Sustainable Removal of BTEX Gas Using Regenerated Metal Containing SiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the Nanocomposites
2.3. Instruments
2.4. Experimental Methodology
3. Results and Discussions
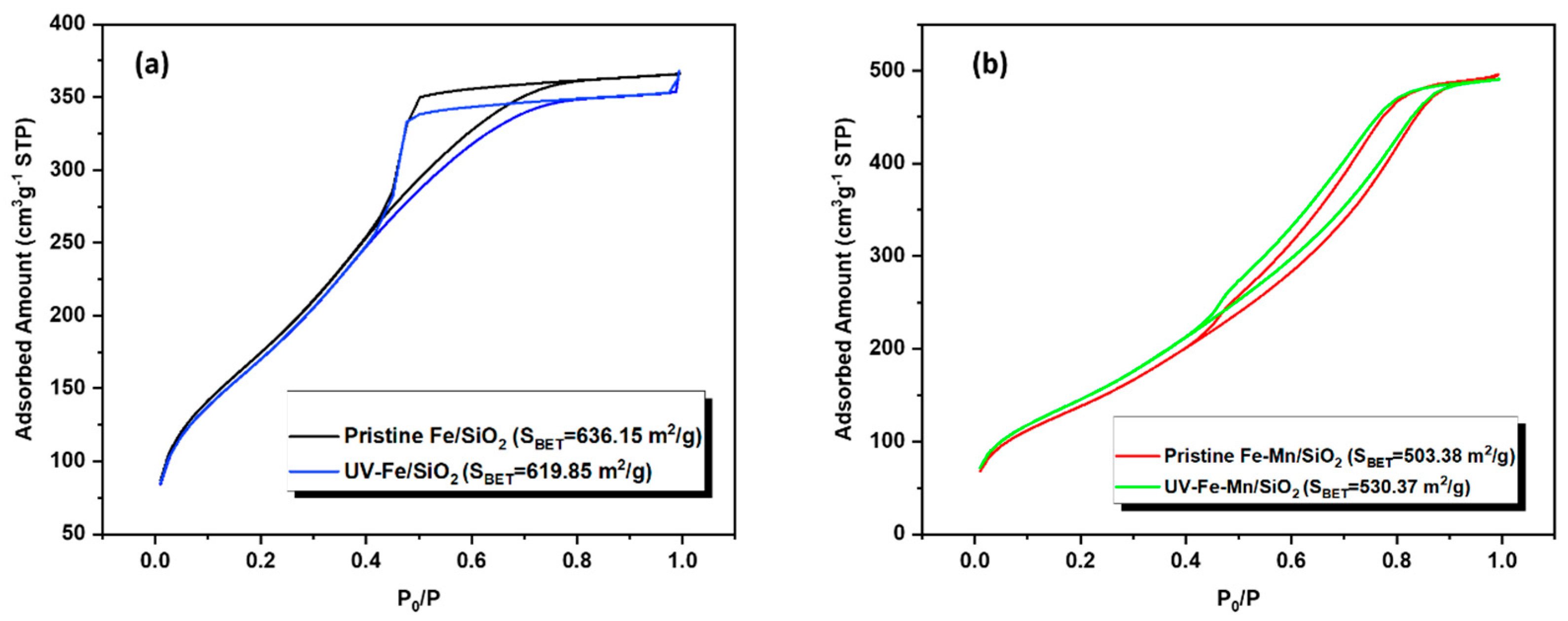
3.1. Pristine Catalyst
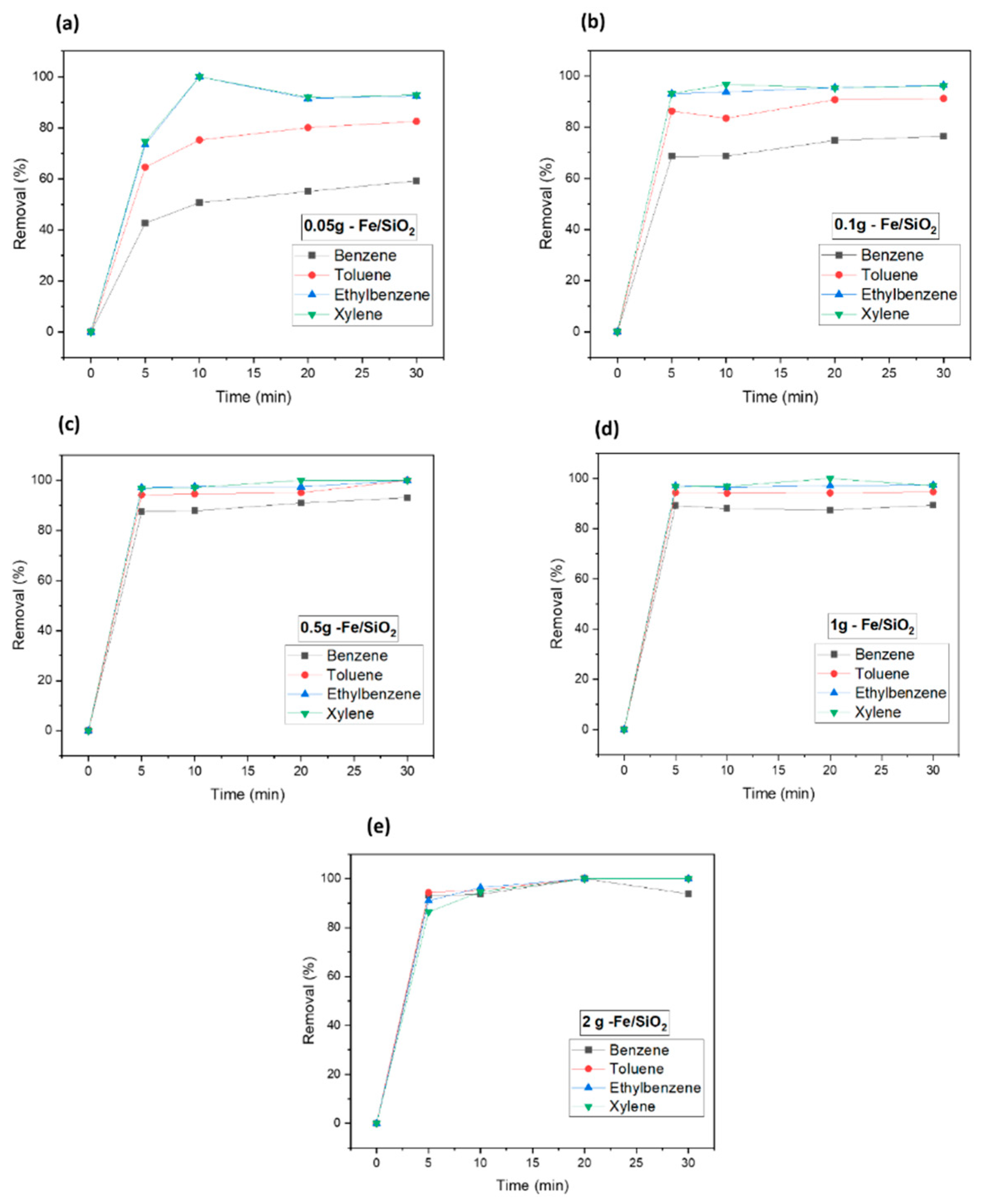
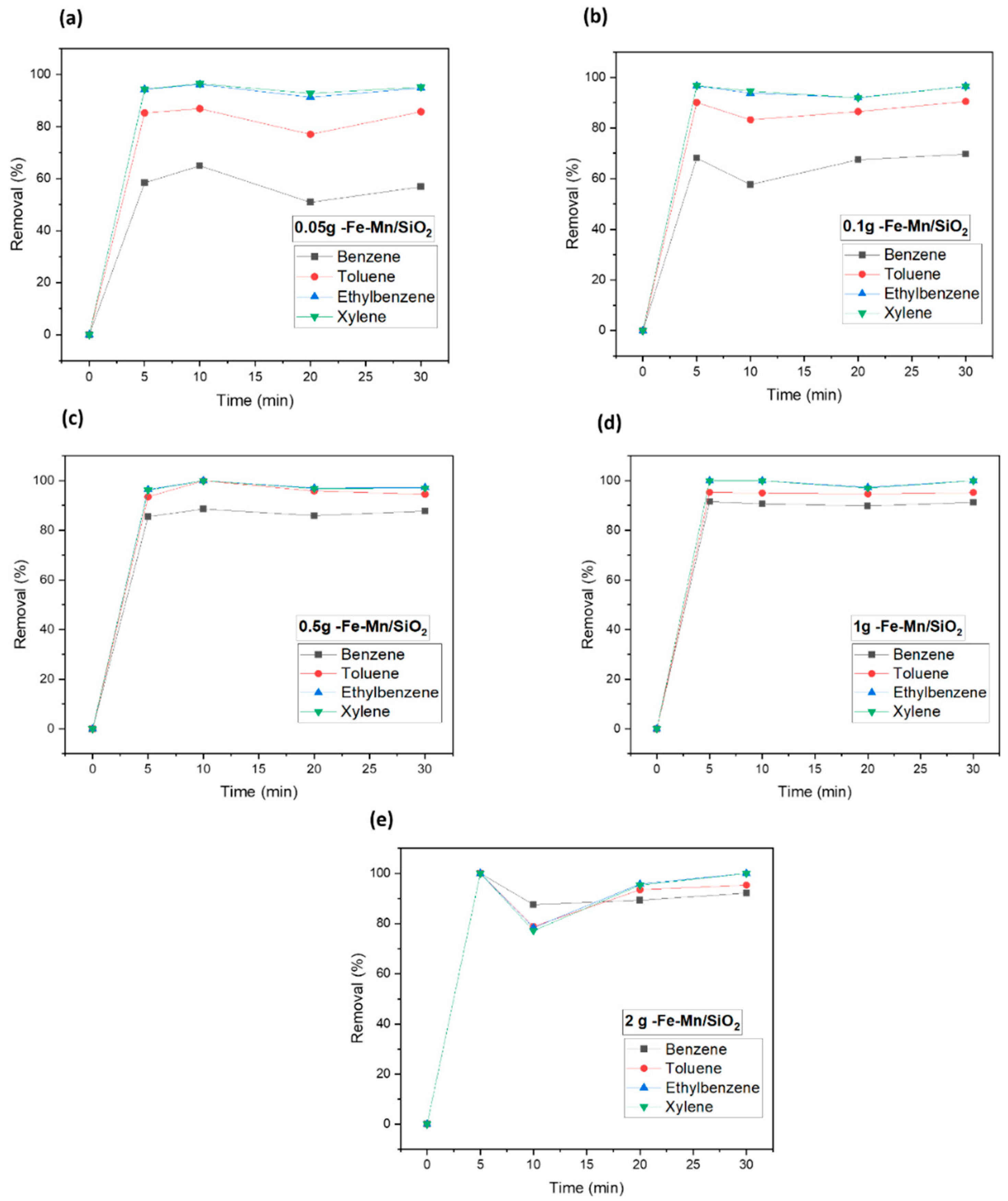
3.2. Catalyst Dosage
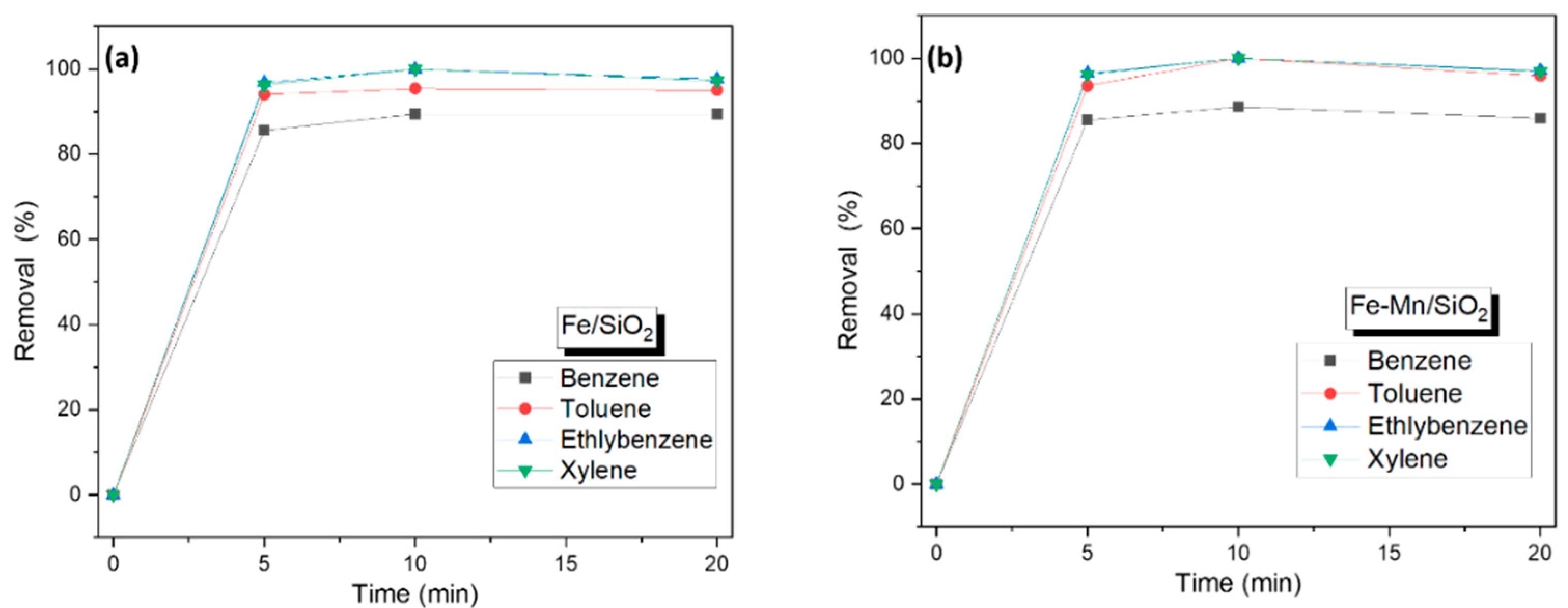
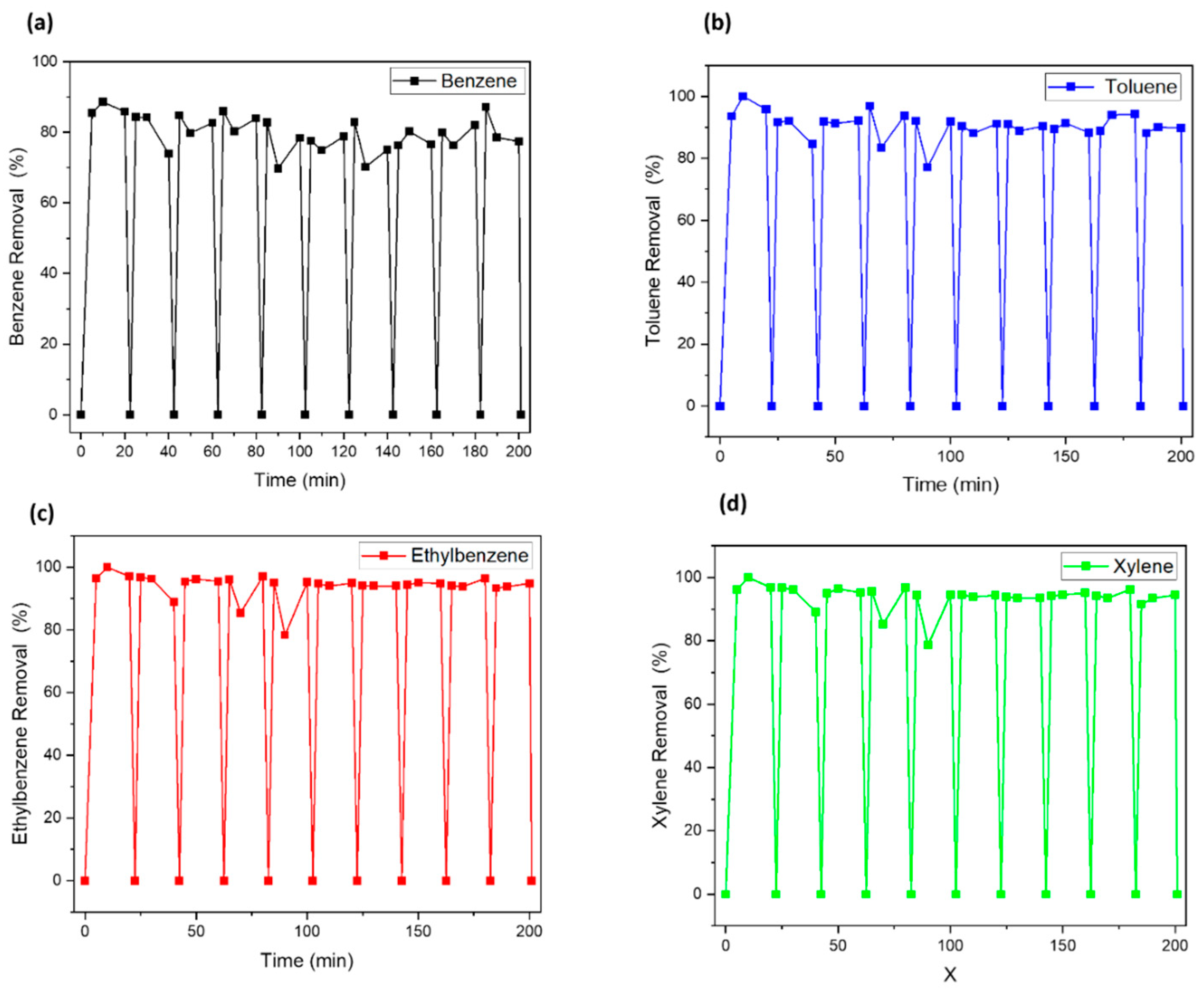
3.3. Performance Test
3.4. Regeneration/Reuse
3.5. Comparison of Pristine and Regenerated Catalyst
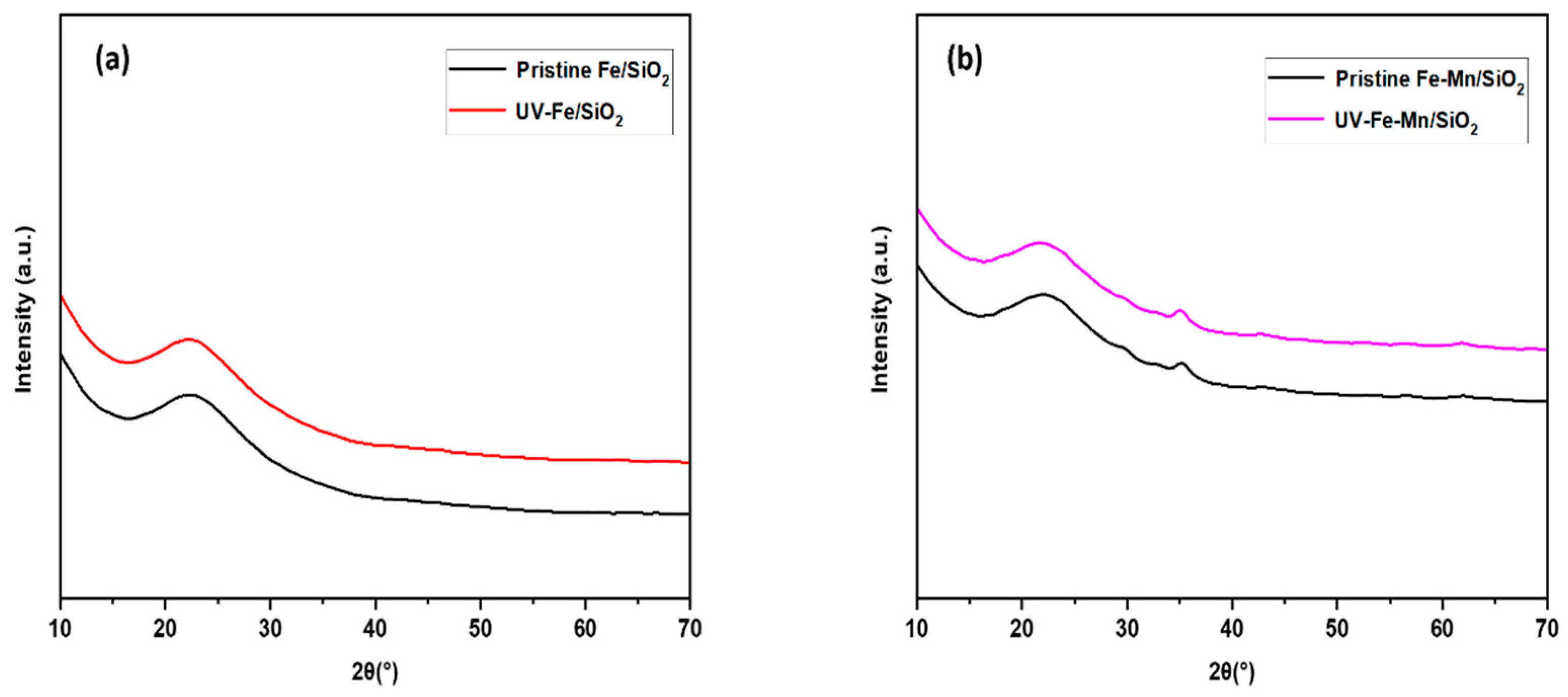
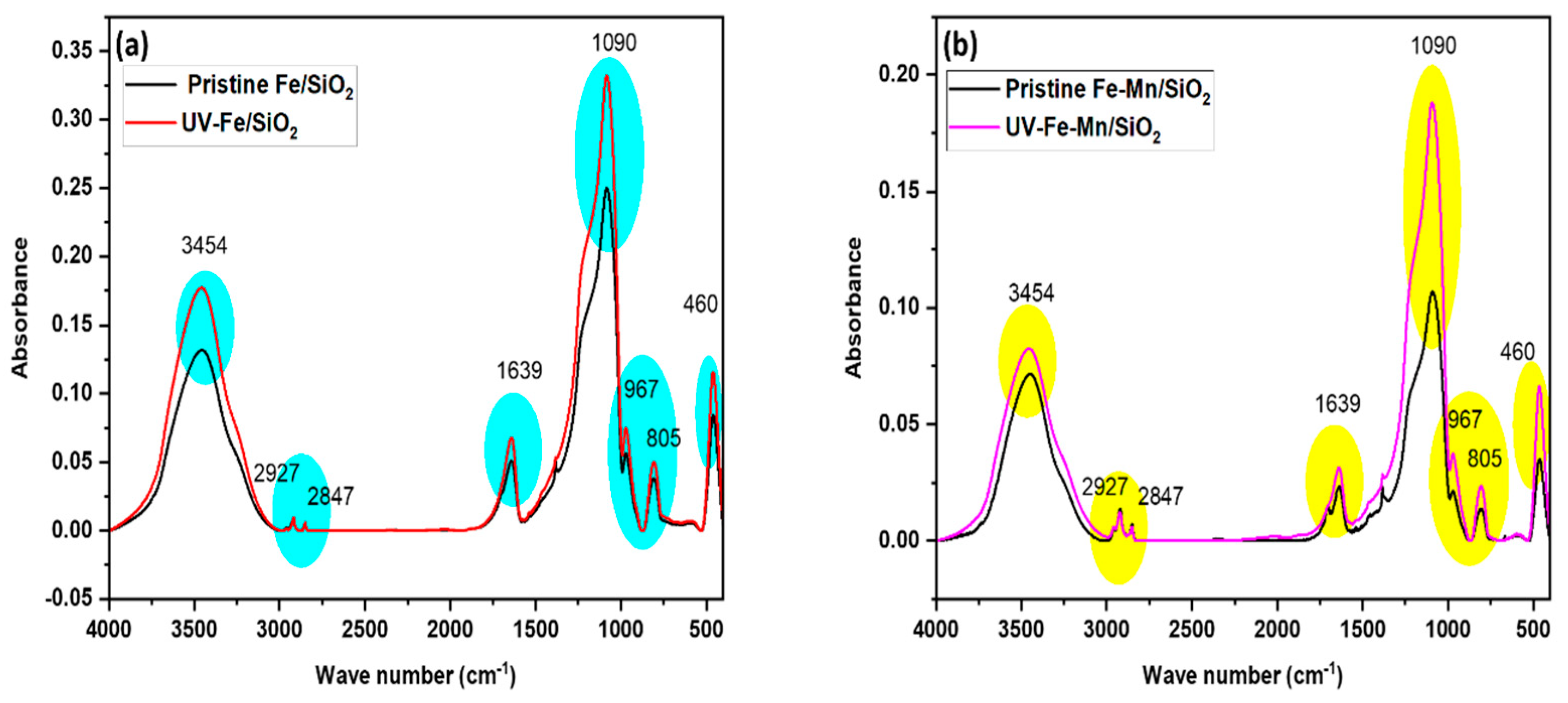
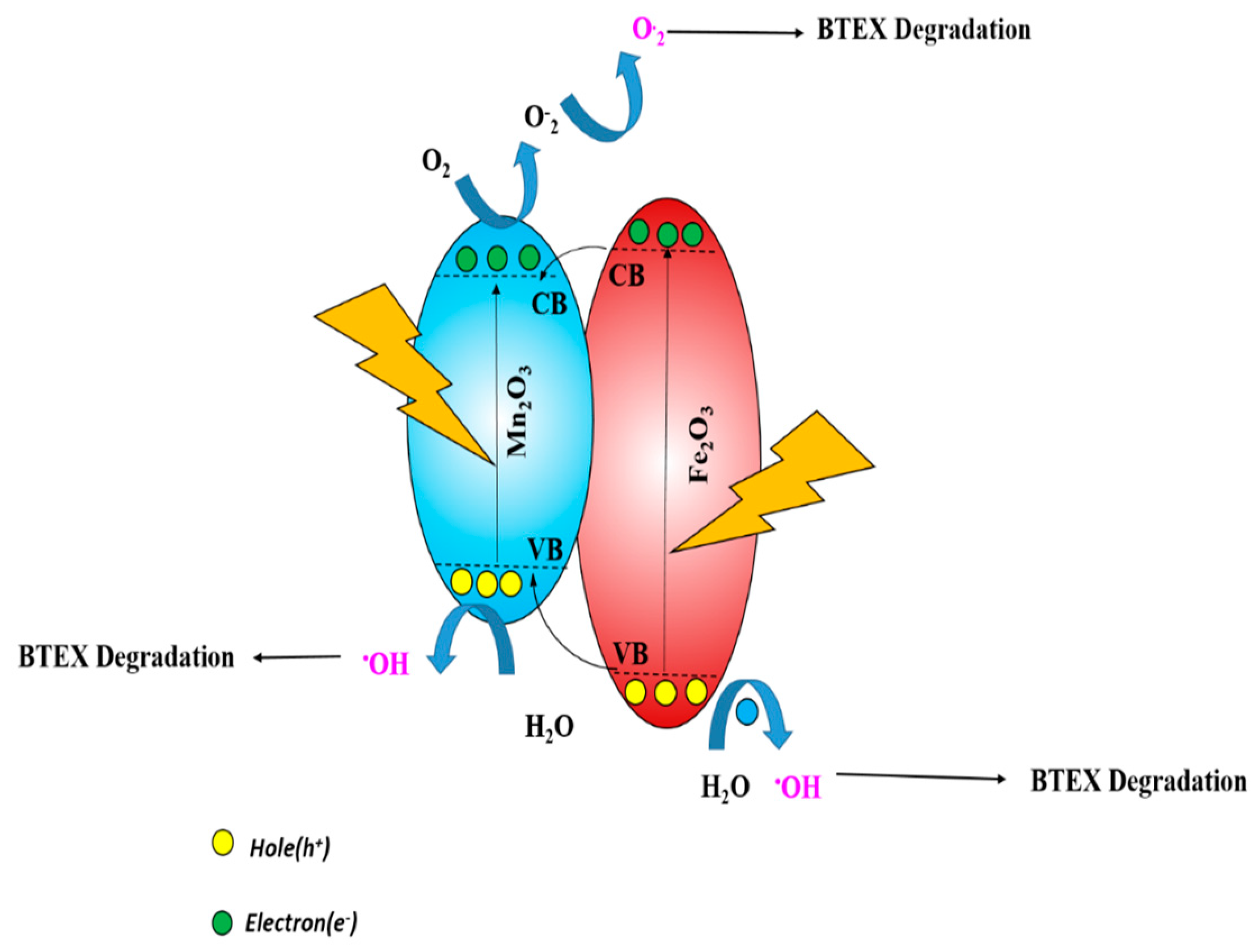
3.6. Mechanism of the Regeneration Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.E.; Ok, Y.S.; Tsang, D.C.W.; Song, J.; Jung, S.-C.; Park, Y.-K. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review. Sci. Total Environ. 2020, 719, 137405. [Google Scholar] [CrossRef] [PubMed]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.-H.; Jeon, M.-J.; Jeon, Y.-W. Study of sulfuric acid treatment of activated carbon used to enhance mixed VOC removal. Int. Biodeterior. Biodegrad. 2016, 113, 195–200. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.-S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Yu, B.; Yuan, Z.; Yu, Z.; Xue-song, F. