Eucalyptus-Mediated Synthesized Silver Nanoparticles-Coated Urinary Catheter Inhibits Microbial Migration and Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Process
2.3. Characterization of AgNPs-Coated Urinary Catheter
2.4. Preparation of Pooled Human Urine
2.5. Agar Inhibition Assay
2.6. Antimicrobial Adhesion
2.7. Antibiofilm Formation
2.8. A Catheter Bridge Model
2.9. A Modified in Vitro Bladder Model
2.10. Cytotoxicity Test
2.11. Ethical Approval
3. Results and Discussion
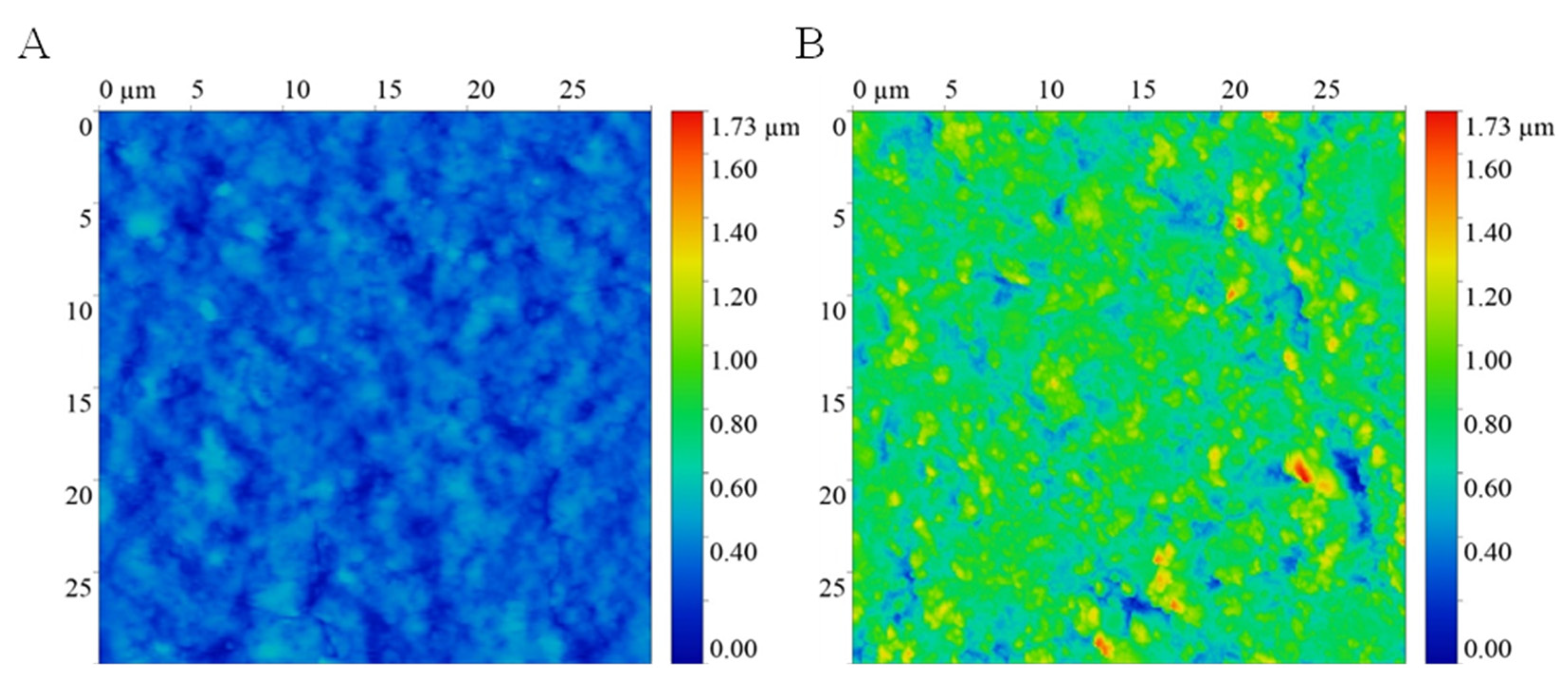
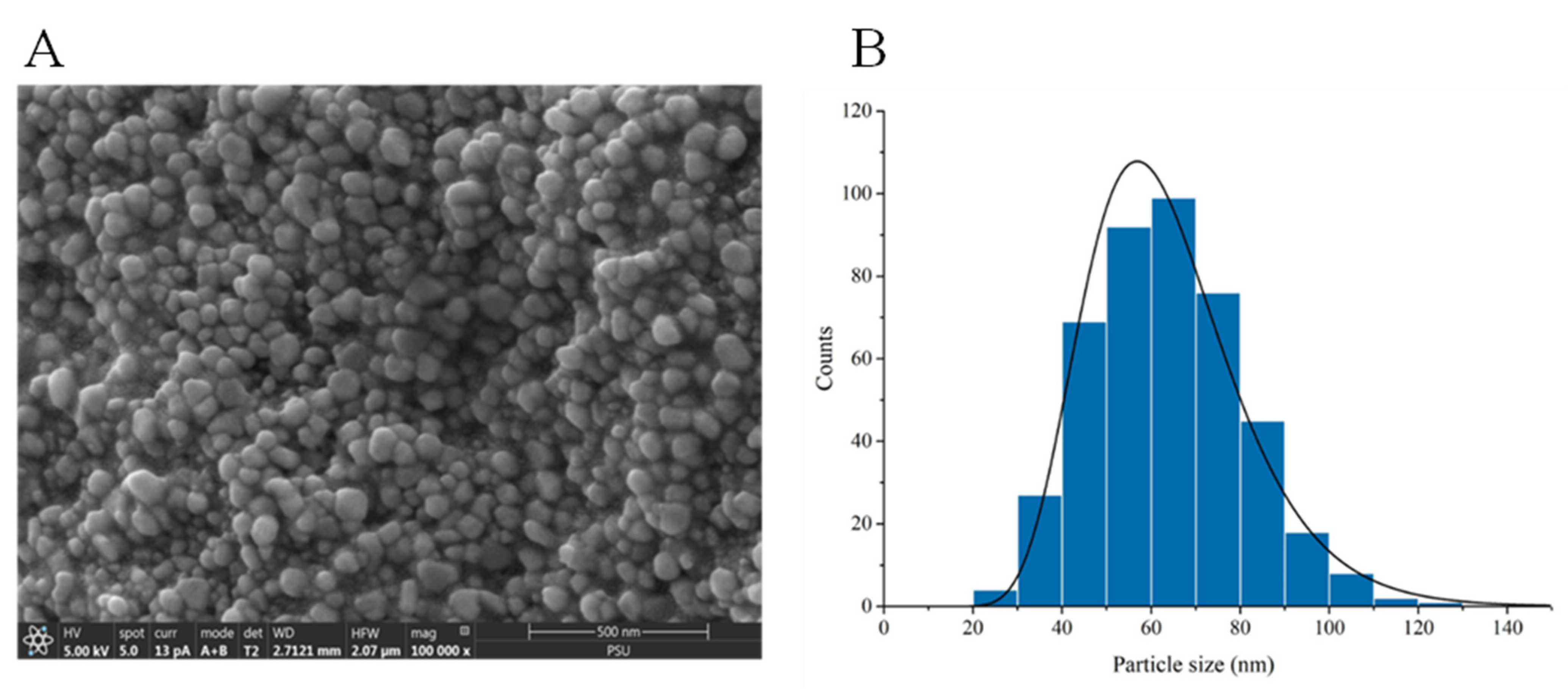
3.1. Surface Characterization
3.2. Antibacterial Activity of the AgNPs-Coated Urinary Catheter
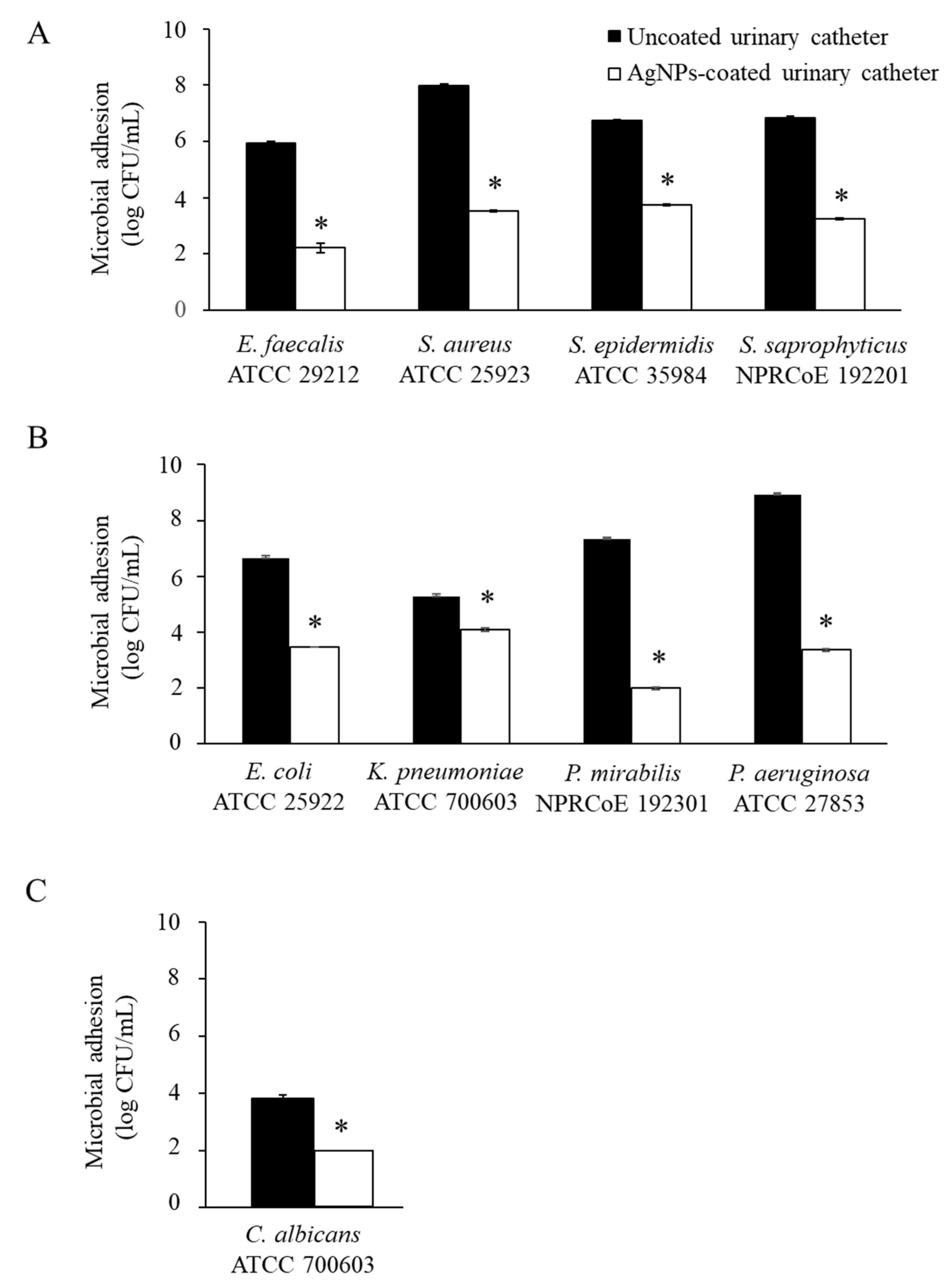
3.3. Antimicrobial Adhesion
3.4. Antibiofilm Formation
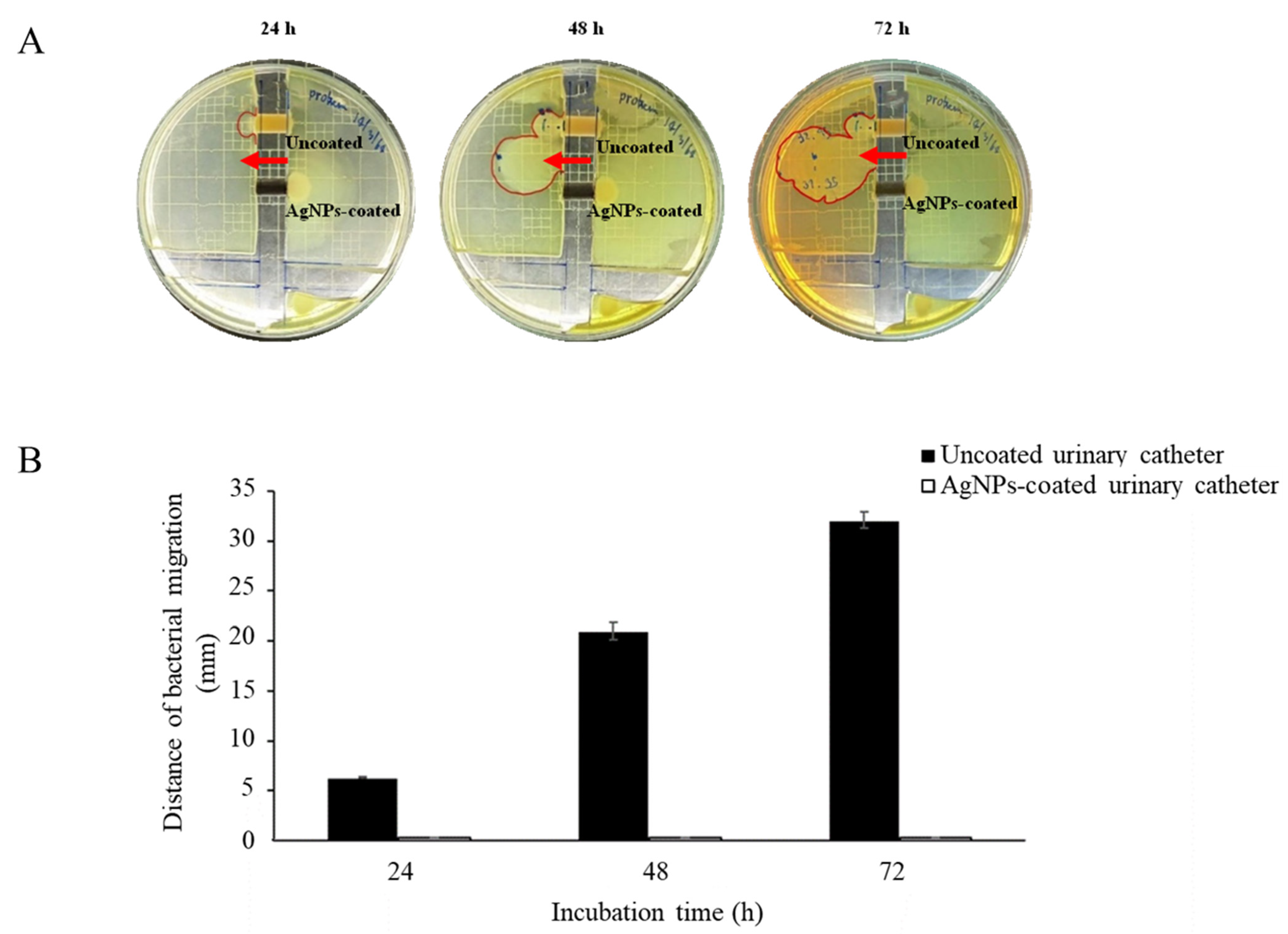
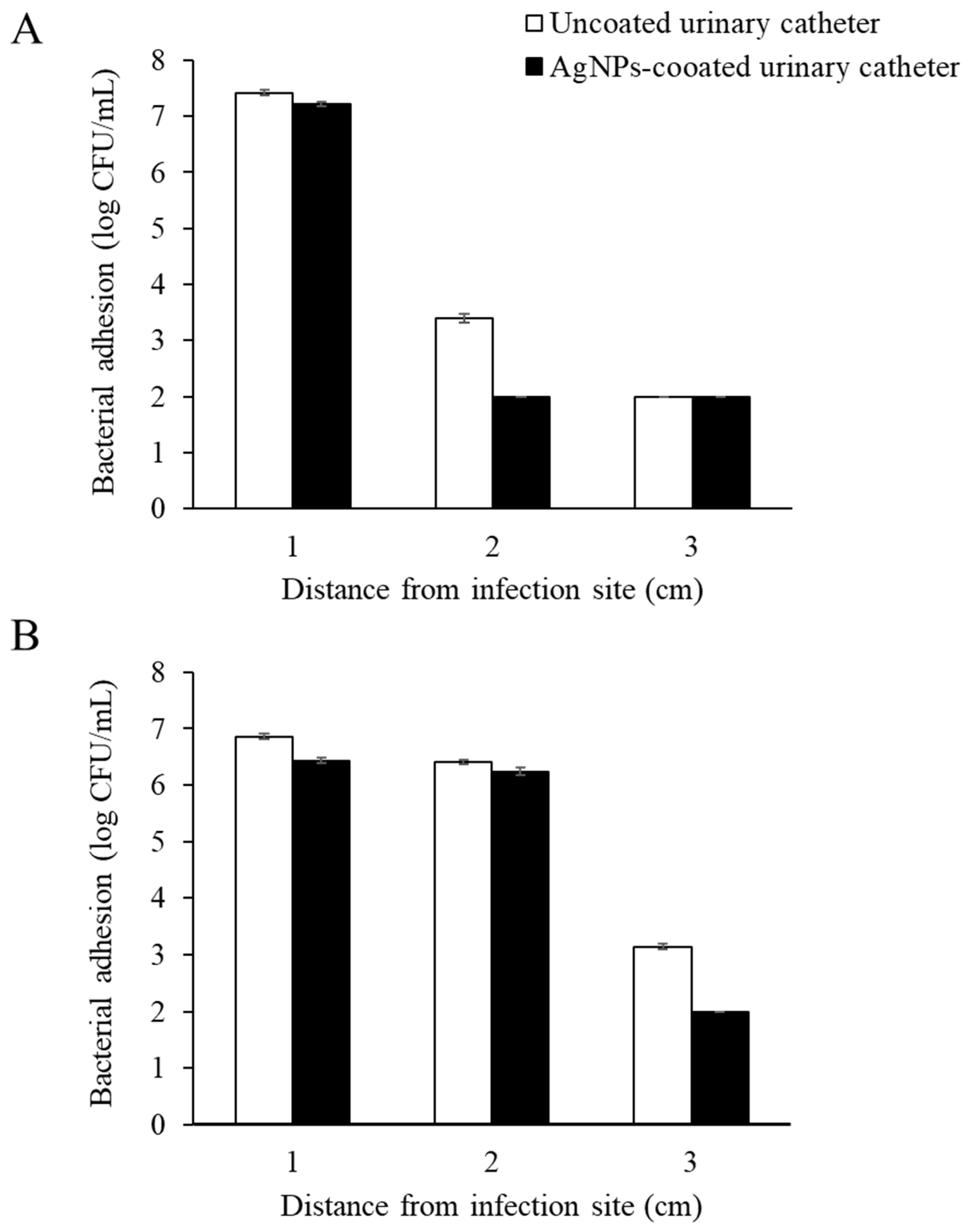
3.5. Antimicrobial Migration
3.6. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, C.V.; Umscheid, C.A.; Agarwal, R.K.; Kuntz, G.; Pegues, D.A. Guideline for prevention of catheter-associated urinary tract infections. Infect. Control Hosp. Epidemiol. 2009, 31, 319–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, D.; Li, X.; Liu, P.; Luo, M.; Chen, S.; Su, K.; Zhang, Z.; He, Q.; Qiu, J.; Li, Y. Epidemiology of pathogens and antimicrobial resistance of catheter-associated urinary tract infections in intensivecare units: A systematic review and meta-analysis. Am. J. Infect. Control 2018, 46, e81–e90. [Google Scholar] [CrossRef] [PubMed]
