Thermodynamic Analysis of Group-III-Nitride Alloying with Yttrium by Hybrid Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Group-IIIa (Ga) Source-Zone Calculation
2.2. Group-IIIa (Al) Source-Zone Calculation
2.3. Group-IIIb Transition-Metal (Y) Source-Zone Calculation
2.4. Growth-Zone Calculation
3. Results
3.1. Input Precursor Control
3.1.1. Precursor from Ga Source Zone
3.1.2. Precursor from Y Source Zone
3.2. Alloy Composition Control
3.2.1. Temperature Effect on YGaN Deposition
3.2.2. Hydrogen Effect on YGaN Deposition
3.2.3. V/III Effect on YGaN Deposition
3.2.4. YAlN Deposition
4. Discussion
4.1. Effect of Temperature and Hydrogen on YGaN Deposition
4.2. Comparison between YGaN and YAlN Deposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, R.C.; Fuierer, P.A.; Newnham, R.E.; Shrout, T.R. Materials for High Temperature Acoustic and Vibration Sensors: A Review. Appl. Acoust. 1994, 41, 299–324. [Google Scholar] [CrossRef]
- Schneider, M.; Bittner, A.; Patocka, F.; Stöger-Pollach, M.; Halwax, E.; Schmid, U. Impact of the Surface-near Silicon Substrate Properties on the Microstructure of Sputter-Deposited AlN Thin Films. Appl. Phys. Lett. 2012, 101, 221602. [Google Scholar] [CrossRef]