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Słomińska, M.; Król, S.; Namieśnik, J. Removal of BTEX compounds from waste gases; destruction and recovery techniques. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1417–1445. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Ganesapillai, M.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. A review on adsorptive removal of oil pollutants (BTEX) from wastewater using carbon nanotubes. J. Mol. Liq. 2019, 277, 1005–1025. [Google Scholar] [CrossRef]
- Li, Y.-A.; Yang, F.; Liu, Z.-C.; Liu, Q.-K.; Dong, Y.-B. A porous Cd (II)-MOF-coated quartz fiber for solid-phase microextraction of BTEX. J. Mater. Chem. A 2014, 2, 13868–13872. [Google Scholar] [CrossRef]
- Li, Y.; Hu, T.; Chen, R.; Xiang, R.; Wang, Q.; Zeng, Y.; He, C. Novel thiol-functionalized covalent organic framework as adsorbent for simultaneous removal of BTEX and mercury (II) from water. Chem. Eng. J. 2020, 398, 125566. [Google Scholar] [CrossRef]
- Maya, F.; Ghani, M. Ordered macro/micro-porous metal-organic framework of type ZIF-8 in a steel fiber as a sorbent for solid-phase microextraction of BTEX. Microchim. Acta 2019, 186, 425. [Google Scholar] [CrossRef] [PubMed]