- Paosen, S.; Saising, J.; Septama, A.W.; Voravuthikunchai, S.P. Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater. Lett. 2017, 209, 201–206. [Google Scholar] [CrossRef]
- Wunnoo, S.; Paosen, S.; Lethongkam, S.; Sukkurd, R.; Waen-Ngoen, T.; Nuidate, T.; Phengmak, M.; Voravuthikunchai, S.P. Biologically rapid synthesized silver nanoparticles from aqueous Eucalyptus camaldulensis leaf extract: Effects on hyphal growth, hydrolytic enzymes, and biofilm formation in Candida albicans. Biotechnol. Bioeng. 2021, 118, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
- Chutrakulwong, F.; Thamaphat, K.; Tantipaibulvut, S.; Limsuwan, P. In situ deposition of green silver nanoparticles on urinary catheters under photo-irradiation for antibacterial properties. Processes 2020, 8, 1630. [Google Scholar] [CrossRef]
- Lethongkam, S.; Daengngam, C.; Tansakul, C.; Siri, R.; Chumpraman, A.; Phengmak, M.; Voravuthikunchai, S.P. Prolonged inhibitory effects against planktonic growth, adherence, and biofilm formation of pathogens causing ventilator-associated pneumonia using a novel polyamide/silver nanoparticle composite-coated endotracheal tube. Biofouling 2020, 36, 292–307. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef] [Green Version]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Voravuthikunchai, S.P. Antibacterial functionalization of nylon monofilament surgical sutures through in situ deposition of biogenic silver nanoparticles. Surf. Coat. Technol. 2021, 413, 127090. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E.; Etemadifar, A. Fabricating multifunctional silver nanoparticles-coated cotton fabric. Arab. J. Chem. 2017, 10, S2355–S2362. [Google Scholar] [CrossRef] [Green Version]
- Daus, M.; Wunnoo, S.; Voravuthikunchai, S.P.; Saithong, S.; Poldorn, P.; Jungsuttiwong, S.; Chomlamay, N.; Yangok, K.; Watanapokasin, R.; Chakthong, S. Phloroglucinol–meroterpenoids from the leaves of Eucalyptus camaldulensis Dehnh. Phytochemistry 2022, 200, 113179. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Superhydrophobic coatings for urinary catheters to delay bacterial biofilm formation and catheter-associated urinary tract infection. ACS Appl. Bio Mater. 2019, 3, 282–291. [Google Scholar] [CrossRef]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goda, R.M.; El-Baz, A.M.; Khalaf, E.M.; Alharbi, N.K.; Elkhooly, T.A.; Shohayeb, M.M. Combating bacterial biofilm formation in catheter by green silver nanoparticle. Antibiotics 2022, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Elkhooly, T.A.; Elsherbiny, S.M.; Reicha, F.M.; Shokeir, A.A. Facile coating of urinary catheter with bio-inspired antibacterial coating. Heliyon 2019, 5, e02986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, M.; Xu, X.; Gao, P.