Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
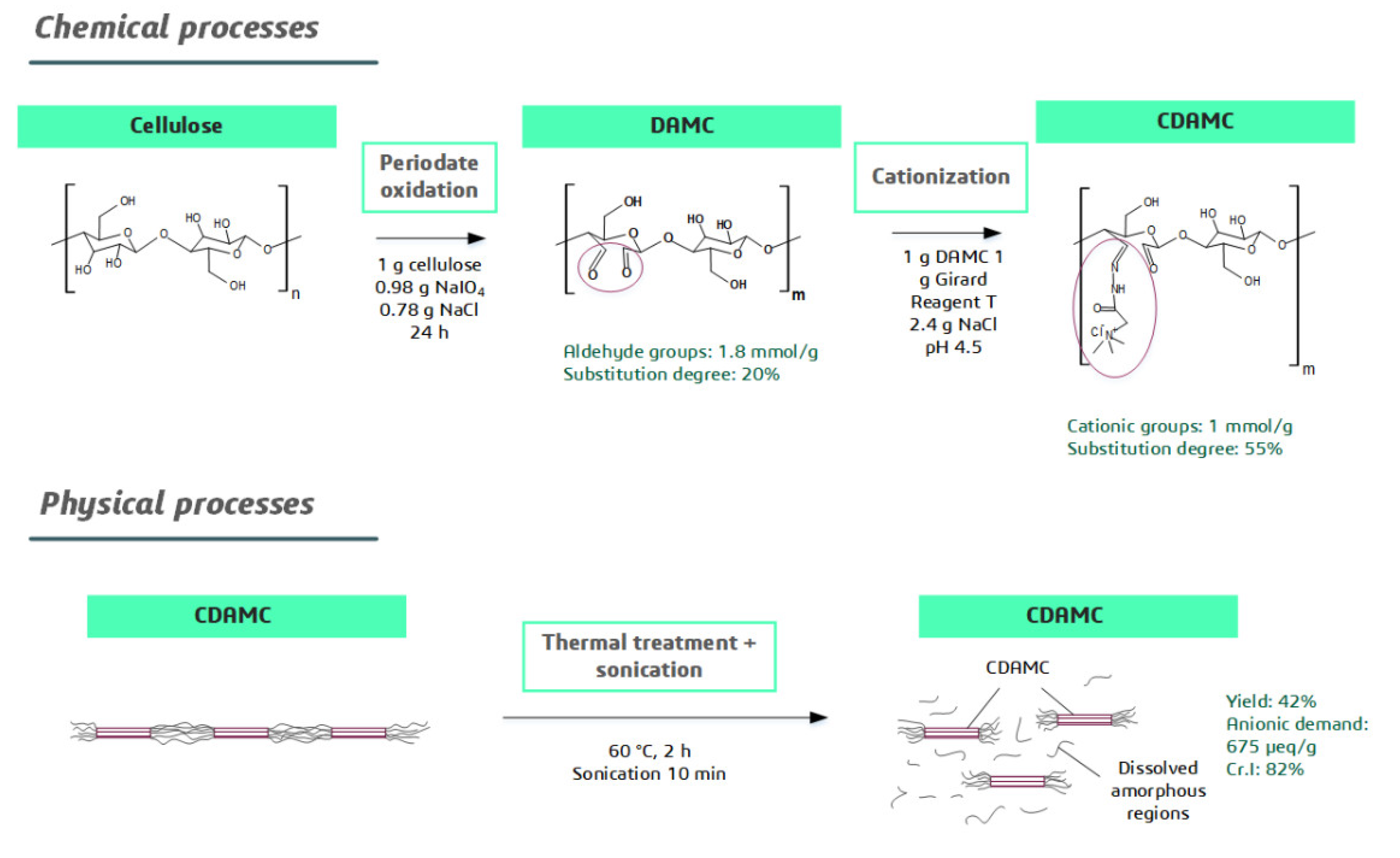
2.2. CCNC Synthesis
2.2.1. Dialdehyde Formation Reaction
2.2.2. Surface Cationization Reaction
2.2.3. Synthesis of Nanocellulose
2.3. CCNC Characterization
2.4. Batch Adsorption Experiments
2.5. Isotherm and Kinetic Data Analysis
3. Results
3.1. CCNC Characterization
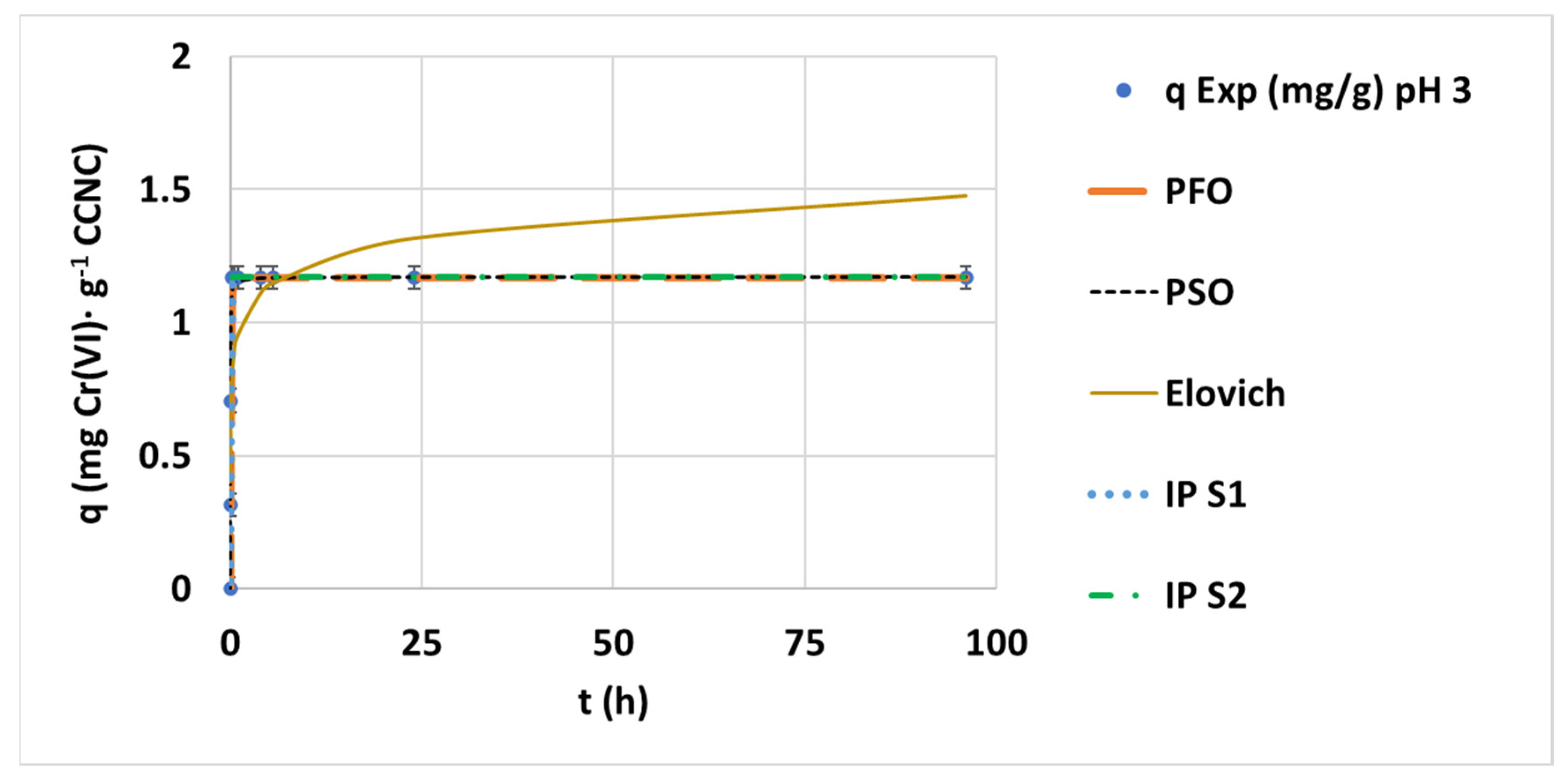
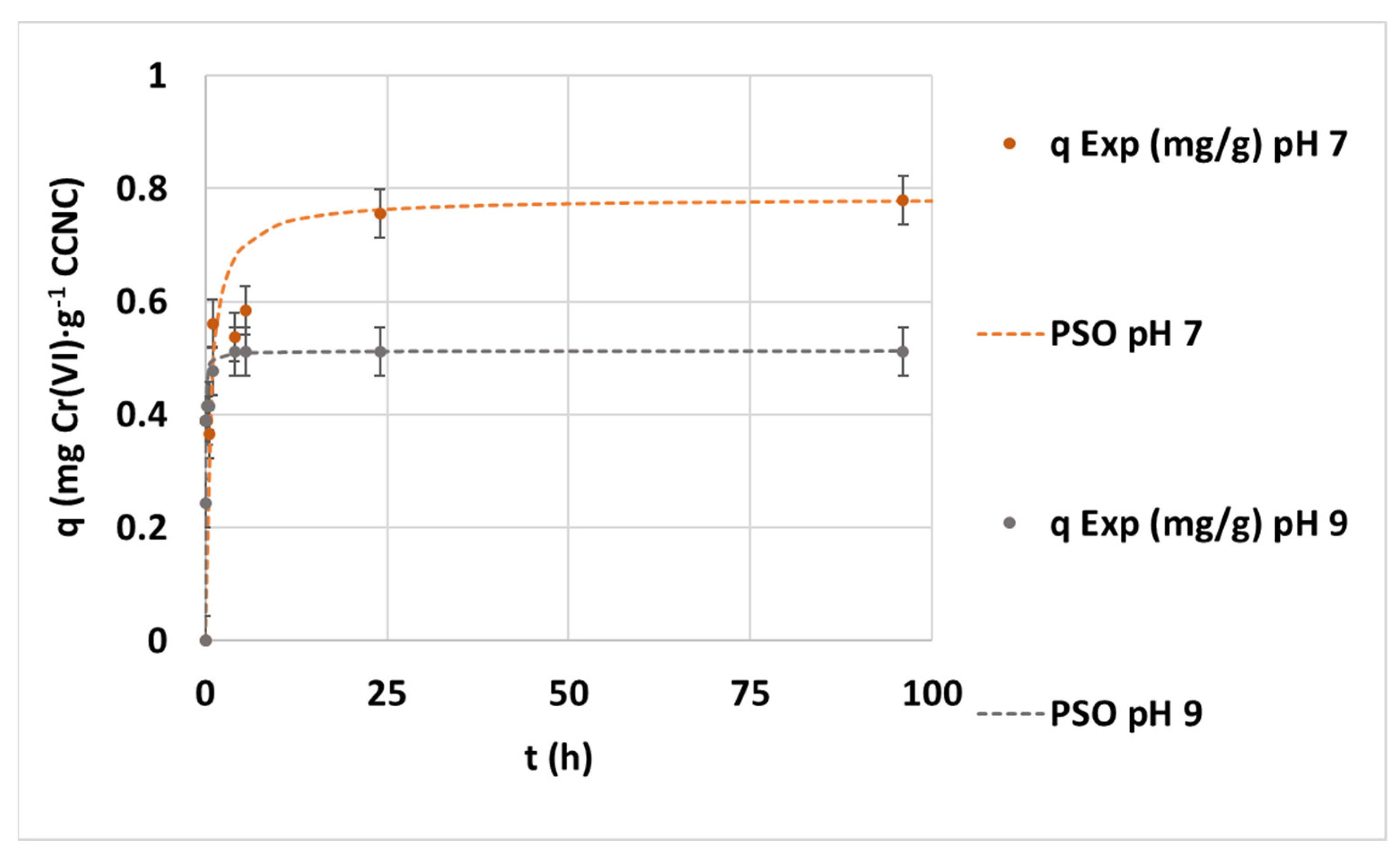
3.2. Hexavalent Chromium Adsorption Kinetics on CCNC
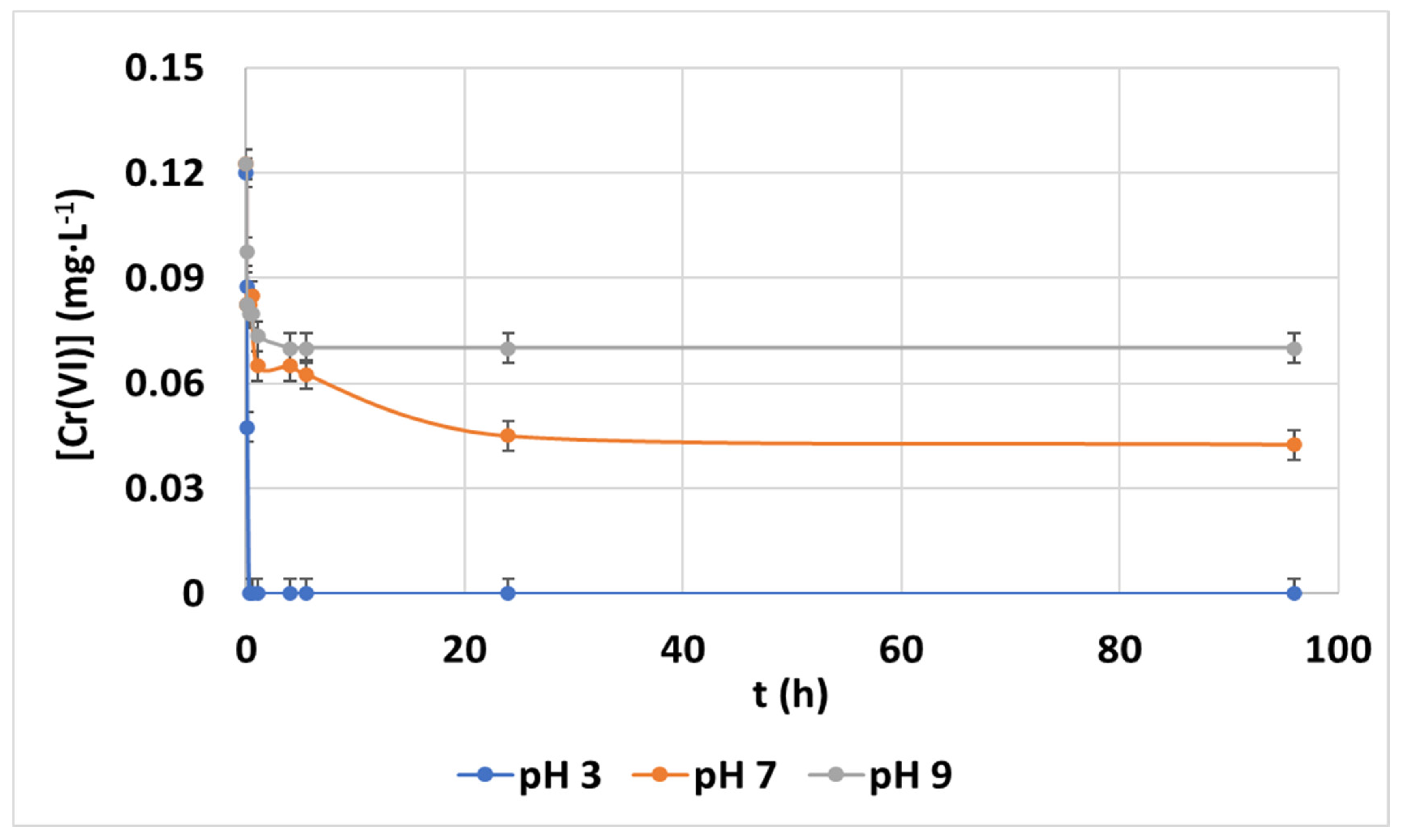
3.2.1. Effect of pH
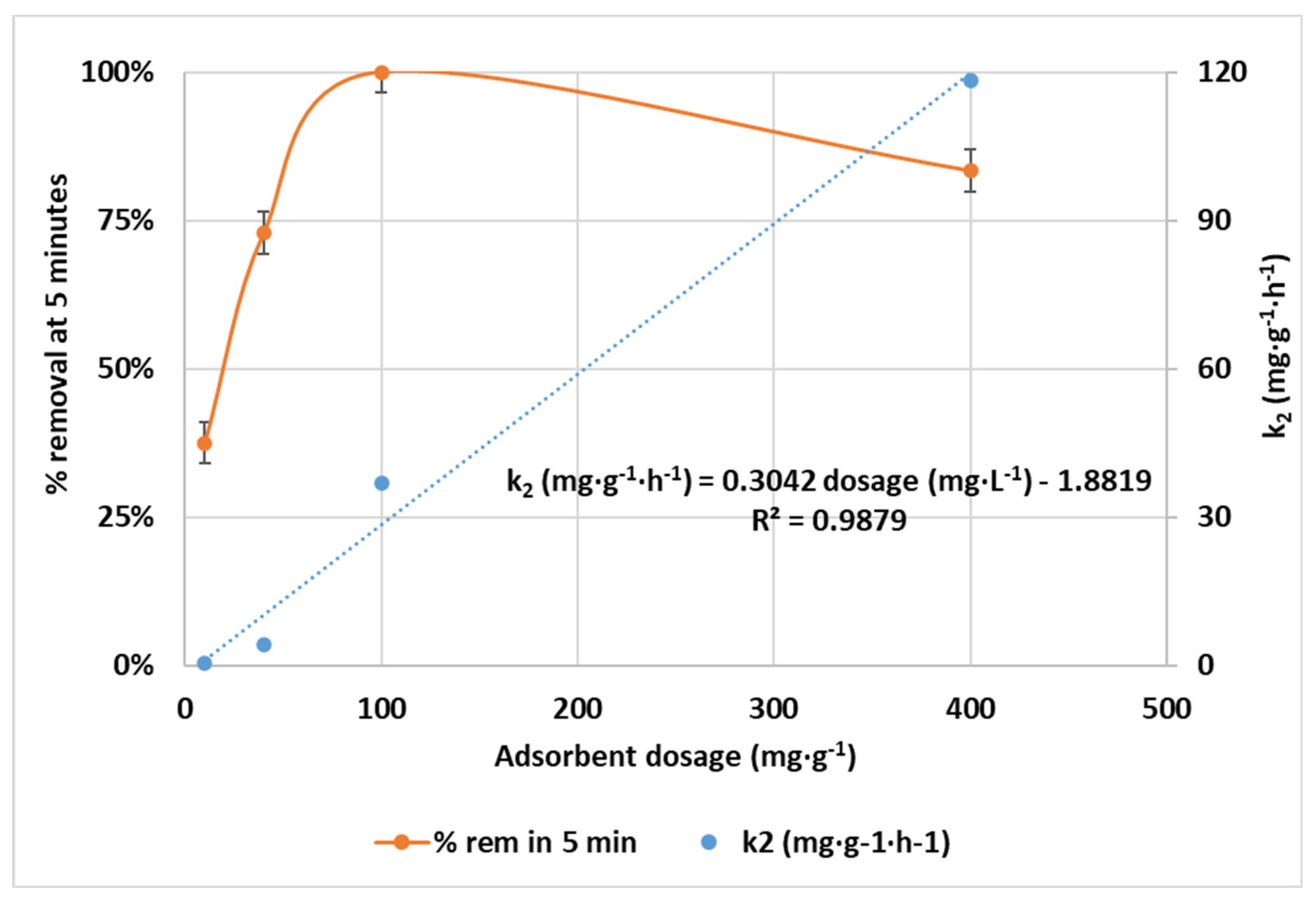
3.2.2. Effect of CCNC Dosage
3.2.3. Effect of Initial Chromium Concentration
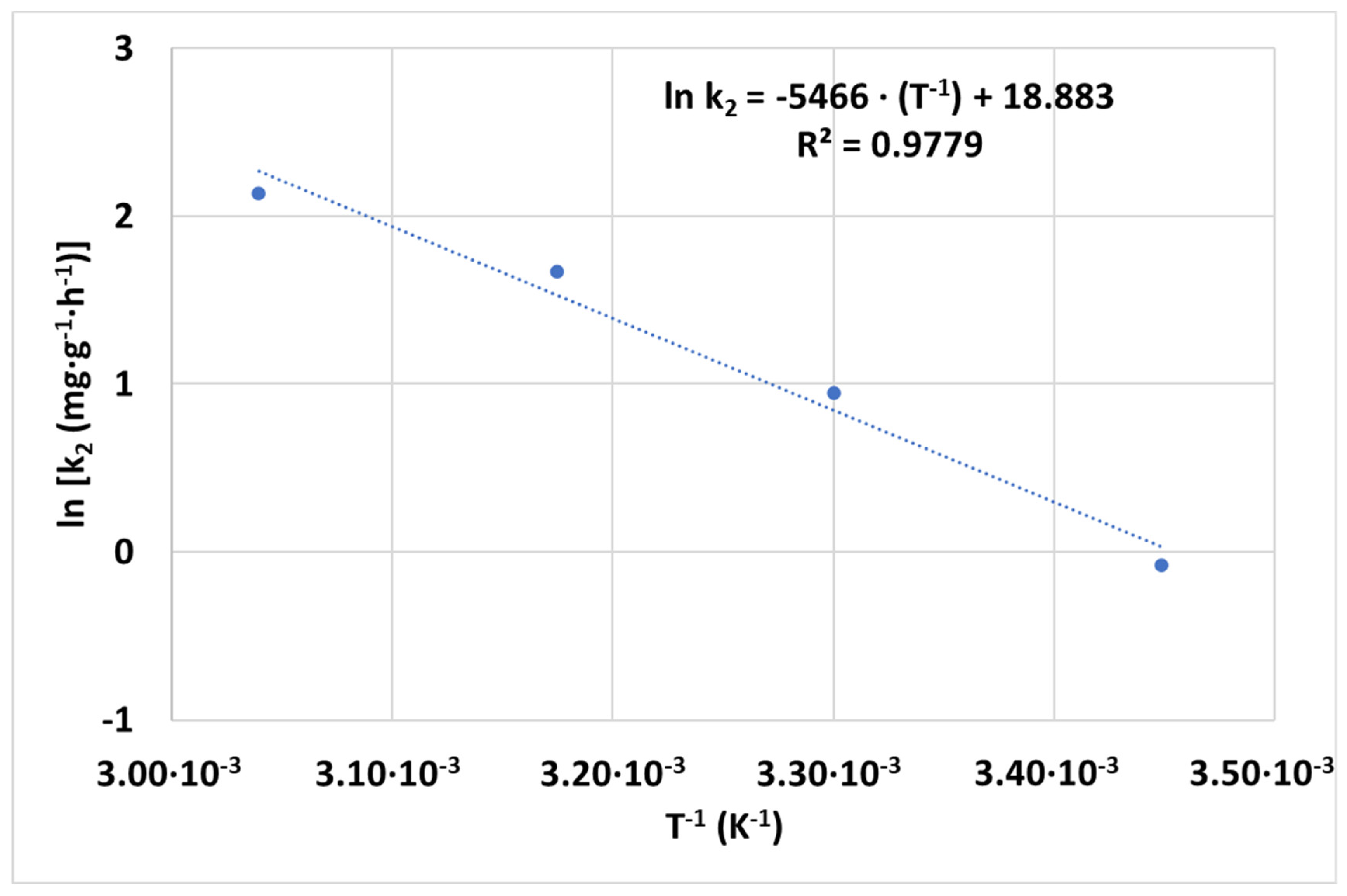
3.2.4. Effect of Temperature
3.3. Hexavalent Chromium Isotherm Study
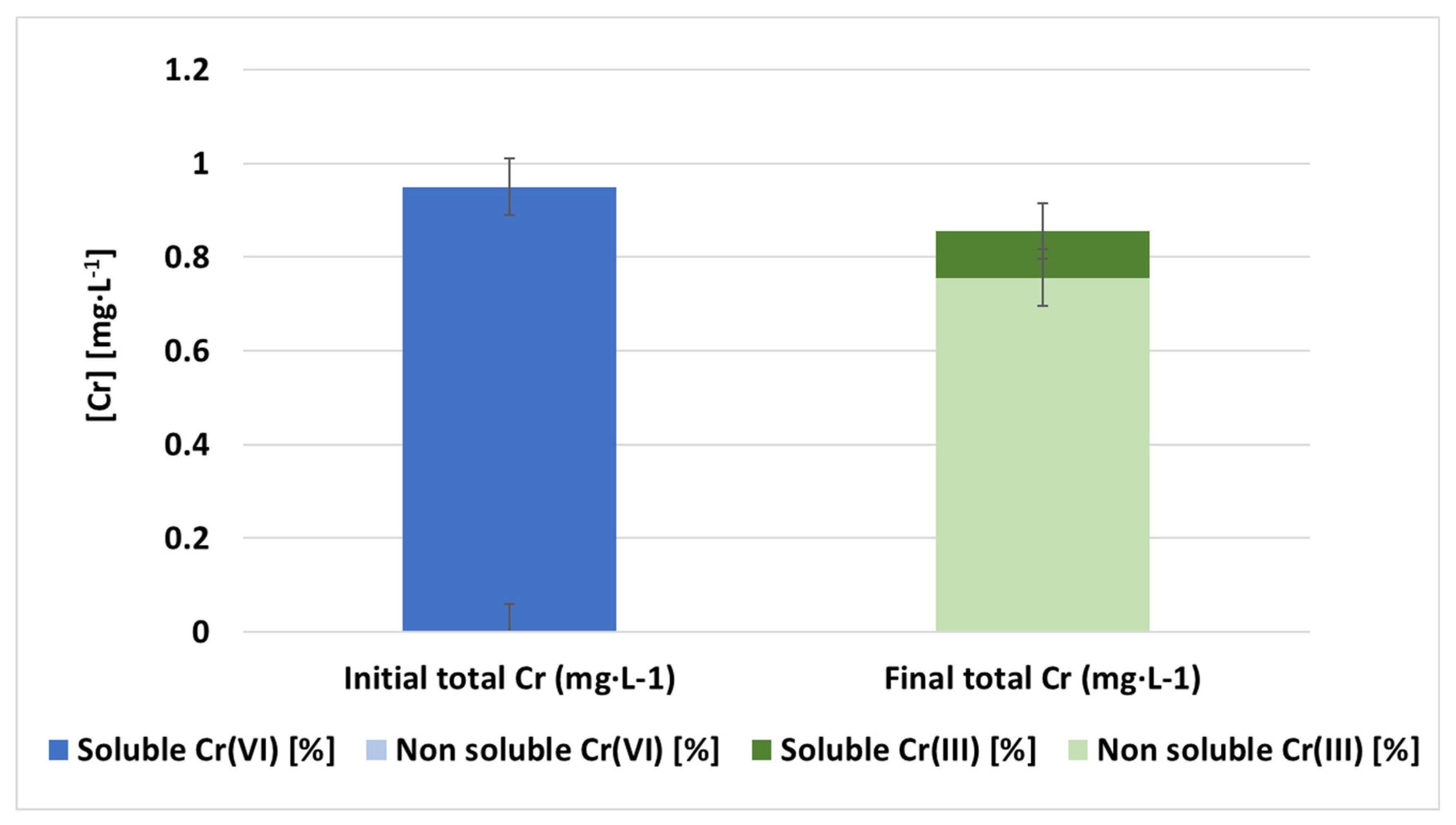
3.4. Determination of Trivalent Chromium in Treated Samples
3.5. Application of CCNC to Urban Wastewater with Tannery Effluents
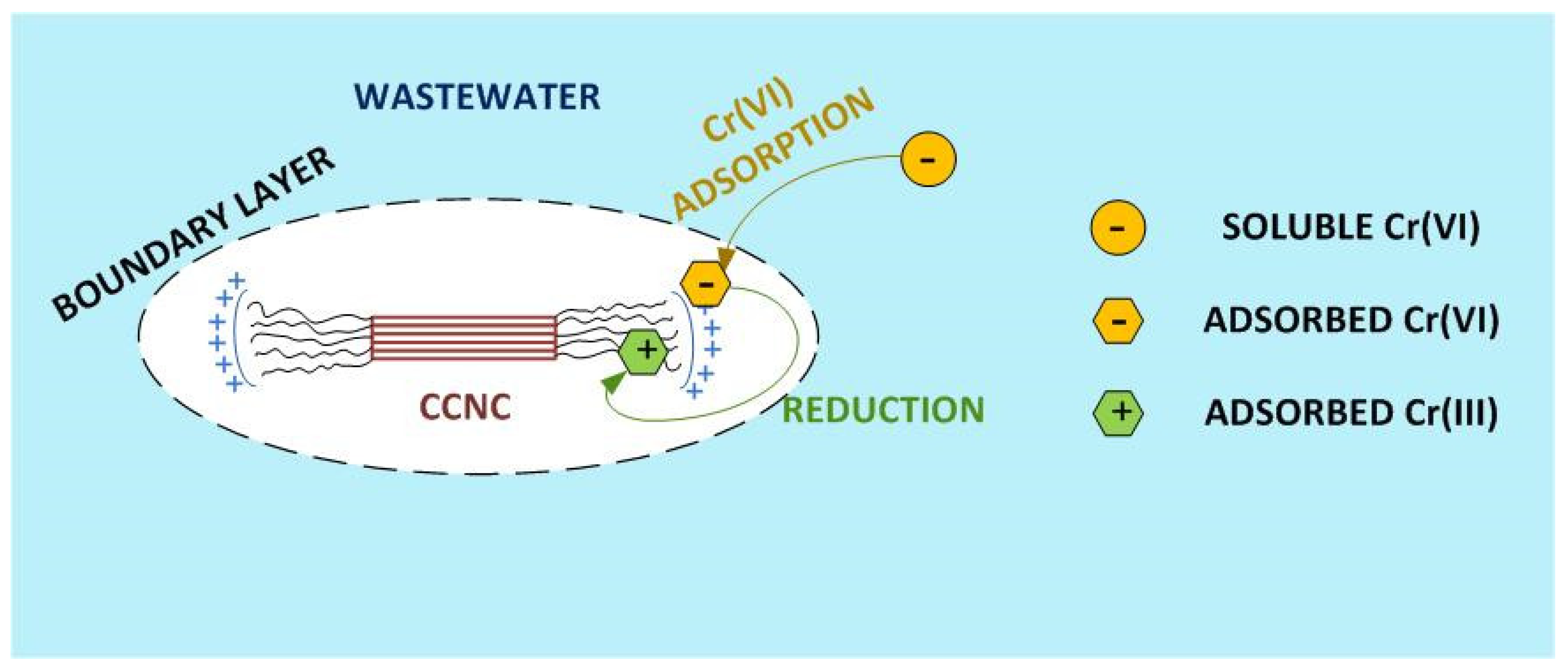
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Kinetic Model | pH Value | (-) | 3 | 7 | 9 |
|---|---|---|---|---|---|
| PFO | Kinetic parameters | k1 (h−1) | 10.56 | 0.1222 | 1.8968 |
| Correlation parameters | R2 | 0.9956 | 0.6173 | 0.7429 | |
| RSS | 2.39·10−2 | 6.8179 | 4.4506 | ||
| PSO | Kinetic parameters | k2 (mg·g−1·h−1) | 62.41 | 1.9935 | 56.24 |
| qe (mg·g−1) | 1.1703 | 0.7838 | 0.5121 | ||
| Correlation parameters | R2 | 0.9505 | 0.7197 | 0.8884 | |
| RSS | 0.1998 | 3.3144 | 3.3371 | ||
| Elovich | Kinetic parameters | α (h·mg·g−1) | 447.99 | 4481.87 | 25,757.06 |
| β (g·mg−1) | 8.6881 | 21.83 | 29.07 | ||