- Sangkhun, W.; Laokiat, L.; Tanboonchuy, V.; Khamdahsag, P.; Grisdanurak, N. Photocatalytic degradation of BTEX using W-doped TiO2 immobilized on fiberglass cloth under visible light. Superlattices Microstruct. 2012, 52, 632–642. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Sun, Y.; Wang, W.; Shao, Z. Perovskite oxides in catalytic combustion of volatile organic compounds: Recent advances and future prospects. Energy Environ. Mater. 2022, 5, 751–776. [Google Scholar] [CrossRef]
- Yang, K.; Xue, F.; Sun, Q.; Yue, R.; Lin, D. Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. J. Environ. Chem. Eng. 2013, 1, 713–718. [Google Scholar] [CrossRef]
- Aziz, A.; Kim, K.S. Synergistic effect of UV pretreated Fe-ZSM-5 catalysts for heterogeneous catalytic complete oxidation of VOC: A technology development for sustainable use. J. Hazard. Mater. 2017, 340, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.H.; Kim, M.J.; Kim, S.; Jung, Y.H.; Moon, H.-R.; Kim, K.-S. Evaluation of Activated Carbon-Coated Electrode in Electrostatic Precipitator and Its Regeneration for Volatile Organic Compounds Removal. Water Air Soil Pollut. 2017, 228, 110. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, Y.; Chen, L.; Chen, Z.; Ma, X. Performance of transition metal (Cu, Fe and Co) modified SCR catalysts for simultaneous removal of NO and volatile organic compounds (VOCs) from coal-fired power plant flue gas. Fuel 2021, 289, 119849. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Du, J.; Sui, H.; He, L. Functionalized Ordered Mesoporous Silica by Vinyltriethoxysilane for the Removal of Volatile Organic Compounds through Adsorption/Desorption Process. Ind. Eng. Chem. Res. 2020, 59, 3511–3520. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, Q.; Zhang, J.-B.; Liu, G.-B. Electrospun SiO2 aerogel/polyacrylonitrile composited nanofibers with enhanced adsorption performance of volatile organic compounds. Appl. Surf. Sci. 2020, 512, 145697. [Google Scholar] [CrossRef]
- Ghaffari, Y.; Beak, S.; Bae, J.; Kim, S.; Saifuddin, M.; Kim, K.S. One-step fabrication of novel ultra porous Mn2O3-Fe2O3 @ SiO2: A versatile material for removal of organic pollutants from industrial wastewater at neutral pH. Sep. Purif. Technol. 2022, 285, 120259. [Google Scholar] [CrossRef]
- Areerob, T.; Grisdanurak, N.; Chiarakorn, S. Utilization of rice husk silica as adsorbent for BTEX passive air sampler under high humidity condition. Environ. Sci. Pollut. Res. 2016, 23, 5538–5548. [Google Scholar] [CrossRef] [PubMed]
- Konggidinata, M.I.; Chao, B.; Lian, Q.; Subramaniam, R.; Zappi, M.; Gang, D.D. Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto Ordered Mesoporous Carbon (OMC). J. Hazard. Mater. 2017, 336, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, D.; Ma, C.; Yang, H. The effect of activated carbon adsorption on the photocatalytic removal of formaldehyde. Build. Environ. 2010, 45, 615–621. [Google Scholar] [CrossRef]
- Miao, J.-L.; Li, C.-B.; Liu, H.-H.; Zhang, X.-X. MnO2/MWCNTs nanocomposites as highly efficient catalyst for indoor formaldehyde removal. J. Nanosci. Nanotechnol. 2018, 18, 3982–3990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kang, H.-J. Photocatalysis of sub-ppm limonene over multiwalled carbon nanotubes/titania composite nanofiber under visible-light irradiation. J. Hazard. Mater. 2015, 283, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gao, X.; Zheng, C.; Wang, Z.; Ni, M.; Tu, X. Plasma-catalytic removal of a low concentration of acetone in humid conditions. RSC Adv. 2014, 4, 37796–37805. [Google Scholar] [CrossRef]
- El-Roz, M.; Kus, M.; Cool, P.; Thibault-Starzyk, F. New Operando IR Technique to Study the Photocatalytic Activity and Selectivity of TiO2 Nanotubes in Air Purification: Influence of Temperature, UV Intensity, and VOC Concentration. J. Phys. Chem. C 2012, 116, 13252–13263. [Google Scholar] [CrossRef]
