High-Selective CO2 Capture in Amine-Decorated Al-MOFs
Abstract
:1. Introduction
2. Experimental Section
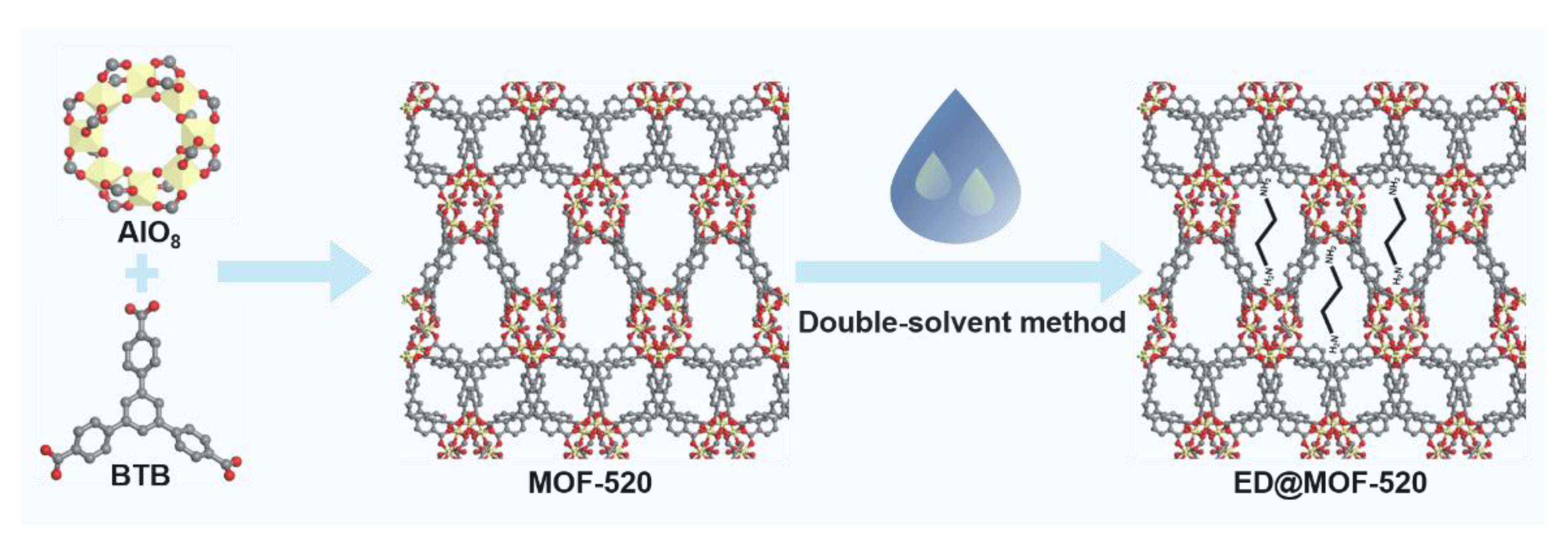
2.1. Synthesis of MOF-520
2.2. Synthesis of ED@MOF-520
2.3. Sample Characterization and Gas Adsorption Measurements
3. Results and Discussion
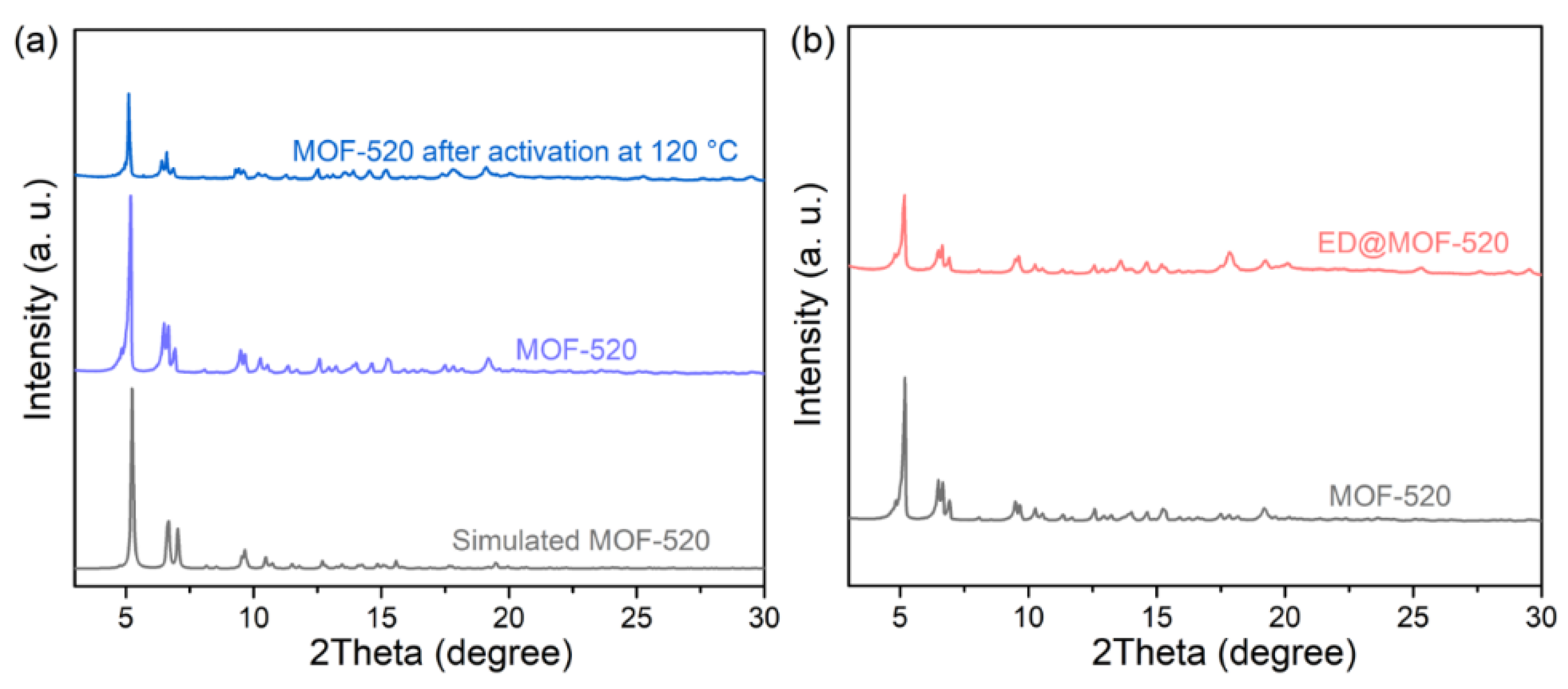
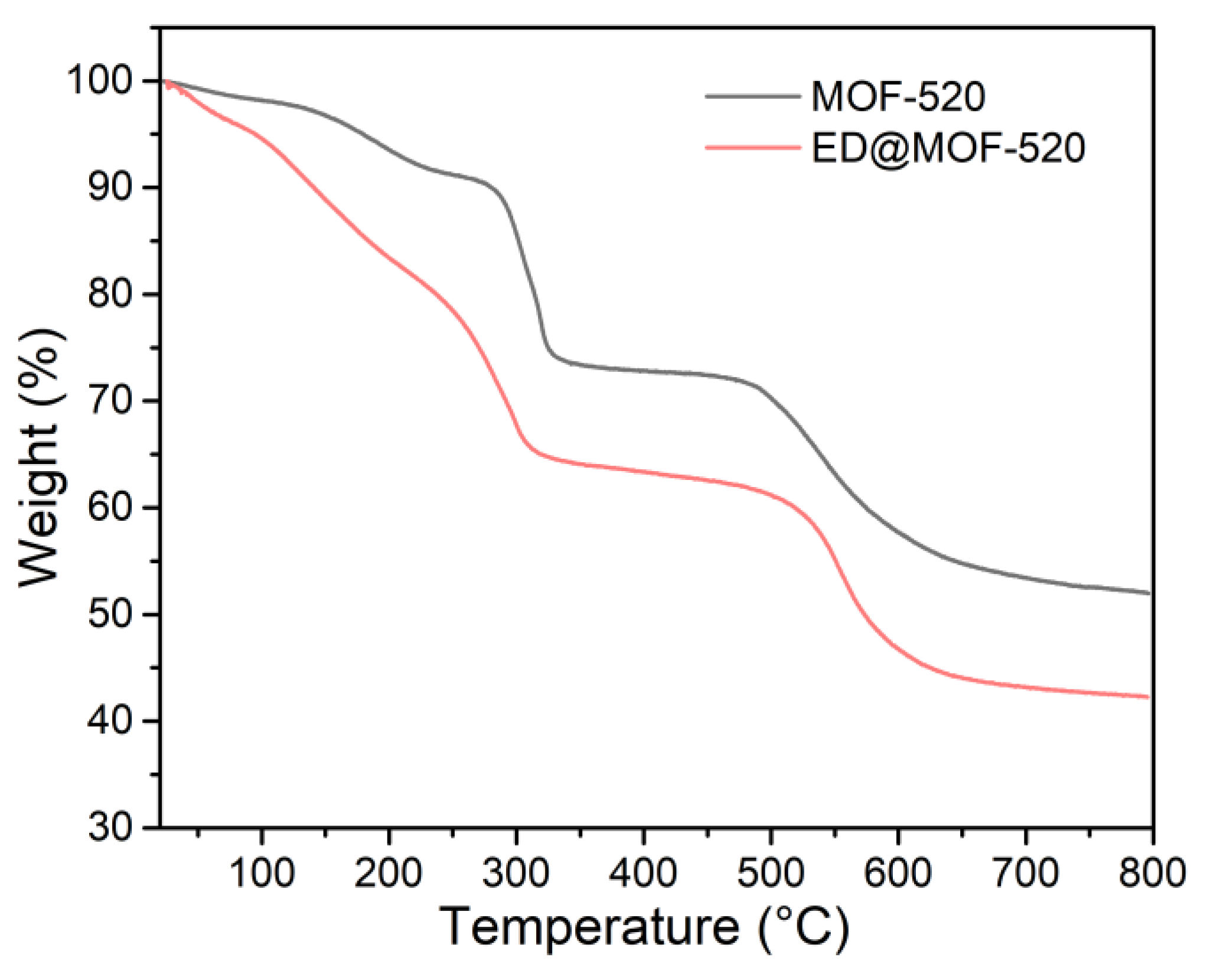
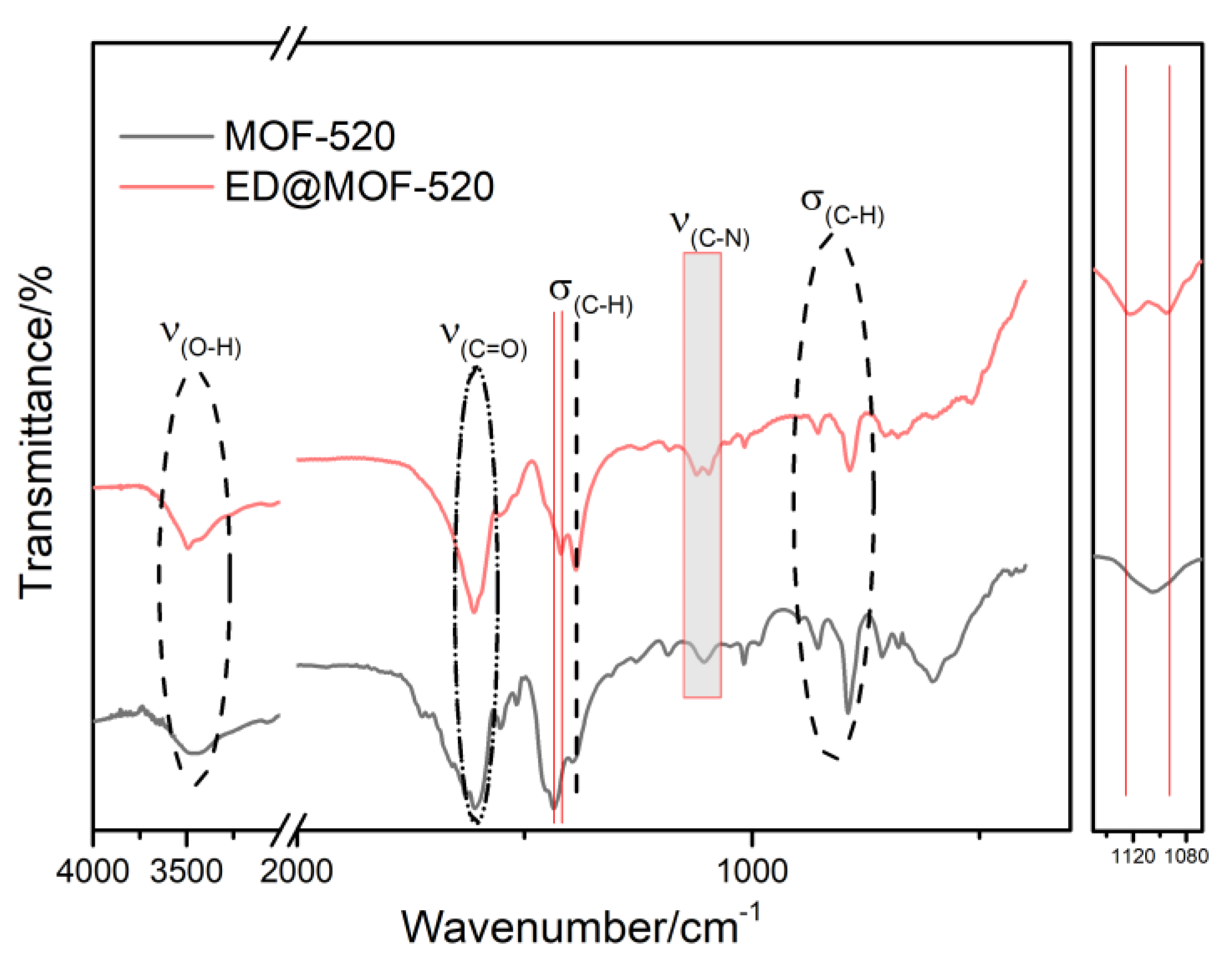
3.1. Structural Analysis of Sample
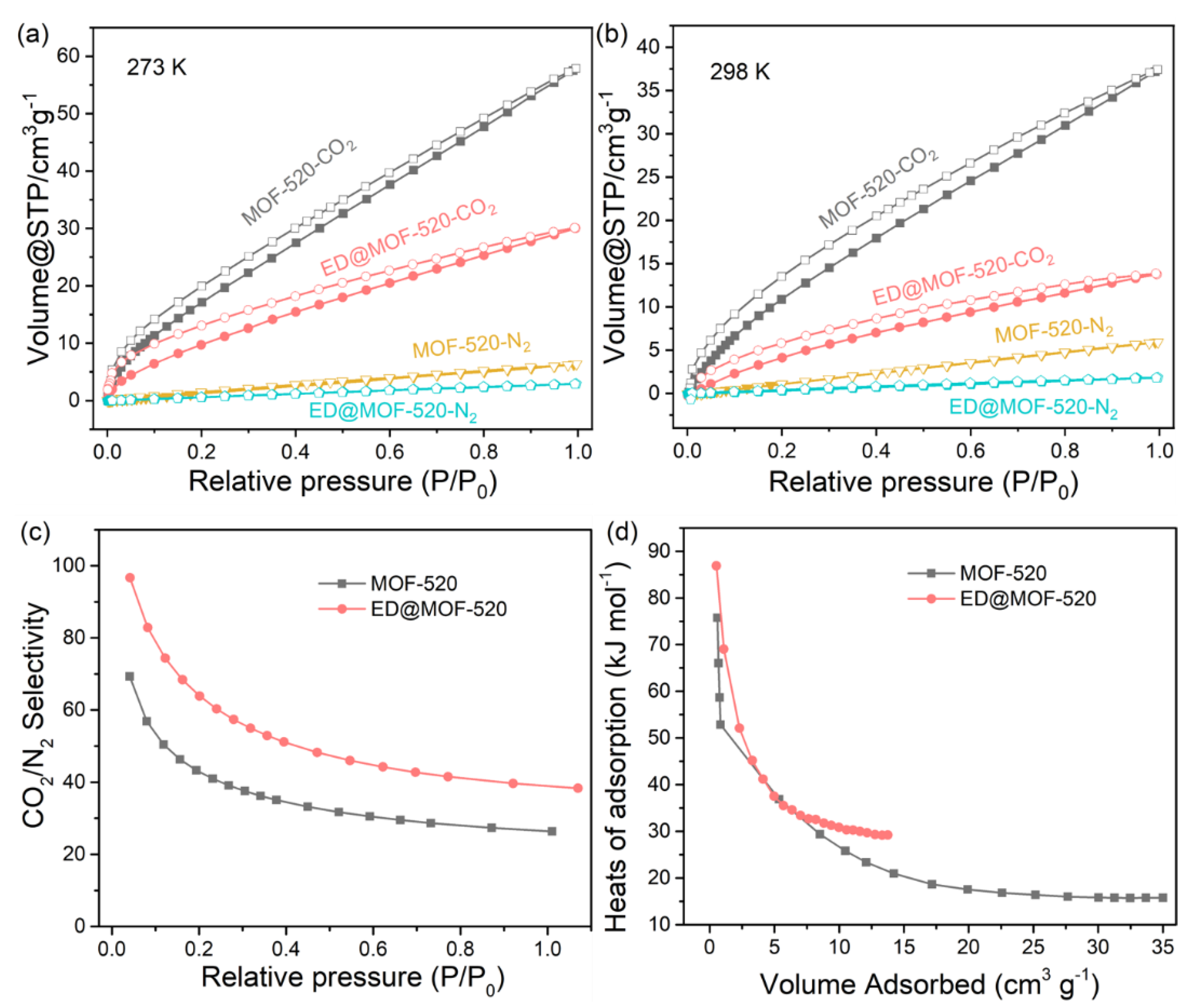
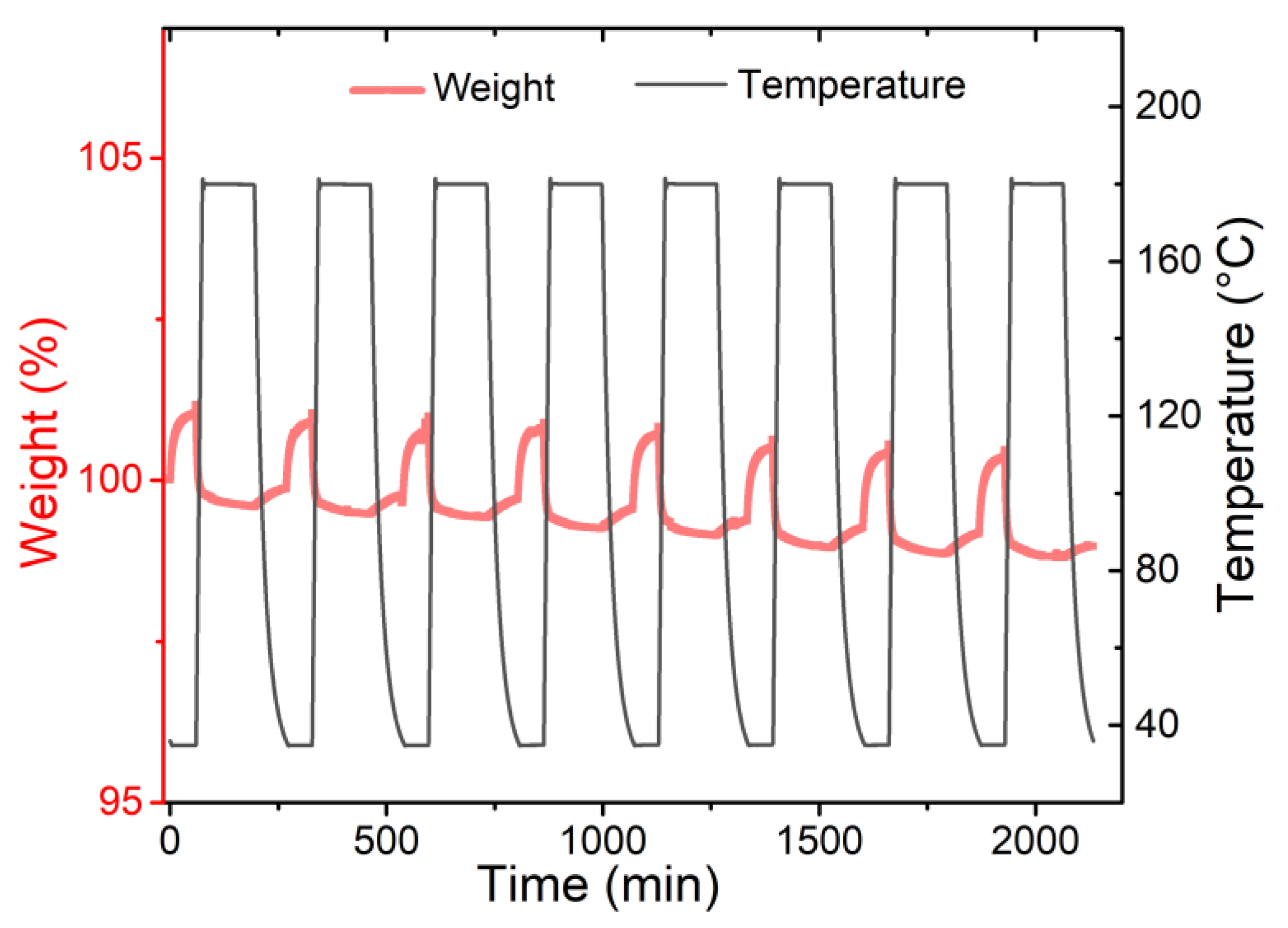
3.2. Gas Adsorption Performance of Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.W.; Zhang, X.S.; Han, W.; Gao, L.; Li, S. A power generation system with integrated supercritical water gasification of coal and CO2 capture. Energy. 2018, 142, 723–730. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Ma, D.F.; Zhu, C.Y.; Fu, T.T.; Ma, Y.G.; Yuan, X.G. Synergistic effect of functionalized ionic liquid and alkanolamines mixed solution on enhancing the mass transfer of CO2 absorption in microchannel. Chem. Eng. J. 2021, 417, 9. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.F.; Neves, L.A.; Chagas, R.; Ferreira, L.M.; Afonso, C.A.M.; Coelhoso, I.M.; Crespo, J.G.; Mota, J.P. Modelling CO2 absorption in aqueous solutions of cholinium lysinate ionic liquid. Chem. Eng. J. 2021, 421, 14. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, C.