Graphene Quantum Dot-Added Thin-Film Composite Membrane with Advanced Nanofibrous Support for Forward Osmosis
Abstract
:Highlights
- The current study establishes that banyan tree leaf-derived B-GQD-incorporated thin-film composite membranes with a SBS NFM substrate show an enhanced FO performance regarding water flux, salt rejection, and chlorine re-sistance ability.
- The membranes developed in the current study have great prospective applications in wastewater treatment, water purification, and desalination.
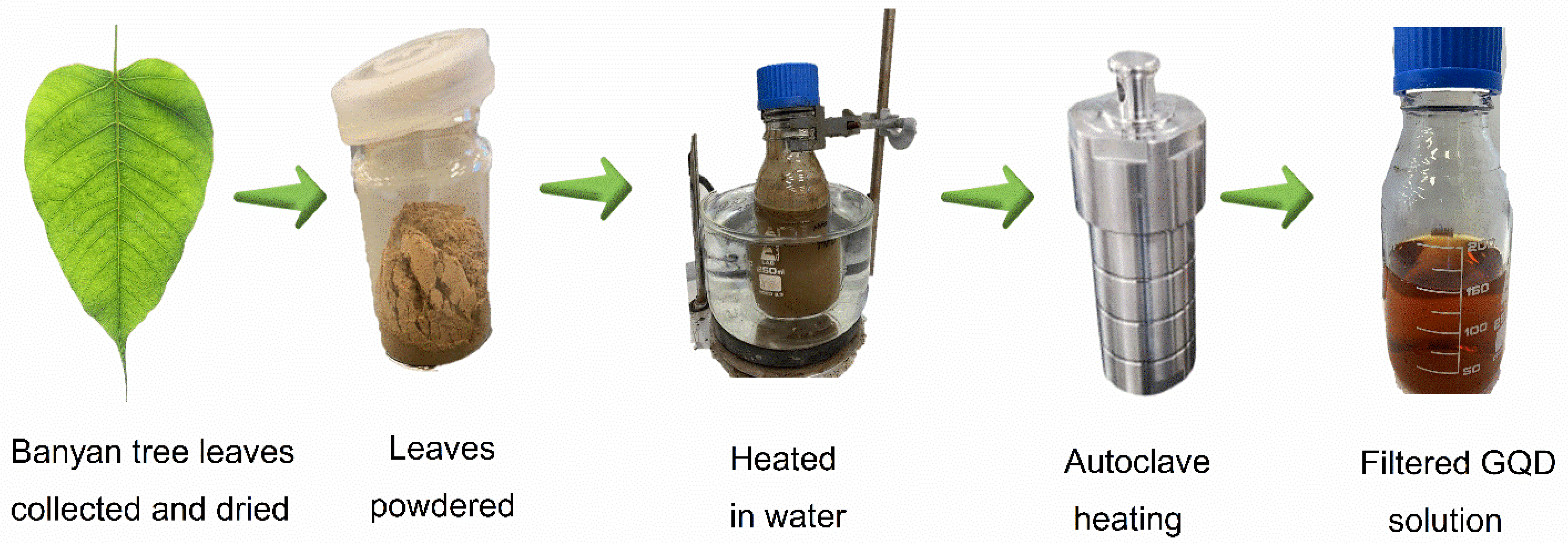
- GQD synthesis from banyan tree leaves was conducted by employing a simple hydrothermal technique.
- No organic solvent or reducing agent was utilized in this GQD synthesis.
- The GQDs were incorporated in a TFC membrane for the FO process.
- The TFC membrane’s support was fabricated by the SBS process.
- The 0.050-B-GQD/PA TFNC membrane demonstrated excellent FO performance and chlorine resistance.