- Deger, C.; Born, E.; Angerer, H.; Ambacher, O.; Stutzmann, M.; Hornsteiner, J.; Riha, E.; Fischerauer, G. Sound Velocity of AlxGa1-xN Thin Films Obtained by Surface Acoustic-Wave Measurements. Appl. Phys. Lett. 1998, 72, 2400–2402. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable Amplified Lead Zirconate Titanate Sensors with Enhanced Piezoelectric Response for Cutaneous Pressure Monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef]
- Akiyama, M.; Kamohara, T.; Kano, K.; Teshigahara, A.; Takeuchi, Y.; Kawahara, N. Enhancement of Piezoelectric Response in Scandium Aluminum Nitride Alloy Thin Films Prepared by Dual Reactive Cosputtering. Adv. Mater. 2009, 21, 593–596. [Google Scholar] [CrossRef]
- Mayrhofer, P.M.; Riedl, H.; Euchner, H.; Stöger-Pollach, M.; Mayrhofer, P.H.; Bittner, A.; Schmid, U. Microstructure and Piezoelectric Response of YxAl1-XN Thin Films. Acta Mater. 2015, 100, 81–89. [Google Scholar] [CrossRef]
- Wingqvist, G.; Tasnádi, F.; Zukauskaite, A.; Birch, J.; Arwin, H.; Hultman, L. Increased Electromechanical Coupling in W−Scx Al1−x N. Appl. Phys. Lett. 2010, 97, 95–98. [Google Scholar] [CrossRef]
- Tasnádi, F.; Alling, B.; Höglund, C.; Wingqvist, G.; Birch, J.; Hultman, L.; Abrikosov, I.A. Origin of the Anomalous Piezoelectric Response in Wurtzite ScxAl1−XN Alloys. Phys. Rev. Lett. 2010, 104, 137601. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.Y.; Holec, D.; Humphreys, C.J.; Moram, M.A. Elastic Constants and Critical Thicknesses of ScGaN and ScAlN. J. Appl. Phys. 2013, 114, 243516. [Google Scholar] [CrossRef]