- Ghaffari, Y.; Beak, S.; Bae, J.; Saifuddin, M.; Kim, K.S. Effect of UV Irradiation on the Structural Variation of Metal Oxide-Silica Nanocomposites for Enhanced Removal of Erythromycin at Neutral pH. Catalysts 2022, 12, 424. [Google Scholar] [CrossRef]
- Kim, S.; Gupta, N.K.; Bae, J.; Kim, K.S. Structural variations and generation of binding sites in Fe-loaded ZSM-5 and silica under the effect of UV-irradiation and their role in enhanced BTEX abatement from gas streams. J. Hazard. Mater. 2020, 384, 121274. [Google Scholar] [CrossRef]
- Hamdan, H.; Muhid, M.N.M.; Endud, S.; Listiorini, E.; Ramli, Z. 29Si MAS NMR, XRD and FESEM studies of rice husk silica for the synthesis of zeolites. J. Non-Cryst. Solids 1997, 211, 126–131. [Google Scholar] [CrossRef]
- Baek, S.; Ghaffari, Y.; Bae, J. Synthesis of Fe2O3/Mn2O3 Nanocomposites and Impregnated Porous Silicates for Dye Removal: Insights into Treatment Mechanisms. Catalysts 2022, 12, 1045. [Google Scholar] [CrossRef]
- Ren, J.-X.; Zhu, J.-L.; Shi, S.-C.; Yin, M.-Q.; Huang, H.-D.; Li, Z.-M. In-situ structuring a robust cellulose hydrogel with ZnO/SiO2 heterojunctions for efficient photocatalytic degradation. Carbohydr. Polym. 2022, 296, 119957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qing, M.; Wang, H.; Liu, X.-W.; Liu, S.; Wan, H.; Li, L.; Gao, X.; Yang, Y.; Wen, X.