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. GQD Synthesis from Banyan Tree Leaves
2.3. PES-Based Nanofiber Fabrication via SBS Technique
2.4. Heat-Press Post-Treatment for PES-Based Nanofiber Mat
2.5. Membrane Fabrication (TFC and TFNC)
2.6. Characterization of B-GQDs and the TFC/TFNC Membranes Developed
2.7. Operation of FO System
2.8. Impact of Varying Concentrations of MPD and B-GQDs during IP Process on the Performance in FO Process
2.9. Water Content Analysis of the Prepared Membranes
2.10. Long-Term Performance Assessment of the TFNC Membranes
2.11. Chlorine Resistance Examination of Developed TFC/TFNC Membranes
2.12. Validation of the FO Experimental Results
3. Results and Discussion
3.1. B-GQD Characterization
3.1.1. Morphological Properties Analysis
3.1.2. Chemical Compositional Analysis
3.1.3. Crystallinity Study
3.2. Characterization of TFC and B-GQD-Incorporated TFNC Membranes
3.2.1. Membrane Morphological Analysis
3.2.2. Membrane Compositional Analysis
3.2.3. Membrane Hydrophilicity Analysis
3.3. FO Membrane Performance of TFC and TFNC Membranes
3.3.1. Impact of MPD Concentration in TFC on FO Membrane Performance
3.3.2. Result of Varying B-GQD wt.% on the FO Performance
3.3.3. Long-Period Performance Assessment of the Developed Membrane
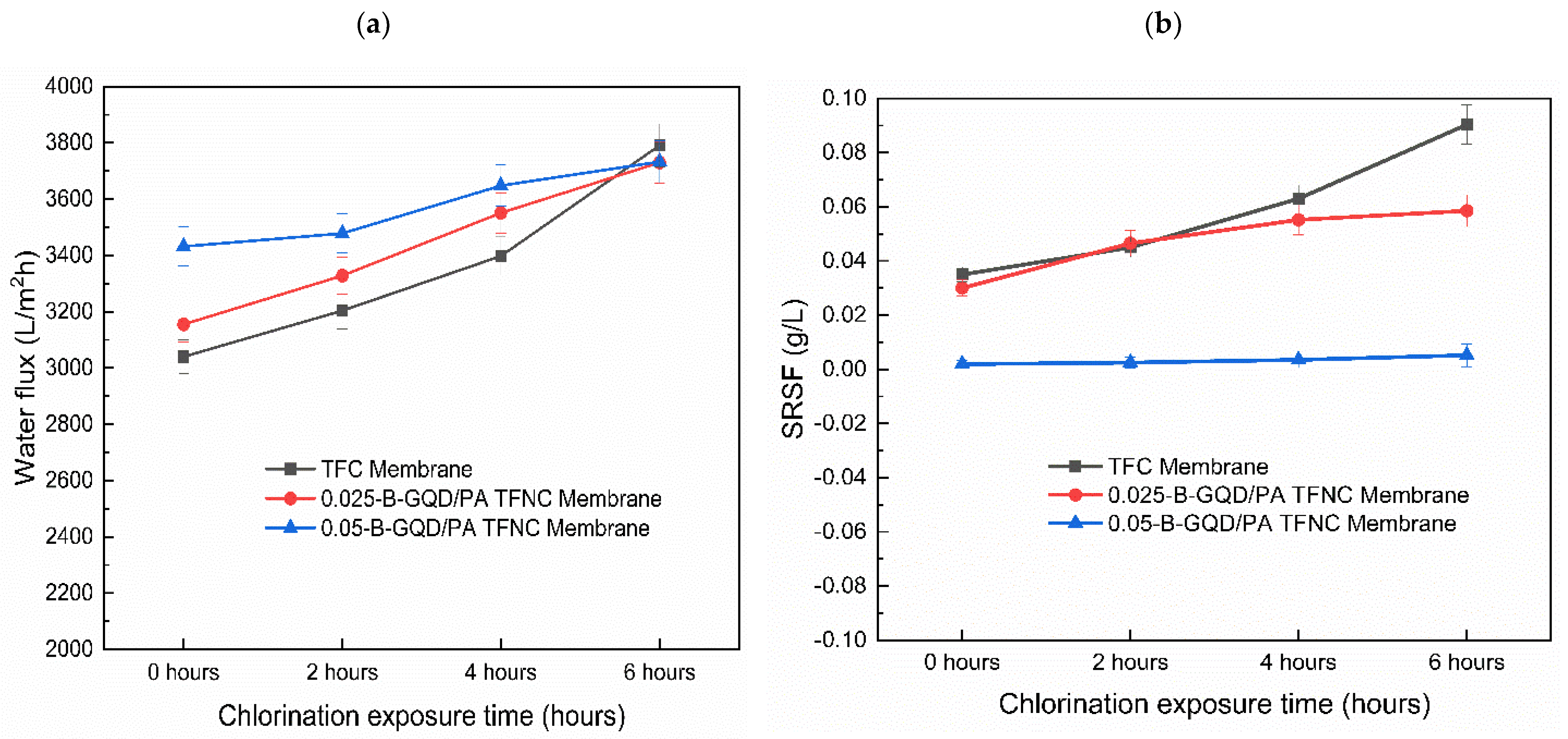
3.3.4. Chlorine Resistance Study of the TFC/TFNC Membranes Developed
3.3.5. Validation Study of the FO Experimental Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane-based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.J.; Saleem, H. Reverse Osmosis Membrane Performance Degradation. J. Reverse Osmosis Syst. 2022, 343–373. [Google Scholar]
- Zaidi, S.J.; Saleem, H. Reverse Osmosis Systems: Design, Optimization and Troubleshooting Guide; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Forward osmosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Saud, A.; Saleem, H.; Zaidi, S.J. Progress and Prospects of Nanocellulose-Based Membranes for Desalination and Water Treatment. Membranes 2022, 12, 462. [Google Scholar] [CrossRef]
- Saleem, H.; Javaid Zaidi, S. Innovative Nanostructured Membranes for Reverse Osmosis Water Desalination. 2020. [Google Scholar] [CrossRef]
- Bakly, S.; Ibrar, I.; Saleem, H.; Yadav, S.; Al-Juboori, R.; Naji, O.; Zaidi, S.J. Polymer-based nano-enhanced forward osmosis membranes. In Advancement in Polymer-Based Membranes for Water Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 471–501. [Google Scholar]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Dawoud, H.D.; Saleem, H.; Alnuaimi, N.A.; Zaidi, S.J. Characterization and Treatment Technologies Applied for Produced Water in Qatar. Water 2021, 13, 3573. [Google Scholar] [CrossRef]
- Kahrizi, M.; Gonzales, R.R.; Kong, L.; Matsuyama, H.; Lu, P.; Lin, J.; Zhao, S. Significant roles of substrate properties in forward osmosis membrane performance: A review. Desalination 2022, 528, 115615. [Google Scholar] [CrossRef]
- Rufuss, D.D.W.; Kapoor, V.; Arulvel, S.; Davies, P. Advances in forward osmosis (FO) technology for enhanced efficiency and output: A critical review. J. Clean. Prod. 2022, 356, 131769. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X. Forward osmosis technology for water treatment: Recent advances and future perspectives. J. Clean. Prod. 2021, 280, 124354. [Google Scholar] [CrossRef]
- Ghazi, Z.M.; Rizvi SW, F.; Shahid, W.M.; Abdulhameed, A.M.; Saleem, H.; Zaidi, S.J. An overview of water desalination systems integrated with renewable energy sources. Desalination 2022, 542, 116063. [Google Scholar] [CrossRef]
- Chia, W.Y.; Chia, S.R.; Khoo, K.S.; Chew, K.W.; Show, P.L. Sustainable membrane technology for resource recovery from wastewater: Forward osmosis and pressure retarded osmosis. J. Water Process Eng. 2020, 39, 101758. [Google Scholar] [CrossRef]
