On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive
Abstract
:1. Introduction
2. Calcium Phosphates
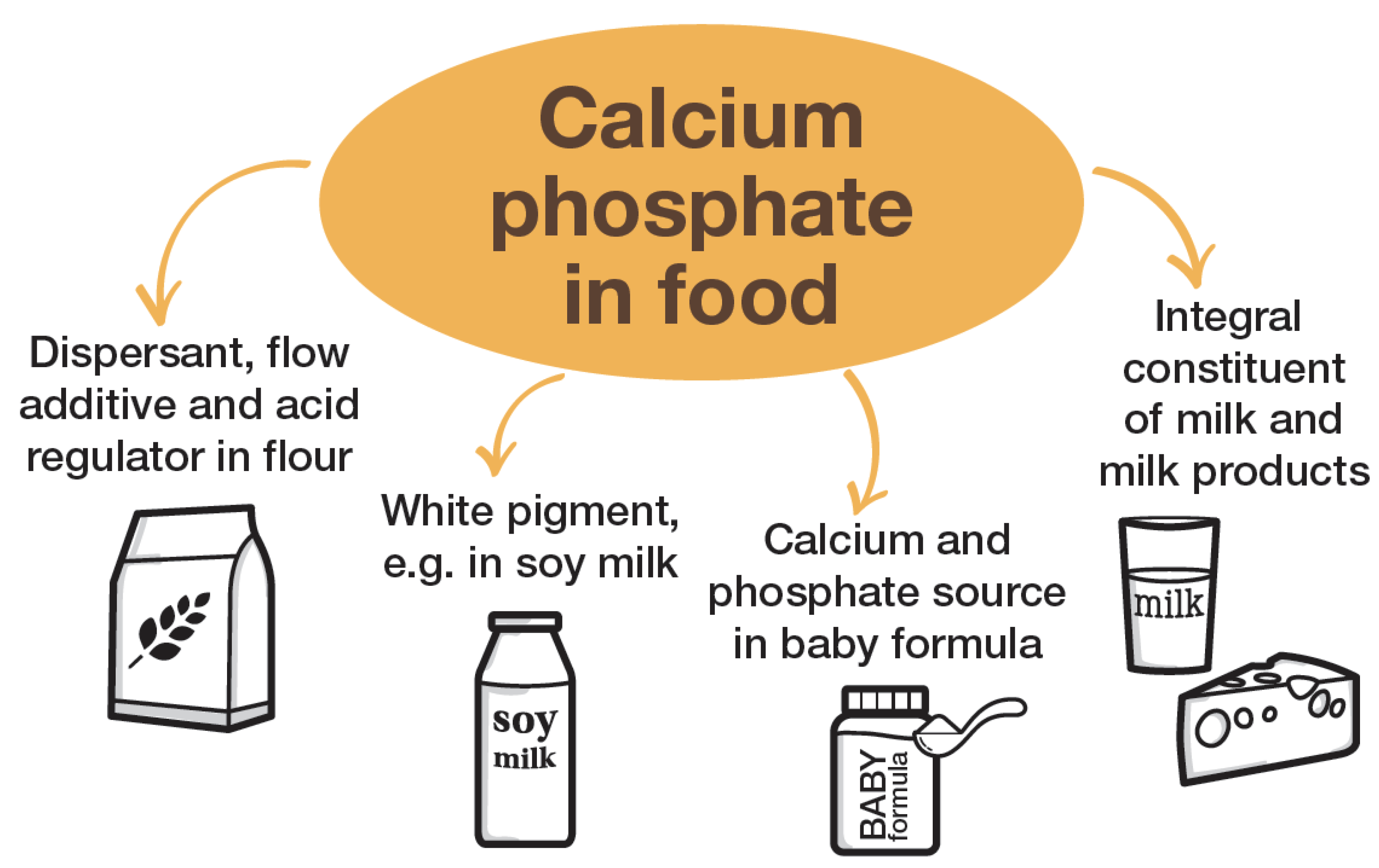
3. Application of Calcium Phosphate as Food Additive
- to improve the consistency and processability of flour;
- as an acid regulator;
- as a white pigment; and
- as a nutritional source of calcium and phosphate.