| Correlation parameters | R2 | 0.8505 | 0.7705 | 0.9152 | |
| RSS | 0.4279 | 2.5517 | 3.2042 | ||
| IP | Kinetic parameters: Step 1 | ki,1 (mg·g−1·min−0.5) | 2.2945 | 8.21·10−2 | 3.8679 |
| Ci,1 (mg·g−1) | 2.93·10−2 | 0.3628 | −0.1093 | ||
| Correlation parameters | R2 | 0.9990 | 0.9633 | 0.9999 | |
| RSS | 2.13·10−10 | 6.23·10−6 | 4.29·10−5 | ||
| Kinetic parameters: Step 2 | ki,2 (mg·g−1·min−0.5) | 0 | 4.98·10−3 | 7.08·10−2 | |
| Ci,2 (mg·g−1) | 1.1700 | 0.7313 | 0.3785 | ||
| Correlation parameters | R2 | 0.9999 | 0.9999 | 0.9111 | |
| RSS | 6.23·10−6 | 4.29·10−5 | 5.36·10−5 | ||
| Kinetic parameters: Step 3 | ki,3 (mg·g−1·min−0.5) | 0 | |||
| Ci,3 (mg·g−1) | 0.5119 | ||||
| Correlation parameters | R2 | 0.9999 | |||
| RSS | 6.74·10−9 |
Appendix B
| Kinetic Model | Adsorbent Dosage | (mg·L−1) | 10 | 40 | 100 | 400 |
|---|---|---|---|---|---|---|
| PFO | Kinetic parameters | k1 (h−1) | 1.1327 | 4.7052 | 10.56 | 3.5902 |
| Correlation parameters | R2 | 0.9772 | 0.9967 | 0.9953 | 0.8034 | |
| RSS | 23.23 | 2.7917 | 0.2356 | 2.60·10−2 | ||
| PSO | Kinetic parameters | k2 (mg·g−1·h−1) | 0.3502 | 4.2168 | 36.87 | 118.33 |
| qe (mg·g−1) | 10.01 | 2.8547 | 1.1763 | 0.2821 | ||
| Correlation parameters | R2 | 0.9807 | 0.9907 | 0.9756 | 0.9352 | |
| RSS | 4.4500 | 0.1818 | 8.84·10−2 | 1.02·10−2 | ||
| Elovich | Kinetic parameters | α (h·mg·g−1) | 184.91 | 122.09 | 349.45 | 193,123 |
| β (g·mg−1) | 0.7049 | 2.3469 | 7.1582 | 62.50 | ||
| Correlation parameters | R2 | 0.9813 | 0.9419 | 0.8505 | 0.9538 | |
| RSS | 1.9938 | 0.5943 | 0.1922 | 6.57·10−4 | ||