- Li, Z.; Wu, C.; Huang, J.; Zhou, R.; Jin, Y. Membrane fouling behavior of forward osmosis for fruit juice concentration. Membranes 2021, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.E.; Yang, Z.; Long, L.; Zhou, S.; Guo, H.; Tang, C.Y. A critical review on porous substrates of TFC polyamide membranes: Mechanisms, membrane performances, and future perspectives. J. Membr. Sci. 2021, 641, 119871. [Google Scholar] [CrossRef]
- Dai, R.; Li, J.; Wang, Z. Constructing interlayer to tailor structure and performance of thin-film composite polyamide membranes: A review. Adv. Colloid Interface Sci. 2020, 282, 102204. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Khalil, A.; Hilal, N. Emerging desalination technologies: Current status, challenges and future trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- AlZainati, N.; Saleem, H.; Altaee, A.; Zaidi, S.J.; Mohsen, M.; Hawari, A.; Millar, G.J. Pressure retarded osmosis: Advancement, challenges and potential. J. Water Process Eng. 2021, 40, 101950. [Google Scholar] [CrossRef]
- Lugito, G.; Ariono, D.; Putra, M.R.T.; Zafra, Z.N. Progress, Challenges, and Prospects of Forward Osmosis (FO) and Pressure Retarded Osmosis (PRO) as An Alternative Solution for Water and Energy Crisis. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1143, p. 012060. [Google Scholar]
- Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.; Sambudi, N.S.; Wirzal, M.D.H.; Yusof, N.; Klaysom, C.; Jaafar, J. Inclined forward osmosis module system for fouling control in sustainable produced water treatment using seawater as draw solution. J. Water Process Eng. 2021, 40, 101752. [Google Scholar] [CrossRef]
- Akhtar, A.; Singh, M.; Subbiah, S.; Mohanty, K. Sugarcane juice concentration using a novel aquaporin hollow fiber forward osmosis membrane. Food Bioprod. Process. 2021, 126, 195–206. [Google Scholar] [CrossRef]
- Feng, X.; Liu, D.; Ye, H.; Peng, D.; Wang, J.; Han, S.; Zhang, Y. High-flux polyamide membrane with improved chlorine resistance for efficient dye/salt separation based on a new N-rich amine monomer. Sep. Purif. Technol. 2021, 278, 119533. [Google Scholar] [CrossRef]
- Ang, W.L.; Chai, P.V. Polymeric Reverse Osmosis and Forward Osmosis Membranes for Water Desalination. Polym. Membr. Water Purif. Gas Sep. 2021, 113, 113–170. [Google Scholar]
- Saleem, H.; Zaidi, S.J.; Alnuaimi, N.A. Recent Advancements in the Nanomaterial Application in Concrete and Its Ecological Impact. Materials 2021, 14, 6387. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Ismail, A.F.; Goh, P.S. Advances of nanomaterials for air pollution remediation and their impacts on the environment. Chemosphere 2022, 287, 132083. [Google Scholar] [CrossRef]
- Saleem, H.; Saud, A.; Munira, N.; Goh, P.S.; Ismail, A.F.; Siddiqui, H.R.; Zaidi, S.J. Improved Forward Osmosis Performance of Thin Film Composite Membranes with Graphene Quantum Dots Derived from Eucalyptus Tree Leaves. Nanomaterials 2022, 12, 3519. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.-F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.-D.; Tang, C.Y. A critical review on thin-film nanocomposite membranes with interlayered structure: Mechanisms, recent developments, and environmental applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Suzaimi, N.D.; Goh, P.S.; Ismail, A.F.; Mamah, S.C.; Malek, N.A.N.N.; Lim, J.W.; Wong, K.C.; Hilal, N. Strategies in forward osmosis membrane substrate fabrication and modification: A review. Membranes 2020, 10, 332. [Google Scholar] [CrossRef]
- Kumar, M.; Khan, M.A.; Arafat, H.A. Recent developments in the rational fabrication of thin film nanocomposite membranes for water purification and desalination. ACS Omega 2020, 5, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhu, J.; Li, X.; Van der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Li, M.-P.; Zhang, X.; Zhang, H.; Liu, W.-L.; Huang, Z.-H.; Xie, F.; Ma, X.-H.; Xu, Z.-L. Hydrophilic yolk-shell ZIF-8 modified polyamide thin-film nanocomposite membrane with improved permeability and selectivity. Sep. Purif. Technol. 2020, 247, 116990. [Google Scholar] [CrossRef]