4. Calcium Phosphate Nanoparticles in Milk
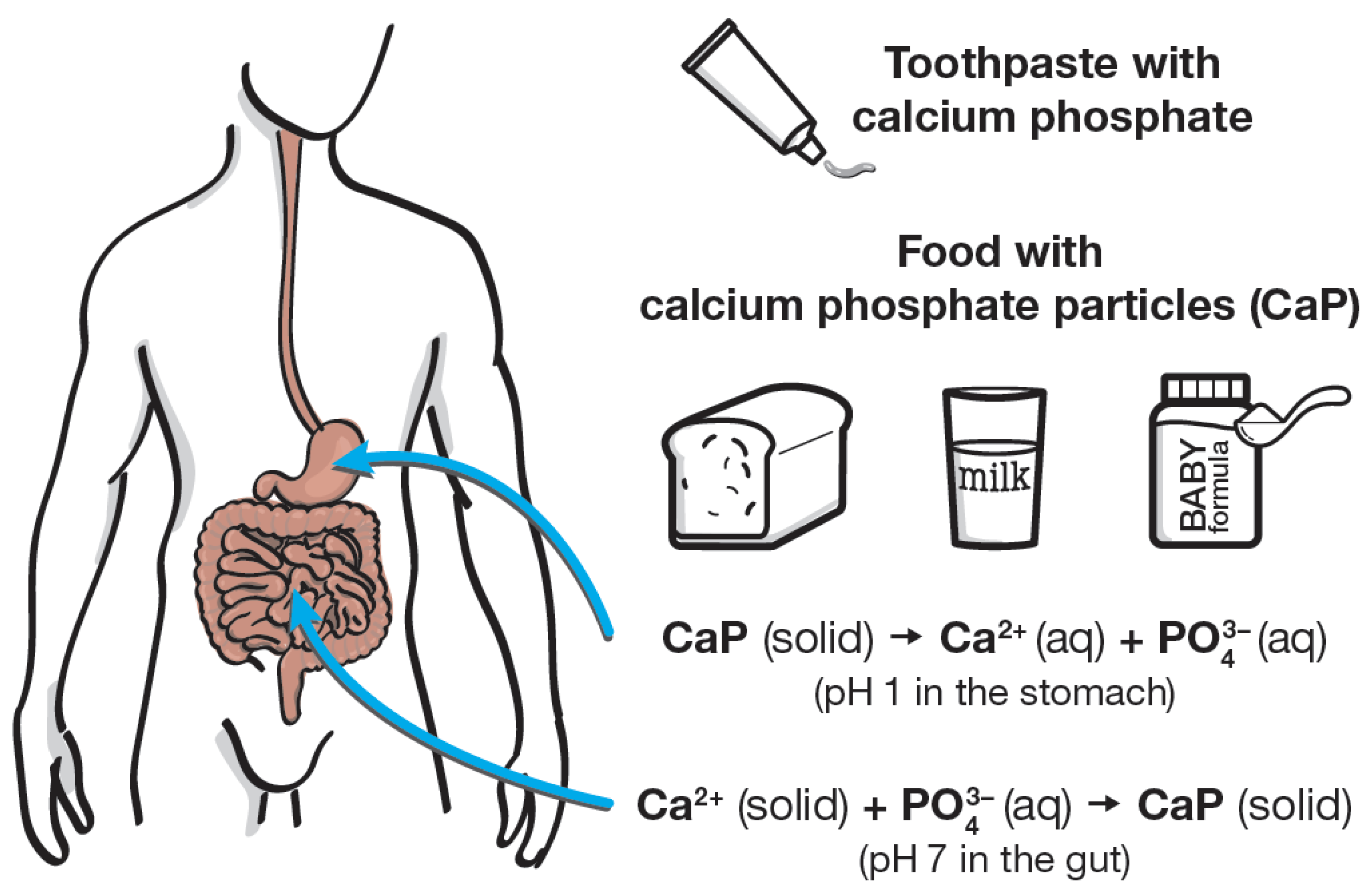
5. Calcium Phosphate Nanoparticles in Blood, Gut, and Saliva
6. Regulatory Aspects of Calcium Phosphate as Food Additive
7. Calcium Phosphate Nanoparticles in Infant Formulae
8. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- Elliott, J.C. Calcium phosphate biominerals. Rev. Mineral. 2002, 48, 427–453. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Fabritius, H.O.; Enax, J.; Meyer, F. Eine Reise ins Innere unserer Zähne / A journey into our teeth; Titus Verlag: Bielefeld, Germany, 2021. [Google Scholar]
- Qi, C.; Lin, J.; Fu, L.H.; Huang, P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Yu, H.J.; Chen, C.Z. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef] [Green Version]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A comprehensive review of bioactive glass coatings: State of the art, challenges and future perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Functionalized calcium orthophosphates (CaPO4) and their biomedical applications. J. Mater. Chem. B 2019, 7, 7471–7489. [Google Scholar] [CrossRef] [PubMed]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating techniques for functional enhancement of metal implants for bone replacement: A review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Biological and medical applications of calcium phosphate nanoparticles. Chem. Eur. J. 2021, 27, 7471–7488. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef] [Green Version]
- Kavasi, R.M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In vitro biocompatibility assessment of nano-hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Egana, J.T.; Reckhenrich, A.K.; Schenck, T.L.; Lohmeyer, J.A.; Schantz, J.T.; Machens, H.G.; Schilling, A.F. Cell-based resorption assays for bone graft substitutes. Acta Biomater. 2012, 8, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.C.; Matsuzaka, K.; Walboomers, X.F.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. Osteoclastic resorption of calcium phosphate coatings applied with electrostatic spray deposition (ESD) in vitro. J. Biomed. Mater. Res. A 2005, 74A, 570–580. [Google Scholar] [CrossRef]
- Leeuwenburgh, A.; Layrolle, P.; Barrere, F.; de Bruijn, J.D.; Schoonman, J.; van Blitterswijk, C.A.; de Groot, K. Osteoclastic resorption of biomimetic calcium phosphate coatings in vitro. J. Biomed. Mater. Res. 2001, 56, 208–215. [Google Scholar] [CrossRef]
- Motskin, M.; Möller, K.H.; Genoud, C.; Monteith, A.G.; Skepper, J.N. The sequestration of hydroxyapatite nanoparticles by human monocyte-macrophages in a compartment that allows free diffusion with the extracellular environment. Biomaterials 2011, 32, 9470–9482. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nano. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Sinfiteli, P.D.; Coutinho, T.C.L.; de Oliveira, P.R.A.; Vasques, W.F.; Azevedo, L.M.; Pereira, A.M.B.; Tostes, M.A. Effect of fluoride dentifrice and casein phosphopeptide-amorphous calcium phosphate cream with and without fluoride in preventing enamel demineralization in a pH cyclic study. J. Appl. Oral Sci. 2017, 25, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.L.; Xie, X.Q.; Wang, Y.; Yin, W.; Antoun, J.S.; Farella, M.; Mei, L. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: A systematic review. J. Dent. 2014, 42, 769–777. [Google Scholar] [CrossRef]