-G.; Wu, Y.-T.; Wang, Y.-T.; Li, A.-M.; Zhang, Z.-B. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem. Eng. J. 2010, 160, 691–697. [Google Scholar]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy. 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Kim, J.; Lu, H.; He, Y.; Zhao, Q.; Scofield, J.; Webley, P.A.; Qiao, G.G. Increasing both selectivity and permeability of mixed-matrix membranes: Sealing the external surface of porous MOF nanoparticles. J. Membr. Sci. 2017, 535, 350–356. [Google Scholar] [CrossRef]
- Anjum, M.W.; Vermoortele, F.; Khan, A.L.; Bueken, B.; De Vos, D.E.; Vankelecom, I.F.J. Modulated UiO-66-Based Mixed-Matrix Membranes for CO2 Separation. ACS Appl. Mater. Interfaces. 2015, 7, 25193–25201. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, W.; Yang, K.; Dai, Y.; Ruan, X.; Yan, X.; He, G. Amino-functional ZIF-8 nanocrystals by microemulsion based mixed linker strategy and the enhanced CO2/N2 separation. Sep. Purif. Technol. 2020, 236, 116209. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Etxeberria Benavides, M.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.J.; Xian, S.K.; Xia, Q.B.; Wang, H.H.; Li, Z.; Li, J. Enhancement of CO2 Adsorption and CO2/N2 Selectivity on ZIF-8 via Postsynthetic Modification. AlChE J. 2013, 59, 2195–2206. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Feng, D.; Chen, Y.-P.; Verdegaal, W.; Zhou, H.