- Asempour, F.; Akbari, S.; Bai, D.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Improvement of stability and performance of functionalized halloysite nano tubes-based thin film nanocomposite membranes. J. Membr. Sci. 2018, 563, 470–480. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Porous metal organic framework CuBDC nanosheet incorporated thin-film nanocomposite membrane for high-performance forward osmosis. J. Membr. Sci. 2018, 573, 46–54. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yu, M.; Wang, Y.; Gao, T.; Yang, F. Conductive thin film nanocomposite forward osmosis membrane (TFN-FO) blended with carbon nanoparticles for membrane fouling control. Sci. Total Environ. 2019, 697, 134050. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Yang, S.; Liu, Y.; Wang, Q.; Li, Y. Layered double hydroxide-modified thin–film composite membranes with remarkably enhanced chlorine resistance and anti-fouling capacity. Sep. Purif. Technol. 2019, 220, 231–237. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2019, 478, 114178. [Google Scholar] [CrossRef]
- Kim, S.; Heath, D.E.; Kentish, S.E. Composite membranes with nanofibrous cross-hatched supports for reverse osmosis desalination. ACS Appl. Mater. Interfaces 2020, 12, 44720–44730. [Google Scholar] [CrossRef]
- Obaid, M.; Abdelkareem, M.A.; Kook, S.; Kim, H.Y.; Hilal, N.; Ghaffour, N.; Kim, I.S. Breakthroughs in the fabrication of electrospun-nanofiber-supported thin film composite/nanocomposite membranes for the forward osmosis process: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1727–1795. [Google Scholar] [CrossRef]
- Shen, L.; Shi, Q.; Zhang, S.; Gao, J.; Cheng, D.C.; Yi, M.; Song, R.; Wang, L.; Jiang, J.; Karnik, R.; et al. Highly porous nanofiber-supported monolayer graphene membranes for ultrafast organic solvent nanofiltration. Sci. Adv. 2021, 7, eabg6263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sahadevan, R.; Crandall, C.; Menkhaus, T.J.; Fong, H. Hot-pressed PAN/PVDF hybrid electrospun nanofiber membranes for ultrafiltration. J. Membr. Sci. 2020, 611, 118327. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fibers Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Wu, W.; Yu, L.; Li, L.; Li, Z.; Kang, J.; Pu, S.; Chen, D.; Ma, R.; An, K.; Liu, G.; et al. Electrospun nanofiber based forward osmosis membrane using graphene oxide as substrate modifier for enhanced water flux and rejection performance. Desalination 2021, 518, 115283. [Google Scholar] [CrossRef]
- Kallem, P.; Banat, F.; Yejin, L.; Choi, H. High performance nanofiber-supported thin film composite forward osmosis membranes based on continuous thermal-rolling pretreated electrospun PES/PAN blend substrates. Chemosphere 2020, 261, 127687. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, M.T.; Velizarov, S.; Van der Bruggen, B. Stability of polyethersulfone membranes to oxidative agents: A review. Polym. Degrad. Stab. 2018, 157, 15–33. [Google Scholar] [CrossRef]
- Hadadpour, S.; Tavakol, I.; Shabani, Z.; Mohammadi, T.; Tofighy, M.A.; Sahebi, S. Synthesis and characterization of novel thin film composite forward osmosis membrane using charcoal-based carbon nanomaterials for desalination application. J. Environ. Chem. Eng. 2021, 9, 104880. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J.; Ismail, A.F.; Goh, P.S.; Vinu, A. Recent advances in the application of carbon nitrides for advanced water treatment and desalination technology. Desalination 2022, 542, 116061. [Google Scholar] [CrossRef]
- Lin, Y.; Shen, Q.; Kawabata, Y.; Segawa, J.; Cao, X.; Guan, K.; Istirokhatun, T.; Yoshioka, T.; Matsuyama, H. Graphene quantum dots (GQDs)-assembled membranes with intrinsic functionalized nanochannels for high-performance nanofiltration. Chem. Eng. J. 2021, 420, 127602. [Google Scholar] [CrossRef]