- Fichtner, S.; Wolff, N.; Lofink, F.; Kienle, L.; Wagner, B. AlScN: A III-V Semiconductor Based Ferroelectric. J. Appl. Phys. 2019, 125, 114103. [Google Scholar] [CrossRef]
- Koleske, D.D.; Creighton, J.R.; Lee, S.R.; Crawford, M.H.; Thaler, G.; Cross, K.C.; Knapp, J.A. Issues Associated with the Metalorganic Chemical Vapor Deposition of ScGaN and YGaN Alloys; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2009. [Google Scholar] [CrossRef]
- Tholander, C.; Birch, J.; Tasnádi, F.; Hultman, L.; Palisaitis, J.; Persson, P.O.Å.; Jensen, J.; Sandström, P.; Alling, B.; Žukauskaitė, A. Ab Initio Calculations and Experimental Study of Piezoelectric YxIn1−XN Thin Films Deposited Using Reactive Magnetron Sputter Epitaxy. Acta Mater. 2016, 105, 199–206. [Google Scholar] [CrossRef]
- Fichtner, S. Development of High Performance Piezoelectric AlScN for Microelectromechanical Systems: Towards a Ferroelectric Wurtzite Structure; Books on Demand: Norderstedt, Germany, 2019; ISBN 9783750431423. [Google Scholar]
- Kim, N.-I.; Chen, J.; Wang, W.; Moradnia, M.; Pouladi, S.; Kwon, M.-K.; Kim, J.-Y.; Li, X.; Ryou, J.-H. Highly-Sensitive Skin-Attachable Eye-Movement Sensor Using Flexible Nonhazardous Piezoelectric Thin Film. Adv. Funct. Mater. 2021, 31, 2008242. [Google Scholar] [CrossRef]