-C. Incorporation of Alkylamine into Metal-Organic Frameworks through a Bronsted Acid-Base Reaction for CO2 Capture. ChemSusChem 2016, 9, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Yang, K.; Yang, G.; Wu, J. Insights into the enhancement of CO2 adsorption on faujasite with a low Si/Al ratio: Understanding the formation sequence of adsorption complexes. Chem. Eng. J. 2021, 404, 127056. [Google Scholar] [CrossRef]
- Martinez, F.; Sanz, R.; Orcajo, G.; Briones, D.; Yangueez, V. Amino-impregnated MOF materials for CO2 capture at post-combustion conditions. Chem. Eng. Sci. 2016, 142, 55–61. [Google Scholar] [CrossRef]
- Frysali, M.G.; Klontzas, E.; Tylianakis, E.; Froudakis, G.E. Tuning the interaction strength and the adsorption of CO2 in metal organic frameworks by functionalization of the organic linkers. Microporous Mesoporous Mater. 2016, 227, 144–151. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.-D.; Yazaydin, A.O. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Ferey, G.; Serre, C.; Devic, T.; Maurin, G.; Jobic, H.; Llewellyn, P.L.; De Weireld, G.; Vimont, A.; Daturi, M.; Chang, J.-S. Why hybrid porous solids capture greenhouse gases? Chem. Soc. Rev. 2011, 40, 550–562. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Snurr, R.Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Y.; Li, P.; Zhao, Y.; Zou, R. Selective H2S/CO2 Separation by Metal-Organic Frameworks Based on Chemical-Physical Adsorption. J. Phys. Chem. C. 2017, 121, 13249–13255. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.M.; Dewil, R.; Baeyens, J.; Fan, X.F. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 19. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Wei, Y.S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef] [Green Version]
- Tranchemontagne, D.J.; Mendoza-Cortes, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Liu, J.; Huang, X.; Yu, X.; Sun, C.; Chen, G.; Zou, R. Experimental and theoretical investigation of a stable zinc-based metal-organic framework for CO2 removal from syngas. CrystEngComm 2015, 17, 8221–8225. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Zhou, Q. Strong Foam-like Composites from Highly Mesoporous Wood and Metal-Organic Frameworks for Efficient CO2 Capture. ACS Appl. Mater. Interfaces 2021, 13, 29949–29959. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zeng, Y.; Liang, Z.; Guo, W.; Zhi, C.; Wu, Y.; Zhong, R.; Qu, C.; Zou, R. Tuning Expanded Pores in Metal-Organic Frameworks for Selective Capture and Catalytic Conversion of Carbon Dioxide. ChemSusChem 2018, 11, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Xu, Z.; Bi, W.; Han, S.; Yu, X.; Zou, R. A charged metal-organic framework for CO2/CH4 and CO2/N2 separation. Inorg. Chim. Acta 2016, 443, 299–303. [Google Scholar] [CrossRef]
- Zou, R.; Li, P.-Z.; Zeng, Y.-F.; Liu, J.; Zhao, R.; Duan, H.; Luo, Z.; Wang, J.-G.; Zou, R.; Zhao, Y. Bimetallic Metal-Organic Frameworks: Probing the Lewis Acid Site for CO2 Conversion. Small 2016, 12, 2334–2343. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef]
- Siegelman, R.L.; McDonald, T.M.; Gonzalez, M.I.; Martell, J.D.; Milner, P.J.; Mason, J.A.; Berger, A.H.; Bhown, A.S.; Long, J.R. Controlling Cooperative CO2 Adsorption in Diamine-Appended Mg2(dobpdc) Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 10526–10538. [Google Scholar] [CrossRef]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 Binding in a Water-Stable, Triazolate-Bridged Metal−Organic Framework Functionalized with Ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef]
- Zhong, R.; Yu, X.; Meng, W.; Liu, J.; Zhi, C.; Zou, R. Amine-Grafted MIL-101(Cr) via Double-Solvent Incorporation for Synergistic Enhancement of CO2 Uptake and Selectivity. ACS Sustain. Chem. Eng. 2018, 6, 16493–16502. [Google Scholar] [CrossRef]
- Zhong, R.; Yu, X.; Meng, W.; Han, S.; Liu, J.; Ye, Y.; Sun, C.; Chen, G.; Zou, R. A solvent ‘squeezing’ strategy to graft ethylenediamine on Cu3(BTC)2 for highly efficient CO2/CO separation. Chem. Eng. Sci. 2018, 184, 85–92. [Google Scholar] [CrossRef]