| IP | Kinetic parameters: Step 1 | ki,1 (mg·g−1·min−0.5) | 5.6060 | 3.5932 | 2.2945 | 9.59·10−2 |
| Ci,1 (mg·g−1) | 1.3471 | 6.38·10−2 | 2.93·10−2 | 0.1849 | ||
| Correlation parameters | R2 | 0.9939 | 0.9999 | 0.9999 | 0.9968 | |
| RSS | 9.16·10−2 | 8.33·10−9 | 1.76·10−9 | 1.19·10−2 | ||
| Kinetic parameters: Step 2 | ki,2 (mg·g−1·min−0.5) | 2.6813 | 0.8322 | 0 | −3·10−16 | |
| Ci,2 (mg·g−1) | 4.1438 | 1.9710 | 1.1700 | 0.2803 | ||
| Correlation parameters | R2 | 0.9999 | 0.9999 | 0.9968 | 0.9999 | |
| RSS | 8.33·10−9 | 1.76·10−9 | 1.19·10−2 | 4.99·10−10 | ||
| Kinetic parameters: Step 3 | ki,3 (mg·g−1·min−0.5) | 0 | 0 | |||
| Ci,3 (mg·g−1) | 9.5065 | 2.8032 | ||||
| Correlation parameters | R2 | 0.9999 | 0.9968 | |||
| RSS | 1.76·10−9 | 1.19·10−2 |
Appendix C
| Kinetic Model | Initial [Cr(VI)] | (mg·L−1) | 0.1 | 1 | 5 | 10 |
|---|---|---|---|---|---|---|
| PFO | Kinetic parameters | k1 (h−1) | 10.56 | 3.3068 | 0.6412 | 0.3066 |
| Correlation parameters | R2 | 0.9953 | 0.9840 | 0.8336 | 0.7091 | |
| RSS | 2.39·10−2 | 5.5219 | 251.84 | 1464.97 | ||
| PSO | Kinetic parameters | k2 (mg·g−1·h−1) | 47.28 | 1.3056 | 0.2626 | 0.1014 |
| qe (mg·g−1) | 1.1711 | 10.37 | 21.11 | 34.59 | ||
| Correlation parameters | R2 | 0.9638 | 0.9886 | 0.9544 | 0.8993 | |
| RSS | 0.1335 | 2.6428 | 33.97 | 227.94 | ||
| Elovich | Kinetic parameters | α (h·mg·g−1) | 46.73 | 5002 | 28,073 | 109,044 |
| β (g·mg−1) | 3.2292 | 0.9992 | 0.5889 | 0.4108 | ||
| Correlation parameters | R2 | 0.7859 | 0.8232 | 0.9370 | 0.9650 | |
| RSS | 2.7155 | 9.8123 | 7.3288 | 9.0262 | ||
| IP | Kinetic parameters: Step 1 | ki,1 (mg·g−1·min−0.5) | 2.2945 | 16.61 | 3.4953 | 2.6270 |