- Akiyama, M.; Umeda, K.; Honda, A.; Nagase, T. Influence of Scandium Concentration on Power Generation Figure of Merit of Scandium Aluminum Nitride Thin Films. Appl. Phys. Lett. 2013, 102, 021915. [Google Scholar] [CrossRef]
- Chen, J.; Oh, S.K.; Nabulsi, N.; Johnson, H.; Wang, W.; Ryou, J.-H. Biocompatible and Sustainable Power Supply for Self-Powered Wearable and Implantable Electronics Using III-Nitride Thin-Film-Based Flexible Piezoelectric Generator. Nano Energy 2019, 57, 670–679. [Google Scholar] [CrossRef]
- Saada, S.; Lakel, S.; Almi, K. Optical, Electronic and Elastic Properties of ScAlN Alloys in WZ and ZB Phases: Prospective Material for Optoelectronics and Solar Cell Applications. Superlattices Microstruct. 2017, 109, 915–926. [Google Scholar] [CrossRef]
- Martin, F.; Muralt, P.; Dubois, M.-A.; Pezous, A. Thickness Dependence of the Properties of Highly c -Axis Textured AlN Thin Films. J. Vac. Sci. Technol. A 2004, 22, 361–365. [Google Scholar] [CrossRef]
- Zukauskaite, A.; Wingqvist, G.; Palisaitis, J.; Jensen, J.; Persson, P.O.Å.; Matloub, R.; Muralt, P.; Kim, Y.; Birch, J.; Hultman, L. Microstructure and Dielectric Properties of Piezoelectric Magnetron Sputtered W-ScxAl1−xN Thin Films. J. Appl. Phys. 2012, 111, 093527. [Google Scholar] [CrossRef]