- Li, Y.; Bi, R.; Su, Y.; Li, Y.; Yang, C.; You, X.; Shen, J.; Yuan, J.; Zhang, R.; Jiang, Z. Tuning the pore size of graphene quantum dots composite nanofiltration membranes by P-aminobenzoic acid for enhanced dye/salt separation. Sep. Purif. Technol. 2021, 263, 118372. [Google Scholar] [CrossRef]
- Dutra, L.V.; Fontoura, C.R.D.O.; da Cruz, J.C.; Nascimento, M.A.; de Oliveira, A.F.; Lopes, R.P. Green synthesis optimization of graphene quantum dots by Doehlert design for dye photodegradation application. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129442. [Google Scholar] [CrossRef]
- Li, S.; Gao, B.; Wang, Y.; Jin, B.; Yue, Q.; Wang, Z. Antibacterial thin film nanocomposite reverse osmosis membrane by doping silver phosphate loaded graphene oxide quantum dots in polyamide layer. Desalination 2019, 464, 94–104. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Shamsabadi, A.A.; Soroush, M. Improved performance and antifouling properties of thin-film composite polyamide membranes modified with nano-sized bactericidal graphene quantum dots for forward osmosis. Chem. Eng. Res. Des. 2018, 139, 321–334. [Google Scholar] [CrossRef]
- Xu, S.; Li, F.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Novel graphene quantum dots (GQDs)-incorporated thin film composite (TFC) membranes for forward osmosis (FO) desalination. Desalination 2019, 451, 219–230. [Google Scholar] [CrossRef]
- Song, X.; Zhou, Q.; Zhang, T.; Xu, H.; Wang, Z. Pressure-assisted preparation of graphene oxide quantum dot-incorporated reverse osmosis membranes: Antifouling and chlorine resistance potentials. J. Mater. Chem. A 2016, 4, 16896–16905. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, R.; Shen, J.; Liu, Y.-N.; He, M.; You, X.; Su, Y.; Jiang, Z. Graphene quantum dots engineered nanofiltration membrane for ultrafast molecular separation. J. Membr. Sci. 2019, 572, 504–511. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property. J. Membr. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Idrees, M.F.; Tariq, U. Enhancing chlorine resistance in polyamide membranes with surface & structure modification strategies. Water Supply 2022, 22, 1199–1215. [Google Scholar]
- Wang, C.-C.; Lu, S.-Y. Carbon black-derived graphene quantum dots composited with carbon aerogel as a highly efficient and stable reduction catalyst for the iodide/tri-iodide couple. Nanoscale 2015, 7, 1209–1215. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, C.X.; Chi, Y.; Li, C.M. Reply to comment on “one-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black”. J. Mater. Chem. 2012, 22, 21777–21778. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Gao, N.; Ren, J.; Qu, X. (2013). Improvement of photoluminescence of graphene quantum dots with a biocompatible photochemical reduction pathway and its bioimaging application. ACS Appl. Mater. Interfaces 2013, 5, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Peng, Z.; Metzger, A.; Lin, J.; Mann, J.A.; Huang, K.; Xiang, C.; Fan, X.; Samuel, E.L.G.; Alemany, L.B.; et al. Bandgap engineering of coal-derived graphene quantum dots. ACS Appl. Mater. Interfaces 2015, 7, 7041–7048. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Periasamy, A.P.; Chuang, C.; Liou, Y.-R.; Chen, Y.-F.; Joly, J.; Liang, C.-T.; Chang, H.-T. Plant leaf-derived graphene quantum dots and applications for white LEDs. New J. Chem. 2014, 38, 4946–4951. [Google Scholar] [CrossRef]
- Khose, R.V.; Chakraborty, G.; Bondarde, M.P.; Wadekar, P.H.; Ray, A.K.; Some, S. Red-fluorescent graphene quantum dots from guava leaf as a turn-off probe for sensing aqueous Hg(ii). New J. Chem. 2021, 45, 4617–4625. [Google Scholar]