| Ci,1 (mg·g−1) | 2.93·10−2 | −0.7973 | 11.18 | 20.58 | ||
| Correlation parameters | R2 | 0.9995 | 1.0000 | 1.0000 | 0.9284 | |
| RSS | 3.49·10−4 | 6.79·10−10 | 1.05·10−7 | 9.67·10−2 | ||
| Kinetic parameters: Step 2 | ki,2 (mg·g−1·min−0.5) | 0 | 4.8746 | 14.98 | 16.844 | |
| Ci,2 (mg·g−1) | 1.17 | 5.2377 | 3.0577 | 10.66 | ||
| Correlation parameters | R2 | 0.9999 | 0.9800 | 1.0000 | 1.0000 | |
| RSS | 3.68·10−9 | 0.1236 | 7.21·10−8 | 4.16·10−7 | ||
| Kinetic parameters: Step 3 | ki,3 (mg·g−1·min−0.5) | 6.79·10−2 | 0.6576 | 1.7653 | ||
| Ci,3 (mg·g−1) | 10.04 | 17.93 | 25.60 | |||
| Correlation parameters | R2 | 0.698 | 0.9985 | 0.9990 | ||
| RSS | 3.98·10−2 | 0.8894 | 5.36·10−2 |
| Kinetic Model | Initial [Cr(VI)] | (mg·L−1) | 25 | 50 | 70 |
|---|---|---|---|---|---|
| PFO | Kinetic parameters | k1 (h−1) | 0.2233 | 0.3162 | 0.4961 |
| Correlation parameters | R2 | 0.8524 | 0.6837 | 0.7066 | |
| RSS | 707.21 | 1407.73 | 1969.33 | ||
| PSO | Kinetic parameters | k2 (mg·g−1·h−1) | 38.81 | 36.74 | 44.77 |
| qe (mg·g−1) | 3.22·10−2 | 9.32·10−2 | 6.41·10−2 | ||
| Correlation parameters | R2 | 0.9507 | 0.9549 | 0.8838 | |
| RSS | 109.16 | 98.19 | 471.17 | ||
| Elovich | Kinetic parameters | α (h·mg·g−1) | 594 | 570,868 | 150947 |
| β (g·mg−1) | 0.2177 | 0.4309 | 0.3233 | ||
| Correlation parameters | R2 | 0.7859 | 0.8232 | 0.9370 | |
| RSS | 2.7086 | 27.24 | 295.94 | ||
| IP | Kinetic parameters: Step 1 | ki,1 (mg·g−1·min−0.5) | 35.31 | 19.42 | 34.60 |
| Ci,1 (mg·g−1) | −3.0290 | 14.96 | 13.90 | ||
| Correlation parameters | R2 | 1.0000 | 1.0000 | 1.0000 | |
| RSS | 7.65·10−8 | 5.82·10−9 | 1.45·10−7 | ||