- Bleek, K.; Taubert, A. New developments in polymer-controlled, bioinspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, J.; Lee, J.; Suh, H.; Lee, H.; Ryu, D.; Lee, C. New analytical approach for the determination of calcium phosphate dibasic and tribasic in processed food by comparison of ion chromatography with high-performance liquid chromatography. Foods 2020, 9, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef]
- Burdock, G.A. Encyclopedia of Food and Color Additives, 1st ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Kumta, P.N.; Sfeir, C.; Lee, D.H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Carson, G.R. Flour Additives. In Food Additives Data Book; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 535–580. [Google Scholar]
- Zawadzka, G.G. Nutritive Additives. In Food Additives Data Book; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 597–681. [Google Scholar]
- Bonjour, J.P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 2011, 30, 438s–448s. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Phosphorus nutrition and the treatment of osteoporosis. Mayo Clin. Proc. 2004, 79, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Serna, J.; Bergwitz, C. Importance of dietary phosphorus for bone metabolism and healthy aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef]
- Dorea, J.G. Calcium and phosphorus in human milk. Nutrition Res. 1999, 19, 709–739. [Google Scholar] [CrossRef]
- Holt, C. Interrelationships of the concentrations of some ionic constituents of human milk and comparison with cow and goat milks. Comp. Biochem. Physiol. Comp. Physiol. 1993, 104, 35–41. [Google Scholar] [CrossRef]
- Neville, M.C.; Keller, R.P.; Casey, C.; Allen, J.C. Calcium partitioning in human and bovine milk. J. Dairy Sci. 1994, 77, 1964–1975. [Google Scholar] [CrossRef]
- Kent, J.C.; Arthur, P.G.; Mitoulas, L.R.; Hartmann, P.E. Why calcium in breastmilk is independent of maternal dietary calcium and vitamin D. Breastfeed. Rev. 2009, 17, 5–11. [Google Scholar]
- Holt, C. An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein micelles and its application to the calculation of the partition of salts in milk. Eur. Biophys. J. 2004, 33, 421–434. [Google Scholar] [CrossRef]
- Carver, J.A.; Holt, C. Functional and dysfunctional folding, association and aggregation of caseins. Adv. Protein Chem. Struct. Biol. 2020, 118, 163–216. [Google Scholar]