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.; Sun, H. Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 3331. [Google Scholar] [CrossRef]
- Jiang, H.S.; Zhang, Y.; Lu, Z.W.; Lebrun, R.; Gontero, B.; Li, W. Interaction between silver nanoparticles and two dehydrogenases: Role of thiol groups. Small 2019, 15, 1900860. [Google Scholar] [CrossRef]
- Säemann, M.D.; Weichhart, T.; Hörl, W.H.; Zlabinger, G.J. Tamm-Horsfall protein: A multilayered defence molecule against urinary tract infection. Eur. J. Clin. Investig. 2005, 35, 227–235. [Google Scholar] [CrossRef]
- Shields-Cutler, R.R.; Crowley, J.R.; Hung, C.S.; Stapleton, A.E.; Aldrich, C.C.; Marschall, J.; Henderson, J.P. Human urinary composition controls antibacterial activity of siderocalin. J. Biol. Chem. 2015, 290, 15949–15960. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.C.; Tseng, C.C.; Wu, A.B.; Lin, W.H.; Teng, C.H.; Yan, J.J.; Wu, J.J. Bacterial characteristics and glycemic control in diabetic patients with Escherichia coli urinary tract infection. J. Microbiol. Immunol. Infect. 2013, 46, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Chambers, S.T.; Peddie, B.A.; Randall, K.; Lever, M. Inhibitors of bacterial growth in urine: What is the role of betaines? Int. J. Antimicrob. Agents 1999, 11, 293–296. [Google Scholar] [CrossRef]