- Akiyama, M.; Tabaru, T.; Nishikubo, K.; Teshigahara, A.; Kano, K. Preparation of Scandium Aluminum Nitride Thin Films by Using Scandium Aluminum Alloy Sputtering Target and Design of Experiments. J. Ceram. Soc. Jpn. 2010, 118, 1166–1169. [Google Scholar] [CrossRef]
- Leone, S.; Ligl, J.; Manz, C.; Kirste, L.; Fuchs, T.; Menner, H.; Prescher, M.; Wiegert, J.; Žukauskaitė, A.; Quay, R.; et al. Metal-Organic Chemical Vapor Deposition of Aluminum Scandium Nitride. Phys. Status Solidi—Rapid Res. Lett. 2020, 14, 1900535. [Google Scholar] [CrossRef]
- Casamento, J.; Xing, H.G.; Jena, D. Oxygen Incorporation in the Molecular Beam Epitaxy Growth of ScxGa1−xN and ScxAl1−xN. Phys. Status Solidi Basic Res. 2020, 257, 1900612. [Google Scholar] [CrossRef]
- Hardy, M.T.; Downey, B.P.; Nepal, N.; Storm, D.F.; Katzer, D.S.; Meyer, D.J. Epitaxial ScAlN Grown by Molecular Beam Epitaxy on GaN and SiC Substrates. Appl. Phys. Lett. 2017, 110, 162104. [Google Scholar] [CrossRef]
- Wang, P.; Laleyan, D.A.; Pandey, A.; Sun, Y.; Mi, Z. Molecular Beam Epitaxy and Characterization of Wurtzite ScxAl1−xN. Appl. Phys. Lett. 2020, 116, 151903. [Google Scholar] [CrossRef]
- Koukitu, A.; Kikuchi, J.; Kangawa, Y.; Kumagai, Y. Thermodynamic Analysis of AlGaN HVPE Growth. J. Cryst. Growth 2005, 281, 47–54. [Google Scholar] [CrossRef]