- Βasdeki, A.M.; Fatouros, D.G.; Βiliaderis, C.G.; Moschakis, T. Physicochemical properties of human breast milk during the second year of lactation. Curr. Res. Food Sci. 2021, 4, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Burrow, K.; Young, W.; McConnell, M.; Carne, A.; Bekhit, A.E. Do dairy minerals have a positive effect on bone health? Compr. Rev. Food. Sci. Food Saf. 2018, 17, 989–1005. [Google Scholar] [CrossRef] [Green Version]
- Gelli, R.; Ridi, F.; Baglioni, P. The importance of being amorphous: Calcium and magnesium phosphates in the human body. Adv. Colloid Interface Sci. 2019, 269, 219–235. [Google Scholar] [CrossRef] [PubMed]
- de Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Markoska, T.; Vasiljevic, T.; Huppertz, T. Unravelling conformational aspects of milk protein structure-contributions from nuclear magnetic resonance studies. Foods 2020, 9, 1128. [Google Scholar] [CrossRef]
- Lenton, S.; Nylander, T.; Teixeira, S.C.; Holt, C. A review of the biology of calcium phosphate sequestration with special reference to milk. Dairy Sci. Technol. 2015, 95, 3–14. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Irlam, J.C.; Holt, C.; Hasnain, S.S.; Hukins, D.W.L. Comparison of the structure of micellar calcium phosphate in milk from six species by extended X-ray absorption fine structure spectroscopy. J. Dairy Res. 1985, 52, 267–273. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Jordan, A.; Thomar, P.; Nicolai, T.; Dittmer, J. The effect of pH on the structure and phosphate mobility of casein micelles in aqueous solution. Food Hydrocoll. 2015, 51, 88–94. [Google Scholar] [CrossRef]
- De Sa Peixoto, P.; Silva, J.V.; Laurent, G.; Schmutz, M.; Thomas, D.; Bouchoux, A.; Gesan-Guiziou, G. How high concentrations of proteins stabilize the amorphous state of calcium orthophosphate: A solid-state nuclear magnetic resonance (NMR) study of the casein case. Langmuir 2017, 33, 1256–1264. [Google Scholar] [CrossRef]
- Aoki, T.; Umeda, T.; Kako, Y. The least number of phosphate groups for crosslinking of casein by colloidal calcium phosphate. J. Dairy Sci. 1992, 75, 971–975. [Google Scholar] [CrossRef]
- Little, E.M.; Holt, C. An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein phosphopeptides. Eur. Biophys. J. 2004, 33, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, M.; Deracinois, B.; Dugardin, C.; Mateos, A.; Romelard, A.; Auger, J.; Boulier, A.; Ravallec, R.; Flahaut, C.; Cudennec, B. Identification, production and bioactivity of casein phosphopeptides—A review. Food Res. Int. 2022, 157, 111360. [Google Scholar] [CrossRef]
- Thierens, L.A.M.; Moerman, S.; Van Elst, C.; Vercruysse, C.; Maes, P.; Temmerman, L.; De Roo, N.M.C.; Verbeeck, R.M.H.; De Pauw, G.A.M. The in vitro remineralizing effect of CPP-ACP and CPP-ACPF after 6 and 12 weeks on initial caries lesion. J. Appl. Oral Sci. 2019, 27, e20180589. [Google Scholar] [CrossRef] [PubMed]