- Aubron, C.; Huet, O.; Ricome, S.; Borderie, D.; Pussard, E.; Leblanc, P.E.; Bouvet, O.; Vicaut, E.; Denamur, E.; Duranteau, J. Changes in urine composition after trauma facilitate bacterial growth. BMC Infect. Dis. 2012, 12, 330. [Google Scholar] [CrossRef] [Green Version]
- Watts, R.E.; Totsika, M.; Challinor, V.L.; Mabbett, A.N.; Ulett, G.C.; De Voss, J.J.; Schembri, M.A. Contribution of siderophore systems to growth and urinary tract colonization of asymptomatic bacteriuria Escherichia coli. Infect. Immun. 2012, 80, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiron, A.; Posteraro, B.; Carrière, M.; Remy, L.; Delporte, C.; La Sorda, M.; Sanguinetti, M.; Juillard, V.; Borezée-Durant, E. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol. Microbiol. 2010, 77, 1246–1260. [Google Scholar] [CrossRef] [PubMed]
- La Bella, A.A.; Andersen, M.J.; Molesan, A.; Stuckey, P.V.; Santiago-Tirado, F.H.; Flores-Mireles, A.L. Catheterized-bladder environment induces hyphal Candida albicans formation, promoting fungal colonization and persistence. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wazait, H.D.; Patel, H.R.; Veer, V.; Kelsey, M.; Van Der Meulen, J.H.; Miller, R.A.; Emberton, M. Catheter-associated urinary tract infections: Prevalence of uropathogens