- Wan, Y.; Miao, Y.; Qiu, T.; Kong, D.; Wu, Y.; Zhang, Q.; Shi, J.; Zhong, R.; Zou, R. Tailoring Amine-Functionalized Ti-MOFs via a Mixed Ligands Strategy for High-Efficiency CO2 Capture. Nanomaterials 2021, 11, 3348. [Google Scholar] [CrossRef] [PubMed]
- Flaig, R.W.; Osborn Popp, T.M.; Fracaroli, A.M.; Kapustin, E.A.; Kalmutzki, M.J.; Altamimi, R.M.; Fathieh, F.; Reimer, J.A.; Yaghi, O.M. The Chemistry of CO2 Capture in an Amine-Functionalized Metal–Organic Framework under Dry and Humid Conditions. J. Am. Chem. Soc. 2017, 139, 12125–12128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, J.A.; McDonald, T.M.; Bae, T.-H.; Bachman, J.E.; Sumida, K.; Dutton, J.J.; Kaye, S.S.; Long, J.R. Application of a High-Throughput Analyzer in Evaluating Solid Adsorbents for Post-Combustion Carbon Capture via Multicomponent Adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Gandara, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High methane storage capacity in aluminum metal-organic frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef]
- Naghdi, S.; Cherevan, A.; Giesriegl, A.; Guillet-Nicolas, R.; Biswas, S.; Gupta, T.; Wang, J.; Haunold, T.; Bayer, B.C.; Rupprechter, G.; et al. Selective ligand removal to improve accessibility of active sites in hierarchical MOFs for heterogeneous photocatalysis. Nat. Commun. 2022, 13, 282. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Fang, H.; Chen, L.; Su, C.-Y. Metal–Organic Gel Material Based on UiO-66-NH2 Nanoparticles for Improved Adsorption and Conversion of Carbon Dioxide. Chem. Asian J. 2016, 11, 2278–2283. [Google Scholar] [CrossRef]