- Chuang, C.; Roy, P.; Ravindranath, R.; Periasamy, A.P.; Chang, H.-T.; Liang, C.-T. Intrinsic magnetic properties of plant leaf-derived graphene quantum dots. Mater. Lett. 2016, 170, 110–113. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots from mangifera indica: Application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391. [Google Scholar] [CrossRef]
- Shinde, H.M.; Bhosale, T.T.; Gavade, N.L.; Babar, S.B.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of ZrO2 nanoparticles from Ficus benghalensis leaf extract for photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 14055–14064. [Google Scholar] [CrossRef]
- Yadav, S.; Rani, N.; Saini, K. Green synthesis of ZnO and CuO NPs using Ficus benghalensis leaf extract and their comparative study for electrode materials for high performance supercapacitor application. Mater. Today Proc. 2022, 49, 2124–2130. [Google Scholar] [CrossRef]
- Manimaran, I.; Ramamoorthy, K. A new synthesis of SWCNTs from Banyan tree leaves for nano gene delivery system–a novel feasibility study. Int. J. Anal. Exp. Modal Anal. 2019, XI, 3132–3145. [Google Scholar]
- Francis, G.; Thombre, R.; Parekh, F.; Leksminarayan, P. Bioinspired synthesis of gold nanoparticles using Ficus benghalensis (Indian Banyan) leaf extract. Chem. Sci. Trans. 2014, 3, 470–474. [Google Scholar]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 2013, 425–426, 30–39. [Google Scholar] [CrossRef]
- Eyvaz, M.; Arslan, S.; İmer, D.; Yüksel, E.; Koyuncu, İ. Forward osmosis membranes–a review: Part I. Osmotically Driven Membrane Processes-Approach. Dev. Curr. Status 2018, 10, 11–40. [Google Scholar]
- Li, Y.; Deng, W.; Li, H.; Su, F.; Huang, X.; Mo, F.; Zhang, R.; Ren, X. Toward enhancing the chlorine resistance of forward osmosis membranes: An effective strategy via grafting cyclohexylamine. Water Supply 2021, 21, 3449–3458. [Google Scholar] [CrossRef]
- HussainZadeh, M.; Mighani, H.; Shakeri, A. High permeable and anti-fouling forward osmosis membranes modified with Grafted Graphene Oxide to Polyacrylamide (GO-PAAm). J. Polym. Res. 2022, 29, 169. [Google Scholar] [CrossRef]
- Meng, Q.-W.; Ge, Q. Enhancing chlorine resistance and water permeability during forward osmosis separation using superhydrophilic materials with conjugated systems. ACS Appl. Mater. Interfaces 2020, 12, 35393–35402. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Hui, H.; Ma, T.; Zhao, G.; Zarak, M.; You, X.; Li, W. A novel nanofiltration membrane with a sacrificial chlorine-resistant nanofilm: Design and characterization of tailored membrane pores and surface charge. Desalination 2022, 538, 115896. [Google Scholar] [CrossRef]
- Kim, W.-J.; Campanella, O.; Heldman, D.R. A stepwise approach to predict the performance of forward osmosis operation: Effect of temperature and flow direction. Desalination 2022, 538, 115889. [Google Scholar] [CrossRef]
- Tshangana, C.S.; Muleja, A.A.; Nxumalo, E.N.; Mhlanga, S.D. Poly (ether) sulfone electrospun nanofibrous membranes embedded with graphene oxide quantum dots with antimicrobial activity. Environ. Sci. Pollut. Res. 2020, 27, 26845–26855. [Google Scholar] [CrossRef] [PubMed]
- Danial, W.H.; Bahri, N.F.M.; Majid, Z.A. Preparation, marriage chemistry and applications of graphene quantum dots–nanocellulose composite: A brief review. Molecules 2021, 26, 6158. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fan, H.; Ai, S.; Zhu, L. Preparation of fluorescent graphene quantum dots from humic acid for bioimaging application. New J. Chem. 2015, 39, 7054–7059. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.; Li, C. Facile Preparation and Upconversion Luminescence of Graphene Quantum Dots. Chem. Commun. 2011, 47, 2580–2582. [Google Scholar] [CrossRef]