| Kinetic parameters: Step 2 | ki,2 (mg·g−1·min−0.5) | 0 | 0 | 2.8476 | |
| Ci,2 (mg·g−1) | 18.31 | 27.09 | 30.11 | ||
| Correlation parameters | R2 | 0.9999 | 0.9999 | 0.9890 | |
| RSS | 1.41·10−10 | 3.18·10−9 | 2.3781 | ||
| Kinetic parameters: Step 3 | ki,3 (mg·g−1·min−0.5) | 4.0010 | 1.9211 | ||
| Ci,3 (mg·g−1) | 18.31 | 27.09 | |||
| Correlation parameters | R2 | 0.9985 | 0.9950 | ||
| RSS | 0.4068 | 0.3077 |
Appendix D
| Kinetic Model | Temperature | (°C) | 17 | 30 | 42 | 56 |
|---|---|---|---|---|---|---|
| PSO | Kinetic parameters | k2 (mg·g−1·h−1) | 0.9243 | 2.5799 | 5.3297 | 8.4657 |
| qe (mg·g−1) | 10.63 | 10.45 | 10.38 | 10.36 | ||
| Correlation parameters | R2 | 0.9961 | 0.9872 | 0.9939 | 0.9997 | |
| RSS | 0.7617 | 2.3274 | 1.0576 | 6.13·10−2 | ||
| Arrhenius equation | Parameters | Values | ||||
| Activation Energy Preexponential factor | EA (J·mol−1) | 45,447 | ||||
| k0 (mg·g−1·h−1) | 1.59·108 | |||||
| Correlation parameters | R2 | 0.9779 | ||||
| RSS | 6.18·10−2 | |||||
Appendix E
| Kinetic Model | Real Wastewater Treatment | ||
|---|---|---|---|
| Pseudo-first order | Kinetic parameters | k1 (h−1) | 2.9262 |
| Correlation parameters | R2 | 0.9920 | |
| RSS | 0.4067 | ||
| PSO | Kinetic parameters | k2 (mg·g−1·h−1) | 1.1405 |
| qe (mg·g−1) | 4.1621 | ||
| Correlation parameters | R2 | 0.9897 | |
| RSS | 0.3201 | ||
| Elovich | Kinetic parameters | α (h·mg·g−1) | 67.42 |
| β (g·mg−1) | 1.3789 | ||
| Correlation parameters | R2 | 0.9730 | |
| RSS | 0.4487 | ||
| IP | Kinetic parameters: Step 1 | ki,1 (mg·g−1·min−0.5) | 3.4863 |
| Ci,1 (mg·g−1) | 0.1324 | ||