and pattern of antimicrobial resistance in a UK hospital (1996–2001). BJU Int. 2003, 91, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Sabbuba, N.; Hughes, G.; Stickler, D.J. The migration of Proteus mirabilis and other urinary tract pathogens over foley catheters. BJU Int. 2002, 89, 55–60. [Google Scholar] [CrossRef]
- Nickel, J.C.; Costerton, J.W.; McLean, R.J.; Olson, M. Bacterial biofilms: Influence on the pathogenesis, diagnosis and treatment of urinary tract infections. J. Antimicrob. Chemother. 1994, 33 (Suppl. A), 31–41. [Google Scholar] [CrossRef]



| Microorganisms | Inhibition Zone (mm) | |
|---|---|---|
| Uncoated Urinary Catheters | AgNPs-Coated Urinary Catheters | |
| Gram-positive bacteria | ||
| Enterococcus faecalis ATCC 29212 | - a | 10.7 ± 0.3 b |
| Staphylococcus aureus ATCC 25923 | - | 10.6 ± 0.1 |
| Staphylococcus epidermidis ATCC 35984 | - | 9.0 ± 0.4 |
| Staphylococcus saprophyticus NPRCoE 192201 | - | 9.7 ± 0.7 |
| Gram-negative bacteria | ||
| Escherichia coli ATCC 25922 | - | 8.0 ± 0.6 |
| Klebsiella pneumoniae ATCC 700603 | - | 12.3 ± 0.9 |
| Proteus mirabilis NPRCoE 192201 | - | 7.5 ± 0.4 |
| Pseudomonas aeruginosa ATCC 27853 | - | 13.3 ± 0.2 |
| Fungi | ||