- Han, G.; Qian, Q.; Mizrahi Rodriguez, K.; Smith, Z.P. Hydrothermal Synthesis of Sub-20 nm Amine-Functionalized MIL-101(Cr) Nanoparticles with High Surface Area and Enhanced CO2 Uptake. Ind. Eng. Chem. Res. 2020, 59, 7888–7900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zou, X.; Gao, X.; Fan, S.; Sun, F.; Ren, H.; Zhu, G. Hydrogen Selective NH2-MIL-53(Al) MOF Membranes with High Permeability. Adv. Funct. Mater. 2012, 22, 3583–3590. [Google Scholar] [CrossRef]
- Kong, L.; Zou, R.; Bi, W.; Zhong, R.; Mu, W.; Liu, J.; Han, R.P.S.; Zou, R. Selective adsorption of CO2/CH4 and CO2/N2 within a charged metal–organic framework. J. Mater. Chem. A 2014, 2, 17771–17778. [Google Scholar] [CrossRef]
- Bae, Y.S.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification. J. Mater. Chem. 2009, 19, 2131–2134. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]

| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | The Elements Content of C, H, and N (wt%) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| MOF-520 | 2699.46 | 1.08 | 56.12 | 3.34 | 0 |
| ED@MOF-520 | 787.44 | 0.39 | 45.53 | 4.12 | 3.39 |
| Samples | BET Area (m2 g−1) | CO2 Capture Capacity @ Testing Conditions | CO2/N2 (CO) Selectivity | Qst (kJ mol−1) | Ref |
|---|---|---|---|---|---|