- Routh, P.; Das, S.; Shit, A.; Bairi, P.; Das, P.; Nandi, A.K. Graphene Quantum Dots from a Facile Sono-Fenton Reaction and Its Hybrid with a Polythiophene Graft Copolymer toward Photovoltaic Application. ACS Appl. Mater. Interfaces 2013, 5, 12672–12680. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A Green and Facile Approach for the Synthesis of Water Soluble Fluorescent Carbon Dots from Banana Juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Hu, Q.; Gong, X.; Liu, L.; Choi, M.M.F. Characterization and analytical separation of fluorescent carbon nanodots. J. Nanomater. 2017, 2017, 1804178. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.; Park, J.; Hyun, D.; Yang, J.; Lee, H. Generation of graphene quantum dots by the oxidative cleavage of graphene oxide using the oxone oxidant. New J. Chem. 2015, 39, 2425–2428. [Google Scholar] [CrossRef]
- Lim, S.; Park, M.J.; Phuntsho, S.; Tijing, L.D.; Nisola, G.M.; Shim, W.-G.; Chung, W.-J.; Shon, H.K. Dual-layered nanocomposite substrate membrane based on polysulfone/graphene oxide for mitigating internal concentration polarization in forward osmosis. Polymer 2017, 110, 36–48. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Bui, N.N.; McCutcheon, J.R. Hydrophilic nanofibers as new supports for thin film composite membranes for engineered osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Arena, J.T.; McCutcheon, J.R. Surface modified PVDF nanofiber supported thin film composite membranes for forward osmosis. J. Membr. Sci. 2016, 499, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Wu, L.; Pan, X.; Zhang, L.; Chen, H.; Gao, C. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Kim, H.J.; Lim, M.-Y.; Jung, K.H.; Kim, D.-G.; Lee, J.-C. High-performance reverse osmosis nanocomposite membranes containing the mixture of carbon nanotubes and graphene oxides. J. Mater. Chem. A 2014, 3, 6798–6809. [Google Scholar] [CrossRef]
- Xia, S.; Yao, L.; Zhao, Y.; Li, N.; Zheng, Y. Preparation of graphene oxide modified polyamide thin film composite membranes with improved hydrophilicity for natural organic matter removal. Chem. Eng. J. 2015, 280, 720–727. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Synthesis and characterization of novel thin film nanocomposite reverse osmosis membranes with improved organic fouling properties for water desalination. RSC Adv. 2015, 5, 21268–21276. [Google Scholar] [CrossRef]
- Ali, M.E.; Wang, L.; Wang, X.; Feng, X. Thin film composite membranes embedded with graphene oxide for water desalination. Desalination 2016, 386, 67–76. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.-J.; Lee, K.-H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Ghorbani, M.; Alizadeh, B.; Salehi, M.A. Study on novel poly(vinyl pyrrolidone) doped MWCNTs/polyrhodanine nanocomposites: Synthesis, characterization, and thermal performance. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 604–609. [Google Scholar] [CrossRef]
- Rahimpour, A.; Seyedpour, S.F.; Aktij, S.A.; Firouzjaei, M.D.; Zirehpour, A.; Shamsabadi, A.A.; Salestan, S.K.; Jabbari, M.; Soroush, M. Simultaneous improvement of antimicrobial, Antifouling, and transport properties of forward osmosis membranes with immobilized highly-compatible polyrhodanine nanoparticles. Environ. Sci. Technol. 2018, 52, 5246–5258. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2013, 1, 349–354. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Ali, A.; Yu CL, M.; Sani NA, M.; Abdullah MA, A.; Awang, M.; Mohamed, N.S.; Yunus MA, C. Preparation, characterization and performance of polyvinylidene fluoride/tetraoctyl phosphonium bromide nanocomposite ultrafiltration membrane. Malays. J. Anal. Sci. 2018, 22, 514–521. [Google Scholar]
- Wei, J.; Liu, X.; Qiu, C.; Wang, R.; Tang, C.Y. Influence of monomer concentrations on the performance of polyamide-based thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 381, 110–117. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Song, P.; Wang, Z. Layer-by-Layer Assembly for Preparation of High-Performance Forward Osmosis Membrane. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 301, p. 012032. [Google Scholar]