- Moradnia, M.; Pouladi, S.; Chen, J.; Kim, N.-I.; Aigbe, O.; Ryou, J.-H. Thermodynamic Analysis of Hybrid Chemical Vapor Deposition of Transition-Metal-Alloyed Group-III-Nitride ScAlN Piezoelectric Semiconductor Films. Cryst. Growth Des. 2021, 22, 2239–2247. [Google Scholar] [CrossRef]
- Assali, A.; Laidoudi, F.; Serhane, R.; Kanouni, F.; Mezilet, O. Highly Enhanced Electro-Acoustic Properties of YAlN/Sapphire Based Surface Acoustic Wave Devices for Next Generation of Microelectromechanical Systems. Mater. Today Commun. 2021, 26, 102067. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Thermochemical Tables, 4th ed.; Journal of Physical and Chemical Reference Data Monographs; Monograph 9 (Part I and Part II); American Institute of Physics: College Park, MD, USA, 1998. [Google Scholar]
- Ruzinov, L.P.; Guljanickij, B.S. Ravnovesnye Prevrasoenija Metallugiceskin Reaktseij; Moskow, Russia, 1975. [Google Scholar]
- Knacke, O.; Kubaschewski, O.H.K. Thermochemical Properties of Inorganic Substances, 2nd ed.; Springer: Berlin, Germany, 1991; pp. 1–1113. [Google Scholar]
- Stringfellow, G.B. Calculation of Ternary and Quaternary III–V Phase Diagrams. J. Cryst. Growth 1974, 27, 21–34. [Google Scholar] [CrossRef]
- Žukauskaitė, A.; Tholander, C.; Pališaitis, J.; Persson, P.O.Å.; Darakchieva, V.; Sedrine, N.B.; Tasnádi, F.; Alling, B.; Birch, J.; Hultman, L. YxAl1−xN Thin Films. J. Phys. D Appl. Phys. 2012, 45, 422001. [Google Scholar] [CrossRef]