- Sleibi, A.; Tappuni, A.R.; Davis, G.R.; Anderson, P.; Baysan, A. Comparison of efficacy of dental varnish containing fluoride either with CPP-ACP or bioglass on root caries: Ex vivo study. J. Dent. 2018, 73, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceci, M.; Mirando, M.; Beltrami, R.; Chiesa, M.; Poggio, C. Protective effect of casein phosphopeptide-amorphous calcium phosphate on enamel erosion: Atomic force microscopy studies. Scanning 2015, 37, 327–334. [Google Scholar] [CrossRef]
- Kent, J.C.; Arthur, P.G.; Retallack, R.W.; Hartmann, P.E. Calcium, phosphate and citrate in human milk at initiation of lactation. J. Dairy Res. 1992, 59, 161–167. [Google Scholar] [CrossRef]
- Aghagolzadeh, P.; Bachtler, M.; Bijarnia, R.; Jackson, C.; Smith, E.R.; Odermatt, A.; Radpour, R.; Pasch, A. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-alpha. Atherosclerosis 2016, 251, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Heiss, A.; Jahnen-Dechent, W.; Endo, H.; Schwahn, D. Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases 2007, 2, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Hunter, L.W.; Charlesworth, J.E.; Yu, S.; Lieske, J.C.; Miller, V.M. Calcifying nanoparticles promote mineralization in vascular smooth muscle cells: Implications for atherosclerosis. Int. J. Nanomedicine 2014, 9, 2689–2698. [Google Scholar]
- Kutikhin, A.G.; Velikanova, E.A.; Mukhamadiyarov, R.A.; Glushkova, T.V.; Borisov, V.V.; Matveeva, V.G.; Antonova, L.V.; Filip’ev, D.E.; Golovkin, A.S.; Shishkova, D.K.; et al. Apoptosis-mediated endothelial toxicity but not direct calcification or functional changes in anti-calcification proteins defines pathogenic effects of calcium phosphate bions. Sci. Rep. 2016, 6, 27255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketteler, M.; Bongartz, P.; Westenfeld, R.; Wildberger, J.E.; Mahnken, A.H.; Böhm, R.; Metzger, T.; Wanner, C.; Jahnen-Dechent, W.; Floege, J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 2003, 361, 827–833. [Google Scholar] [CrossRef]
- Powell, J.J.; Thomas-McKay, E.; Thoree, V.; Robertson, J.; Hewitt, R.E.; Skepper, J.N.; Brown, A.; Hernandez-Garrido, J.C.; Midgley, P.A.; Gomez-Morilla, I.; et al. An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nat. Nanotechnol. 2015, 10, 361–369. [Google Scholar] [CrossRef]
- Hidaka, S.; Oishi, A. An in vitro study of the effect of some dietary components on calculus formation: Regulation of calcium phosphate precipitation. Oral Dis. 2007, 13, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M. Natural enamel wear—A physiological source of hydroxylapatite nanoparticles for biofilm management and tooth repair? Med. Hypotheses 2010, 74, 670–672. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Yang, X.; Xu, S.; Zhang, X.; Gou, Z. A facile pollutant-free approach toward a series of nutritionally effective calcium phosphate nanomaterials for food and drink additives. J. Nanopart. Res. 2011, 13, 1039–1048. [Google Scholar] [CrossRef]
- FDA. Code of Federal Regulations Title 21, Chapter I, Subchapter B, Part 182, Subpart B, Sec. 182.1217 Calcium Phosphate; Federal Drug Administration (FDA): Silver Spring, MD, USA, 2022.