| Candida albicans ATCC 90028 | - | 8.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lethongkam, S.; Paosen, S.; Bilhman, S.; Dumjun, K.; Wunnoo, S.; Choojit, S.; Siri, R.; Daengngam, C.; Voravuthikunchai, S.P.; Bejrananda, T. Eucalyptus-Mediated Synthesized Silver Nanoparticles-Coated Urinary Catheter Inhibits Microbial Migration and Biofilm Formation. Nanomaterials 2022, 12, 4059. https://doi.org/10.3390/nano12224059
Lethongkam S, Paosen S, Bilhman S, Dumjun K, Wunnoo S, Choojit S, Siri R, Daengngam C, Voravuthikunchai SP, Bejrananda T. Eucalyptus-Mediated Synthesized Silver Nanoparticles-Coated Urinary Catheter Inhibits Microbial Migration and Biofilm Formation. Nanomaterials. 2022; 12(22):4059. https://doi.org/10.3390/nano12224059
Chicago/Turabian StyleLethongkam, Sakkarin, Supakit Paosen, Siwaporn Bilhman, Krittima Dumjun, Suttiwan Wunnoo, Suntree Choojit, Ratchaneewan Siri, Chalongrat Daengngam, Supayang P. Voravuthikunchai, and Tanan Bejrananda. 2022. "Eucalyptus-Mediated Synthesized Silver Nanoparticles-Coated Urinary Catheter Inhibits Microbial Migration and Biofilm Formation" Nanomaterials 12, no. 22: 4059. https://doi.org/10.3390/nano12224059
APA StyleLethongkam, S., Paosen, S., Bilhman, S., Dumjun, K., Wunnoo, S., Choojit, S., Siri, R., Daengngam, C., Voravuthikunchai, S. P., & Bejrananda, T. (2022). Eucalyptus-Mediated Synthesized Silver Nanoparticles-Coated Urinary Catheter Inhibits Microbial Migration and Biofilm Formation. Nanomaterials, 12(22), 4059. https://doi.org/10.3390/nano12224059