| MOF-520 | 2699.46 | 2.58/1.67 mmol g−1 @ 273/298 K &1 bar | 27 | This work | |
| ED@MOF-520 | 787.44 | 1.55/0.61 mmol g−1 @ 273/298 K &1 bar | 50 | 29 | This work |
| ZrBDC-NH2-1:1-0.2 | 930–1040 | 4.20/2.68 mmol g−1 @ 273/298 K &1 bar | 71.6 | 26.9 | [47] |
| MIP-207 | 563 | 3.28/2.03 mmol g−1@ 273/298 K &1 bar | 59 | – | [41] |
| MIP-207-NH2 -25% | 735 | 3.96/2.91 mmol g−1@ 273/298 K &1 bar | 77 | 30–35 | [41] |
| ED@Cu3(BTC)2-1 | 444 | 4.28/2.15 mmol g−1 @ 273/298 K &1 bar | 21.5 | 39 | [40] |
| ED@Cu3 (BTC)2-2 | 163 | 1.03/0.54 mmol g−1 @ 273/298 K &1 bar | 2.68 | – | [40] |
| ED@MIL-101 | 1584.6 | 3.93/1.93 mmol g−1 @ 273/298 K &1 bar | 17.3 | – | [39] |
| MIL-101(Cr)-NH2 | ~2800 | 3.4 mmol g−1 @ 289 K and 1 bar | 26.5 | 54.6 | [48] |
| NH2-MIL-53 | – | ~1.5/~1.1 mmol g−1@ 273/298 K &1 bar | – | ~56 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Miao, Y.; Zhong, R.; Zou, R. High-Selective CO2 Capture in Amine-Decorated Al-MOFs. Nanomaterials 2022, 12, 4056. https://doi.org/10.3390/nano12224056
Wan Y, Miao Y, Zhong R, Zou R. High-Selective CO2 Capture in Amine-Decorated Al-MOFs. Nanomaterials. 2022; 12(22):4056. https://doi.org/10.3390/nano12224056
Chicago/Turabian StyleWan, Yinji, Yefan Miao, Ruiqin Zhong, and Ruqiang Zou. 2022. "High-Selective CO2 Capture in Amine-Decorated Al-MOFs" Nanomaterials 12, no. 22: 4056. https://doi.org/10.3390/nano12224056