| Correlation parameters | R2 | 0.9983 | |
| RSS | 5.03·10−2 | ||
| Kinetic parameters: Step 2 | ki,2 (mg·g−1·min−0.5) | 0.9718 | |
| Ci,2 (mg·g−1) | 2.5269 | ||
| Correlation parameters | R2 | 0.9999 | |
| RSS | 5.36·10−11 | ||
| Kinetic parameters: Step 3 | ki,3 (mg·g−1·min−0.5) | 0.1801 | |
| Ci,3 (mg·g−1) | 3.4965 | ||
| Correlation parameters | R2 | 0.9999 | |
| RSS | 1.20·10−9 |
References
- Zhang, Y.; Zheng, P.; Su, Z.; Hu, G.; Jia, G. Perspectives of Genetic Damage and Epigenetic Alterations by Hexavalent Chromium: Time Evolution Based on a Bibliometric Analysis. Chem. Res. Toxicol. 2021, 34, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.T.; Bani Mfarrej, M.F.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of chromium in plants and its repercussion in animals and humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Yasir, M.W.; Siddique, M.B.A.; Shabbir, Z.; Ullah, H.; Riaz, L.; Nisa, W.-U.; ur Rahman, S.; Shah, A.A. Biotreatment potential of co-contaminants hexavalent chromium and polychlorinated biphenyls in industrial wastewater: Individual and simultaneous prospects. Sci. Total Environ. 2021, 779, 146345. [Google Scholar] [CrossRef]
- Kerur, S.S.; Bandekar, S.; Hanagadakar, M.S.; Nandi, S.S.; Ratnamala, G.M.; Hegde, P.G. Removal of hexavalent Chromium-Industry treated water and Wastewater: A review. Mater. Today Proc. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- USEPA. IRIS Toxicological Review of Hexavalent Chromium (2010 External Review Draft). 2010. Available online: https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=221433 (accessed on 25 October 2022).