- WHO. Report TRS 683-JECFA 26/25 (Calcium Phosphate); World Health Organization (WHO): Geneva, Switzerland, 1982. [Google Scholar]
- Scheel, H. For little ones to grow. Wellness Foods Eur. 2011, 26–38. [Google Scholar]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Husøy, T.; Mennes, W.; et al. EFSA Panel on Food Additives Flavourings. Re-evaluation of phosphoric acid–phosphates—di-, tri- and polyphosphates (E 338–341, E 343, E 450–452) as food additives and the safety of proposed extension of use. EFSA J. 2019, 17, e05674. [Google Scholar] [PubMed] [Green Version]
- Epple, M.; Enax, J.; Meyer, F. Prevention of caries and dental erosion by fluorides: A critical discussion based on physico-chemical data and principles. Dent. J. 2022, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Peitsch, T.; Matthes, M.; Brandenburg, V.; Epple, M. An in-vitro crystallization setup to assess the efficiency of different phosphate binders in nephrology. Anal. Meth. 2010, 2, 901–911. [Google Scholar] [CrossRef]
- Maynard, A. 2016. Available online: http://www.foe.org/projects/food-and-technology/nanotechnology/baby-formula (accessed on 1 November 2022).
- Schoepf, J.J.; Bi, Y.; Kidd, J.; Herckes, P.; Hristovski, K.; Westerhoff, P. Detection and dissolution of needle-like hydroxyapatite nanomaterials in infant formula. NanoImpact 2017, 5, 22–28. [Google Scholar] [CrossRef]
- FDA. Code of Federal Regulations Title 21, Chapter I, Subchapter B, Part 107 Infant Formula, Subpart D, Sec. 107.100 Nutrient Specifications; Federal Drug Administration (FDA): Silver Spring, MD, USA, 2022.
- EU. Official Journal of the European Union, Commission Directive 2006/141/EC as of 22.12.2006 on Infant Formulae and Follow-On Formulae; European Union (EU): Maastricht, The Netherlands, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; Epple, M. On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials 2022, 12, 4075. https://doi.org/10.3390/nano12224075
Enax J, Meyer F, Schulze zur Wiesche E, Epple M. On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials. 2022; 12(22):4075. https://doi.org/10.3390/nano12224075
Chicago/Turabian StyleEnax, Joachim, Frederic Meyer, Erik Schulze zur Wiesche, and Matthias Epple. 2022. "On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive" Nanomaterials 12, no. 22: 4075. https://doi.org/10.3390/nano12224075
APA StyleEnax, J., Meyer, F., Schulze zur Wiesche, E., & Epple, M. (2022). On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials, 12(22), 4075. https://doi.org/10.3390/nano12224075