- Ding, R.; Zhang, H.; Li, Y.; Wang, J.; Shi, B.; Mao, H.; Dang, J.; Liu, J. Graphene oxide-embedded nanocomposite membrane for solvent resistant nanofiltration with enhanced rejection ability. Chem. Eng. Sci. 2015, 138, 227–238. [Google Scholar] [CrossRef]
- Shi, S.-J.; Pan, Y.-H.; Wang, S.-F.; Dai, Z.-W.; Gu, L.; Wu, Q.-Y. Aluminosilicate nanotubes embedded polyamide thin film nanocomposite forward osmosis membranes with simultaneous enhancement of water permeability and selectivity. Polymers 2019, 11, 879. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6, 6 nanofibers supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Fabrication of layered silica–polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Adha, R.S.; Lee, C.; Kim, D.-H.; Kim, I.S. Quantifying the influence of divalent cations mass transport on critical flux and organic fouling mechanism of forward osmosis membrane. Desalination 2021, 512, 115146. [Google Scholar] [CrossRef]
- Ali, H.M.; Gadallah, H.; Ali, S.S.; Sabry, R.; Gadallah, A.G. Pilot-scale investigation of forward/reverse osmosis hybrid system for seawater desalination using impaired water from steel industry. Int. J. Chem. Eng. 2016, 2016, 8745943. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zou, L.; Mulcahy, D. Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 2012, 284, 175–181. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Han, M.; Wang, D.; Han, S.; Liu, Z.; Zhou, N.; Shang, R.; Xie, C. Graphene oxide incorporated forward osmosis membranes with enhanced desalination performance and chlorine resistance. Front. Chem. 2020, 7, 877. [Google Scholar] [CrossRef]
- Gu, J.E.; Jun, B.M.; Kwon, Y.N. Effect of chlorination condition and permeability of chlorine species on the chlorination of a polyamide membrane. Water Res. 2012, 46, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Effects of chlorine exposure conditions on physiochemical properties and performance of a polyamide membrane Mechanisms and implications. Environ. Sci. Technol. 2012, 46, 13184–13192. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Gómez, V.; Vankelecom, I.F. Chlorine-resistance of reverse osmosis (RO) polyamide membranes. Prog. Polym. Sci. 2017, 72, 1–15. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]




















| Water Content of the Membrane (%) | |
|---|---|
| Nanofiber Membrane | 62.00 |
| TFC membrane | 64.25 |
| 0.025-B-GQD/PA TFNC membrane | 65.09 |
| 0.050-B-GQD/PA TFNC membrane | 67.03 |
| 0.075-B-GQD/PA TFNC membrane | 70.23 |
| Water Permeability (A) in LMHB | Salt Rejection Obtained in RO System in % (R) | Salt Membrane Permeability (B) in LMH | Structural Parameter (S) in m | |
|---|---|---|---|---|
| TFC membrane | 44.43 | 89.01 | 1.51 | 1 × 10−6 |
| 0.025-B-GQD/PA TFNC membrane | 46.11 | 90.65 | 1.43 | 2 × 10−6 |
| 0.050-B-GQD/PA TFNC membrane | 50.16 | 91.89 | 0.09 | 2 × 10−6 |
| 0.075-B-GQD/PA TFNC membrane | 48.64 | 90.45 | 0.11 | 4 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, H.; Goh, P.S.; Saud, A.; Khan, M.A.W.; Munira, N.; Ismail, A.F.; Zaidi, S.J. Graphene Quantum Dot-Added Thin-Film Composite Membrane with Advanced Nanofibrous Support for Forward Osmosis. Nanomaterials 2022, 12, 4154. https://doi.org/10.3390/nano12234154
Saleem H, Goh PS, Saud A, Khan MAW, Munira N, Ismail AF, Zaidi SJ. Graphene Quantum Dot-Added Thin-Film Composite Membrane with Advanced Nanofibrous Support for Forward Osmosis. Nanomaterials. 2022; 12(23):4154. https://doi.org/10.3390/nano12234154
Chicago/Turabian StyleSaleem, Haleema, Pei Sean Goh, Asif Saud, Mohammad Aquib Wakeel Khan, Nazmin Munira, Ahmad Fauzi Ismail, and Syed Javaid Zaidi. 2022. "Graphene Quantum Dot-Added Thin-Film Composite Membrane with Advanced Nanofibrous Support for Forward Osmosis" Nanomaterials 12, no. 23: 4154. https://doi.org/10.3390/nano12234154






