 500 | Zn-Co-LDH/BC | Gemifloxacin | 0.75 | UV light | 92.7% within 100 min | [161] |
| Palm seeds | 600 | MnFe-LDO/BC | Metronidazole | 0.5 | UV light | 98% within 60 min | [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Xue, Y.; Ma, Q.; Chen, Y.; Yuan, S.; Fan, J. Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal. Nanomaterials 2022, 12, 4045. https://doi.org/10.3390/nano12224045
Ma R, Xue Y, Ma Q, Chen Y, Yuan S, Fan J. Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal. Nanomaterials. 2022; 12(22):4045. https://doi.org/10.3390/nano12224045
Chicago/Turabian StyleMa, Raner, Yinghao Xue, Qian Ma, Yanyan Chen, Shiyin Yuan, and Jianwei Fan. 2022. "Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal" Nanomaterials 12, no. 22: 4045. https://doi.org/10.3390/nano12224045
APA StyleMa, R., Xue, Y., Ma, Q., Chen, Y., Yuan, S., & Fan, J. (2022). Recent Advances in Carbon-Based Materials for Adsorptive and Photocatalytic Antibiotic Removal. Nanomaterials, 12(22), 4045. https://doi.org/10.3390/nano12224045