-D.; et al. Comprehensive understanding of SiO2-promoted Fe Fischer-Tropsch synthesis catalysts: Fe-SiO2 interaction and beyond. Catal. Today 2021, 368, 96–105. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Vasilyeva, M.S.; Zemnukhova, L.A.; Timochkina, A.S. Heterogeneous photo-Fenton oxidation of lignin of rice husk alkaline hydrolysates using Fe-impregnated silica catalysts. Environ. Technol. 2021, 42, 2220–2228. [Google Scholar] [CrossRef]
- Garg, D.; Kaur, M.; Sharma, S.; Verma, V. Effect of CTAB coating on structural, magnetic and peroxidase mimic activity of ferric oxide nanoparticles. Bull. Mater. Sci. 2018, 41, 134. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gu, X.; Sun, C.; Li, H.; Deng, Y.; Gao, F.; Dong, L. In situ loading of ultra-small Cu2O particles on TiO2 nanosheets to enhance the visible-light photoactivity. Nanoscale 2012, 4, 6351–6359. [Google Scholar] [CrossRef]
- Degefu, D.M.; Liao, Z. Photocatalytic degradation of volatile organic compounds using nanocomposite of P-type and N-type transition metal semiconductors. J. Sol-Gel Sci. Technol. 2021, 98, 605–614. [Google Scholar] [CrossRef]



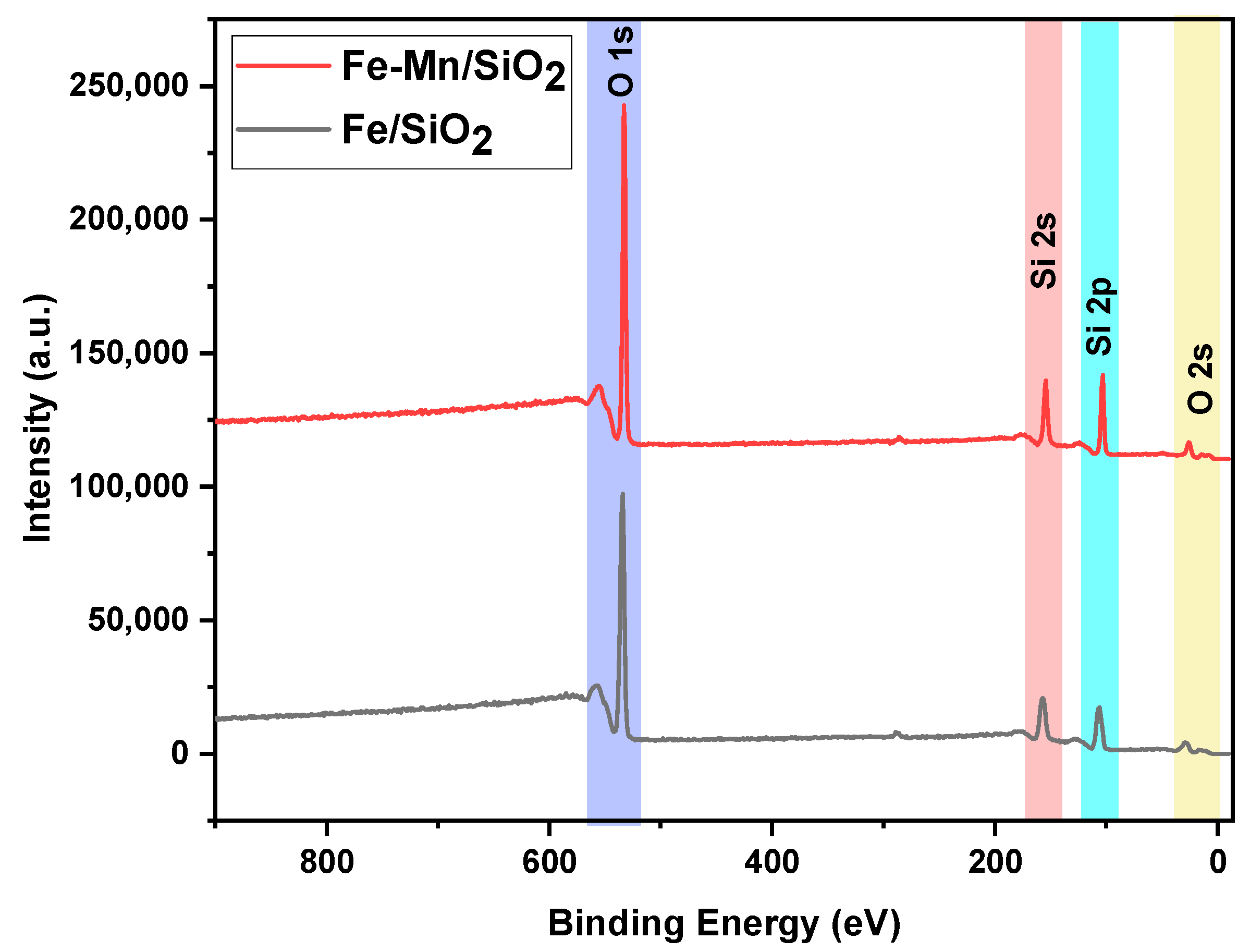

| Element/Nanocomposites | Atomic% (Fe2O3/SiO2) | Atomic% (Fe2O3-Mn2O3/SiO2) |
|---|---|---|
| O1s | 63.08 | 65.33 |
| C1s | 5.13 | 2.79 |
| Si2p | 31.1 | 30.71 |
| Fe2p | 0.68 | 0.7 |