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; et al. A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 2022, 809, 152055. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- González-López, M.E.; Laureano-Anzaldo, C.M.; Pérez-Fonseca, A.A.; Arellano, M.; Robledo-Ortíz, J.R. Chemically Modified Polysaccharides for Hexavalent Chromium Adsorption. Sep. Purif. Rev. 2021, 50, 333–362. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium immobilization in soil using quaternary ammonium cations modified montmorillonite: Characterization and mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Balea, A.; Fuente, E.; Monte, M.C.; Merayo, N.; Campano, C.; Negro, C.; Blanco, A. Industrial Application of Nanocelluloses in Papermaking: A Review of Challenges, Technical Solutions, and Market Perspectives. Molecules 2020, 25, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, L.; Jin, L.; Huang, L.; He, Y.; Tang, J.; Yang, W.; Wang, H. In-situ functionalization of poly(m-phenylenediamine) nanoparticles on bacterial cellulose for chromium removal. Chem. Eng. J. 2018, 344, 441–452. [Google Scholar] [CrossRef]
- Zeng, H.; Hu, Z.; Peng, C.; Deng, L.; Liu, S. Effective Adsorption and Sensitive Detection of Cr(VI) by Chitosan/Cellulose Nanocrystals Grafted with Carbon Dots Composite Hydrogel. Polymers 2021, 13, 3788. [Google Scholar] [CrossRef]
- Ojembarrena, F.d.B.; Sánchez-Salvador, J.L.; Mateo, S.; Balea, A.; Blanco, A.; Merayo, N.; Negro, C. Modeling of Hexavalent Chromium Removal with Hydrophobically Modified Cellulose Nanofibers. Polymers 2022, 14, 3425. [Google Scholar] [CrossRef]
- Yang, H.; van de Ven, T.G.M. Preparation of hairy cationic nanocrystalline cellulose. Cellulose 2016, 23, 1791–1801. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, W.; Cai, J.; Cheng, S.-Y.; Ding, W.-P. Citric acid-incorporated cellulose nanofibrous mats as food materials-based biosorbent for removal of hexavalent chromium from aqueous solutions. Int. J. Biol. Macromol. 2020, 149, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lim, S.-R.; Yun, Y.-S.; Park, J.M. Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosphere 2007, 70, 298–305. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods: 3500-Cr CHROMIUM. Chromium by colororimetry. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Petzold, G.; Schwarz, S.; Buchhammer, H.-M.; Lunkwitz, K. A very effective method for the cationic modification of cellulose. Angew. Makromol. Chem. 1997, 253, 1–15. [Google Scholar] [CrossRef]
- Balea, A.; Sanchez-Salvador, J.L.; Monte, M.C.; Merayo, N.; Negro, C.; Blanco, A. In Situ Production and Application of Cellulose Nanofibers to Improve Recycled Paper Production. Molecules 2019, 24, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campano, C.; Lopez-Exposito, P.; Blanco, A.; Negro, C.; van de Ven, T.G.M. Hairy cationic nanocrystalline cellulose as a novel flocculant of clay. J. Colloid Interf. Sci. 2019, 545, 153–161. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, Á. Low-fibrillated bacterial cellulose nanofibers as a sustainable additive to enhance recycled paper quality. Int. J. Biol. Macromol. 2018, 114, 1077–1083. [Google Scholar] [CrossRef]
- Duranoğlu, D.; Trochimczuk, A.W.; Beker, U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chem. Eng. J. 2012, 187, 193–202. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, M.; Park, D.; Park, H. Use of a Low-Energy Electron Beam for Degree of Polymerization Control of Cotton Linter. Ind. Eng. Chem. Res. 2013, 52, 692–695. [Google Scholar] [CrossRef]
- Lourenço, A.; Araujo, S.; Gominho, J.; Evtuguin, D. Cellulose Structural Changes during Mild Torrefaction of Eucalyptus Wood. Polymers 2020, 12, 2831. [Google Scholar] [CrossRef]
- Otoni, C.G.; Figueiredo, J.S.L.; Capeletti, L.B.; Cardoso, M.B.; Bernardes, J.S.; Loh, W. Tailoring the Antimicrobial Response of Cationic Nanocellulose-Based Foams through Cryo-Templating. ACS Appl. Bio Mater. 2019, 2, 1975–1986. [Google Scholar] [CrossRef]
- Wahlström, R.; Kalliola, A.; Heikkinen, J.; Kyllönen, H.; Tamminen, T. Lignin cationization with glycidyltrimethylammonium chloride aiming at water purification applications. Ind. Crops Prod. 2017, 104, 188–194. [Google Scholar] [CrossRef]
- Bharimalla, A.; Deshmukh, S.; Patil, P.G.; Nadanathangam, V. Micro/nano-fibrillated cellulose from cotton linters as strength additive in unbleached kraft paper: Experimental, semi-empirical, and mechanistic studies. BioResources 2017, 12, 5682–5696. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Luo, J.; Qin, W.; Tong, Z. Elemental analysis, chemical composition, cellulose crystallinity, and FT-IR spectra of Toona sinensis wood. Mon. Für Chem. Chem. Mon. 2014, 145, 175–185. [Google Scholar] [CrossRef]
- Qiu, B.; Gu, H.; Yan, X.; Guo, J.; Wang, Y.; Sun, D.; Wang, Q.; Khan, M.; Zhang, X.; Weeks, B.L.; et al. Cellulose derived magnetic mesoporous carbon nanocomposites with enhanced hexavalent chromium removal. J. Mater. Chem. A 2014, 2, 17454–17462. [Google Scholar] [CrossRef]
- Peng, X.; Yan, Z.; Hu, L.; Zhang, R.; Liu, S.; Wang, A.; Yu, X.; Chen, L. Adsorption behavior of hexavalent chromium in aqueous solution by polyvinylimidazole modified cellulose. Int. J. Biol. Macromol. 2020, 155, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Aigbe, U.O.; Osibote, O.A. A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J. Environ. Chem. Eng. 2020, 8, 104503. [Google Scholar] [CrossRef]