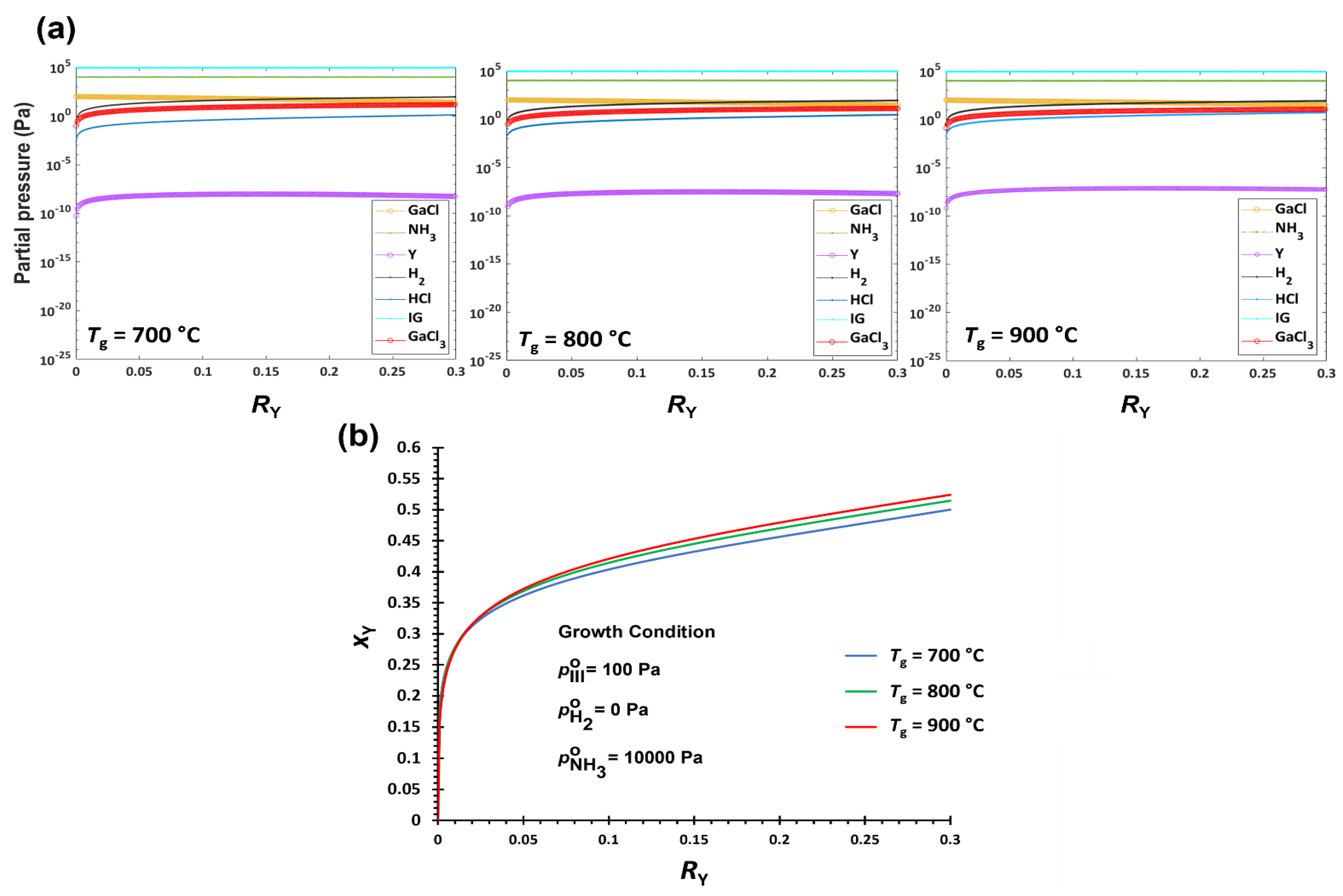
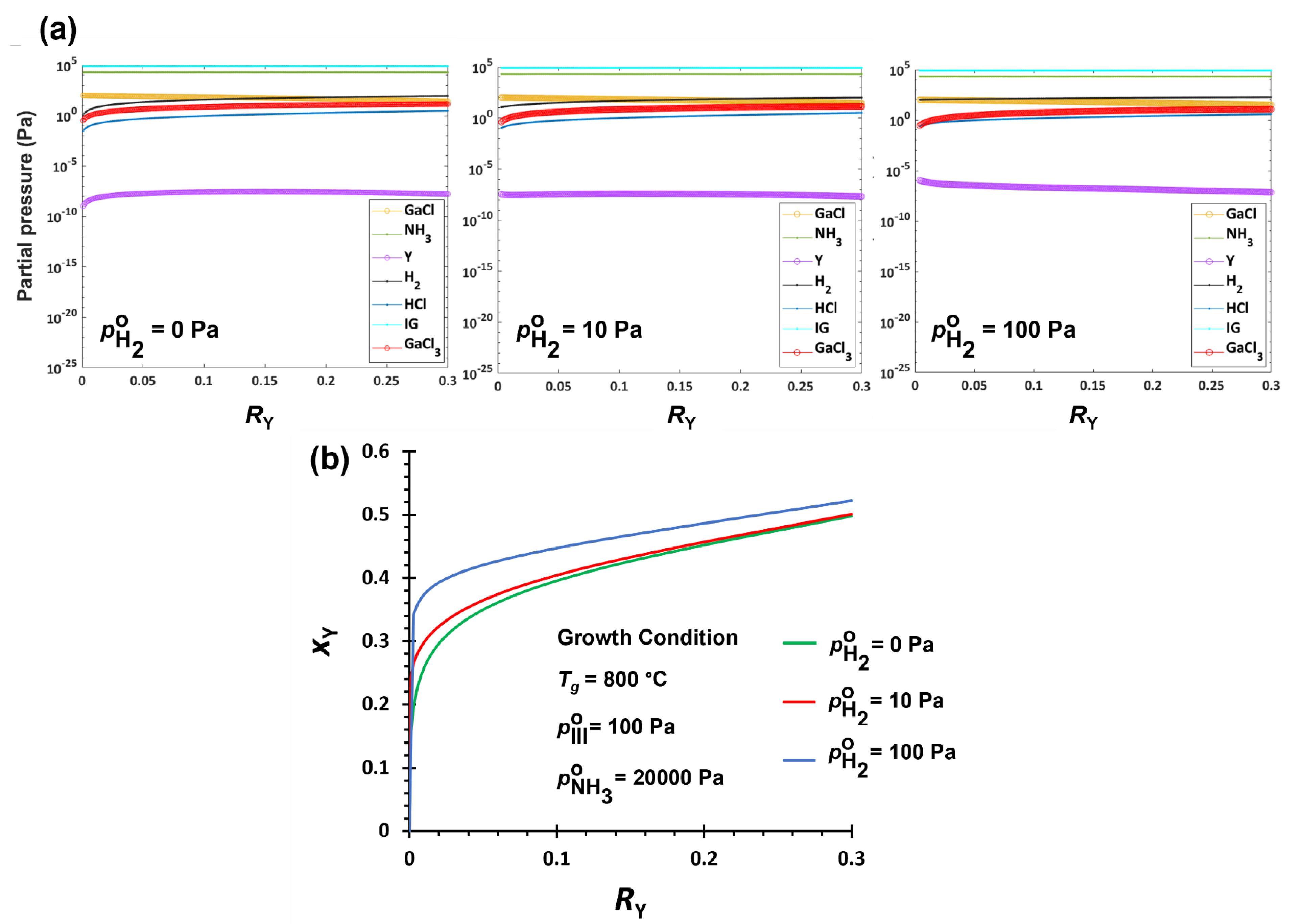
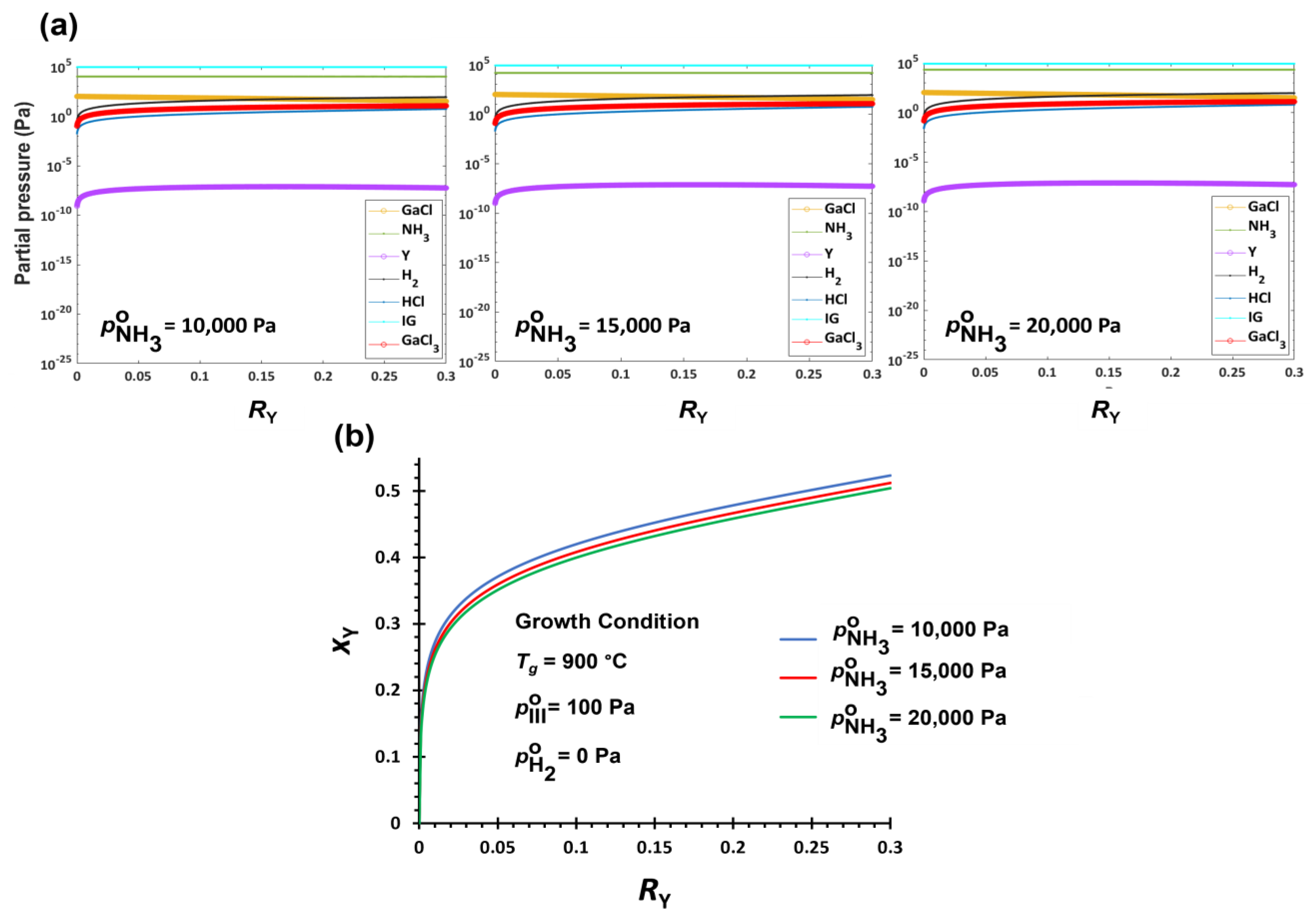
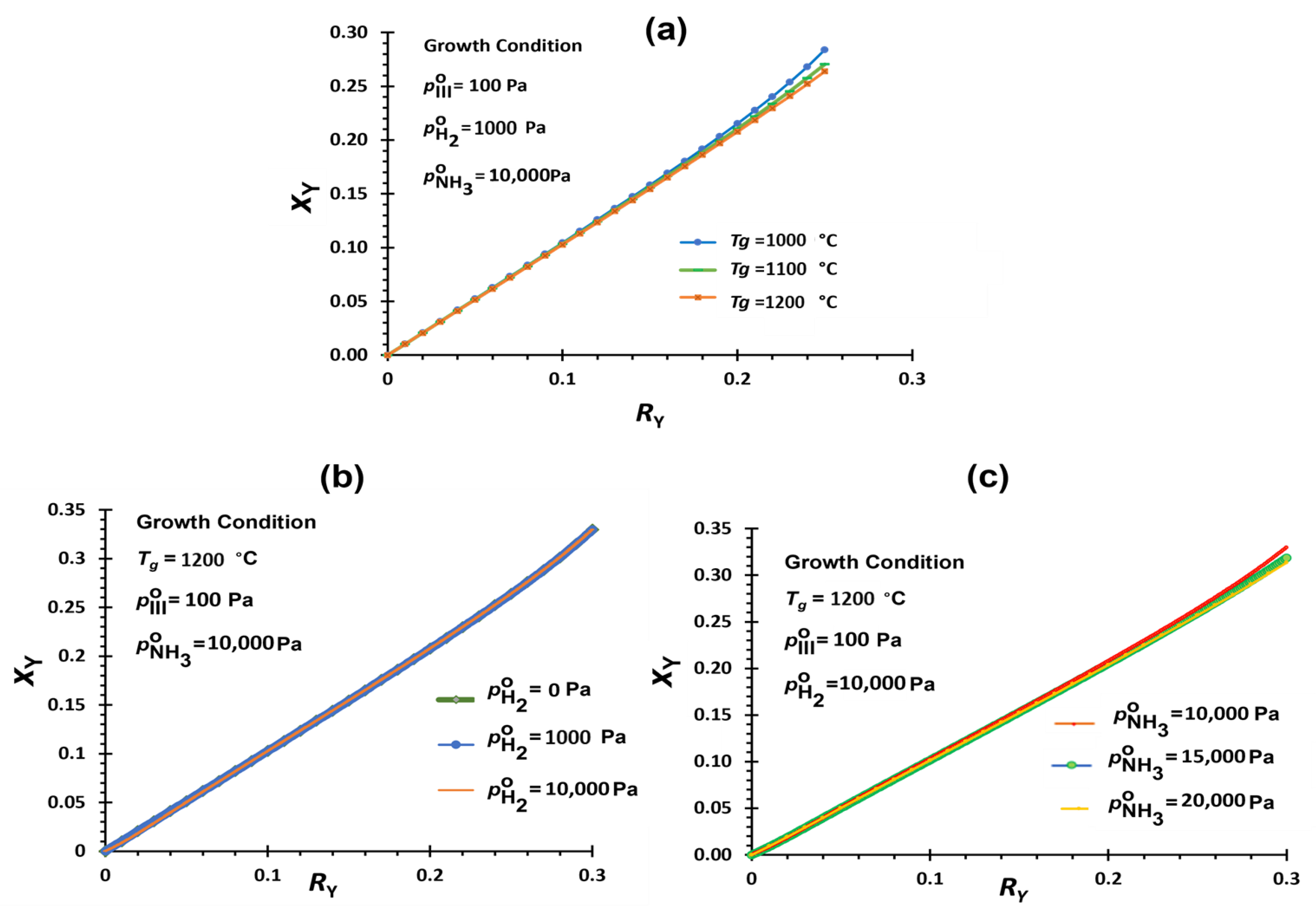
| Tg ↑ | H2 ↑ | V/III ↑ | |
|---|---|---|---|
| xY in YxGa1−xN | Increase | Increase | Decrease |
| xY in YxAl1−xN | Marginal decrease | Nearly same | Marginal decrease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradnia, M.; Pouladi, S.; Aqib, M.; Ryou, J.-H. Thermodynamic Analysis of Group-III-Nitride Alloying with Yttrium by Hybrid Chemical Vapor Deposition. Nanomaterials 2022, 12, 4053. https://doi.org/10.3390/nano12224053
Moradnia M, Pouladi S, Aqib M, Ryou J-H. Thermodynamic Analysis of Group-III-Nitride Alloying with Yttrium by Hybrid Chemical Vapor Deposition. Nanomaterials. 2022; 12(22):4053. https://doi.org/10.3390/nano12224053
Chicago/Turabian StyleMoradnia, Mina, Sara Pouladi, Muhammad Aqib, and Jae-Hyun Ryou. 2022. "Thermodynamic Analysis of Group-III-Nitride Alloying with Yttrium by Hybrid Chemical Vapor Deposition" Nanomaterials 12, no. 22: 4053. https://doi.org/10.3390/nano12224053
APA StyleMoradnia, M., Pouladi, S., Aqib, M., & Ryou, J.-H. (2022). Thermodynamic Analysis of Group-III-Nitride Alloying with Yttrium by Hybrid Chemical Vapor Deposition. Nanomaterials, 12(22), 4053. https://doi.org/10.3390/nano12224053