| Mn2p | - | 0.48 |
| Nanomaterial | VOC | Performance | Ref. |
|---|---|---|---|
| TiO2/AC | Formaldehyde 1 ppm | 33.9% | [23] |
| MnO2/MWCNT | Formaldehyde 10 ppm | 43% | [24] |
| P25/graphene | Benzene 156 ppm | 8% | [25] |
| CNTs/TiO2 | Limonene 1.6 ppm | 42% | [26] |
| 10 wt.% CoOx/Al2O3 | Acetone 200 ppm | 75% | [27] |
| 10% SiO2/TiO2 fiber | Toluene 7 ppm | 90.6% | [28] |
| Fe2O3-Mn2O3/SiO2 | Ethylbenzene 5 ppm | 97.07% | This study |
| Fe2O3-Mn2O3/SiO2 | m-xylene ppm | 96.85% | This study |
| Fe2O3-Mn2O3/SiO2 | Toluene ppm | 95.82% | This study |
| Fe2O3-Mn2O3/SiO2 | Benzene ppm | 85.89% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beak, S.; Ghaffari, Y.; Kim, S.; Kim, E.J.; Kim, K.S.; Bae, J. Sustainable Removal of BTEX Gas Using Regenerated Metal Containing SiO2. Nanomaterials 2022, 12, 4113. https://doi.org/10.3390/nano12234113
Beak S, Ghaffari Y, Kim S, Kim EJ, Kim KS, Bae J. Sustainable Removal of BTEX Gas Using Regenerated Metal Containing SiO2. Nanomaterials. 2022; 12(23):4113. https://doi.org/10.3390/nano12234113
Chicago/Turabian StyleBeak, Soyoung, Yasaman Ghaffari, Suho Kim, Eun Ji Kim, Kwang Soo Kim, and Jiyeol Bae. 2022. "Sustainable Removal of BTEX Gas Using Regenerated Metal Containing SiO2" Nanomaterials 12, no. 23: 4113. https://doi.org/10.3390/nano12234113