- Pourfadakari, S.; Jorfi, S.; Ahmadi, M.; Takdastan, A. Experimental data on adsorption of Cr(VI) from aqueous solution using nanosized cellulose fibers obtained from rice husk. Data Brief 2017, 15, 887–895. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Tran, H.N.; Juang, R.-S. Revisiting temperature effect on the kinetics of liquid–phase adsorption by the Elovich equation: A simple tool for checking data reliability. J. Taiwan Inst. Chem. Eng. 2022, 136, 104403. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Szafran, R.G.; Modelski, S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 2011, 265, 126–134. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.-S.; Chen, M.-L. Application of the Elovich Equation to the Kinetics of Metal Sorption with Solvent-Impregnated Resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Su, Z.; Tu, Y.; Wang, J.; Liu, S.; Liu, J.; Jiang, T. The comprehensive investigation on removal mechanism of Cr(VI) by humic acid-Fe(II) system structured on V, Ti-bearing magnetite surface. Adv. Powder Technol. 2021, 32, 37–51. [Google Scholar] [CrossRef]
- Popović, M.; Veličković, Z.S.; Bogdanov, J.; Marinković, A.D.; Casas Luna, M.; Trajković, I.; Obradović, N.; Pavlović, V. Removal of the As (V) and Cr (VI) from the Water Using Magnetite/3D-Printed Wollastonite Hybrid Adsorbent. Sci. Sinter. 2022, 54, 105–124. [Google Scholar] [CrossRef]
- Nollet, H.; Roels, M.; Lutgen, P.; Van der Meeren, P.; Verstraete, W. Removal of PCBs from wastewater using fly ash. Chemosphere 2003, 53, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, A.; Singh, S.K. Critical analysis of adsorption data statistically. Appl. Water Sci. 2017, 7, 3191–3196. [Google Scholar] [CrossRef] [Green Version]
- Shahnaz, T.; Fazil, S.M.M.; Padmanaban, V.C.; Narayanasamy, S. Surface modification of nanocellulose using polypyrrole for the adsorptive removal of Congo red dye and chromium in binary mixture. Int. J. Biol. Macromol. 2020, 151, 322–332. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Katubi, K.M.; Alzahrani, F.M.; Amari, A.; Osman, H.; Rebah, F.B.; Tahoon, M.A. Synthesis, Characterization and Application of Polypyrrole Functionalized Nanocellulose for the Removal of Cr(VI) from Aqueous Solution. Polymers 2021, 13, 3691. [Google Scholar] [CrossRef]
- Liu, C.; Jin, R.-N.; Ouyang, X.-k.; Wang, Y.-G. Adsorption behavior of carboxylated cellulose nanocrystal—Polyethyleneimine composite for removal of Cr(VI) ions. Appl. Surf. Sci. 2017, 408, 77–87. [Google Scholar] [CrossRef]
- Xu, Q.H.; Wang, Y.L.; Jin, L.Q.; Wang, Y.; Qin, M.H. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Rao, D.G.; Senthikumar, R.; Byrne, J.A.; Feroz, S. Wastewater Treatment: Advanced Processes and Technologies; IWA Publishing: London, UK, 2012. [Google Scholar]
- Peng, J.; Yuan, H.; Ren, T.; Liu, Z.; Qiao, J.; Ma, Q.; Guo, X.; Ma, G.; Wu, Y. Fluorescent nanocellulose-based hydrogel incorporating titanate nanofibers for sorption and detection of Cr(VI). Int. J. Biol. Macromol. 2022, 215, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Varshney, S.; Srivastava, S. Functionalized nanobiomaterials: High-performance sorbents for chromium remediation from water streams. Int. J. Environ. Sci. Technol. 2016, 13, 2893–2904. [Google Scholar] [CrossRef]
- Ren, T.; Peng, J.; Yuan, H.; Liu, Z.; Li, Q.; Ma, Q.; Li, X.; Guo, X.; Wu, Y. Nanocellulose-based hydrogel incorporating silver nanoclusters for sensitive detection and efficient removal of hexavalent chromium. Eur. Polym. J. 2022, 175, 111343. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D. Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J. Environ. Chem. Eng. 2018, 6, 2335–2343. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Boaventura, R.A.R.; Vilar, V.J.P. As(III) and Cr(VI) oxyanion removal from water by advanced oxidation/reduction processes—A review. Environ. Sci. Pollut. Res. 2019, 26, 2203–2227. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.A.; Lakshmipathy, R.; Sarada, N.C. Application of Citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution. Alex. Eng. J. 2014, 53, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, G.-R.R.; Rene, R.-M.J.; Catalina, A.-D.L.T.M.; Ma. Catalina, A.-D.l.T. Chromium (III) uptake by agro-waste biosorbents: Chemical characterization, sorption–desorption studies, and mechanism. J. Hazard. Mater. 2009, 170, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- Kumar, N.; Kardam, A.; Rajawat, D.S.; Jain, V.K.; Suman. Carboxymethyl nanocellulose stabilized nano zero-valent iron: An effective method for reduction of hexavalent chromium in wastewater. Mater. Res. Express 2019, 6, 1150f1153. [Google Scholar] [CrossRef]
- Dupont, L.; Guillon, E. Removal of Hexavalent Chromium with a Lignocellulosic Substrate Extracted from Wheat Bran. Environ. Sci. Technol. 2003, 37, 4235–4241. [Google Scholar] [CrossRef]
- Labied, R.; Benturki, O.; Eddine Hamitouche, A.Y.; Donnot, A. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): Kinetic, equilibrium, and thermodynamic study. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef]


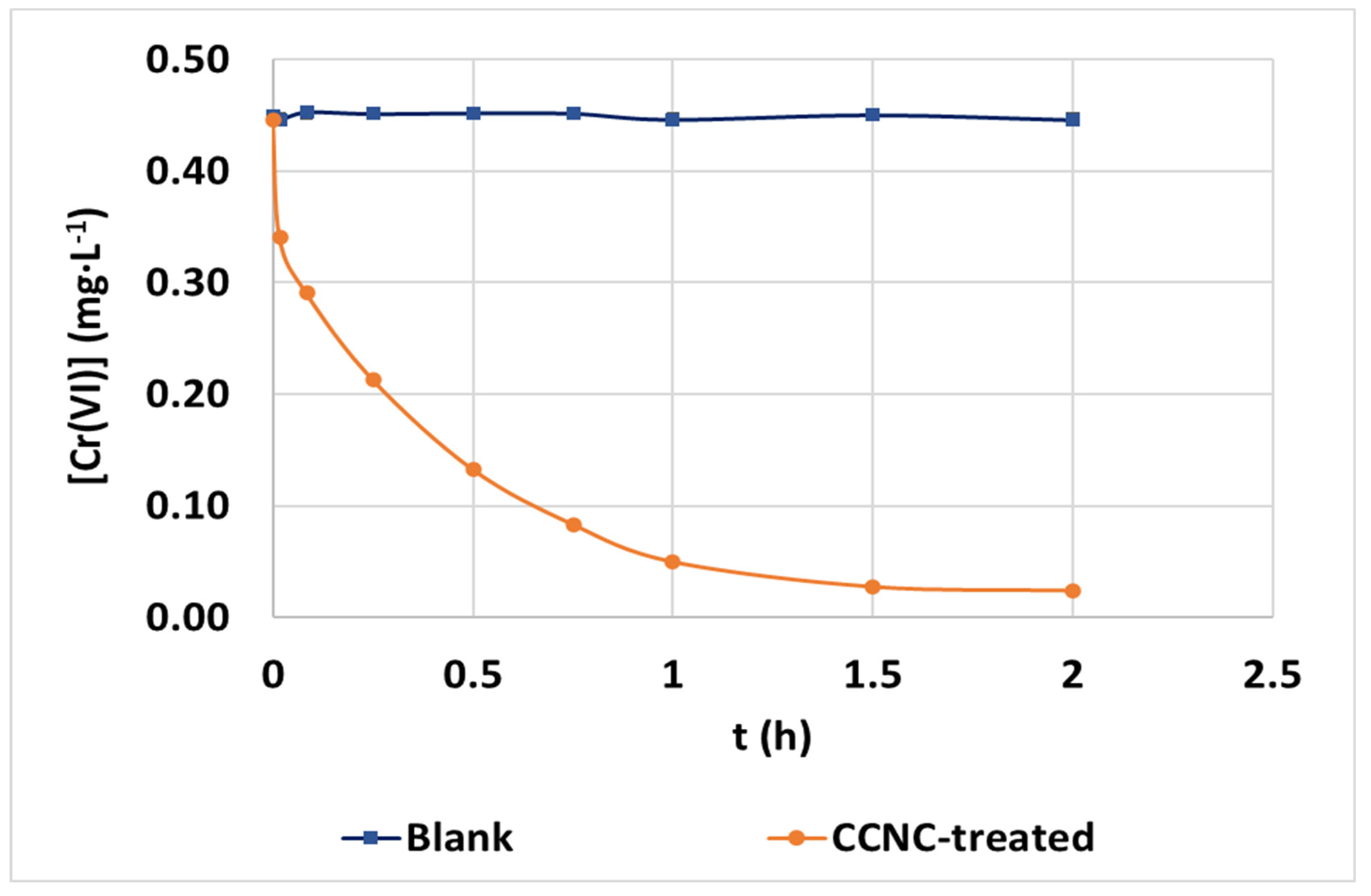
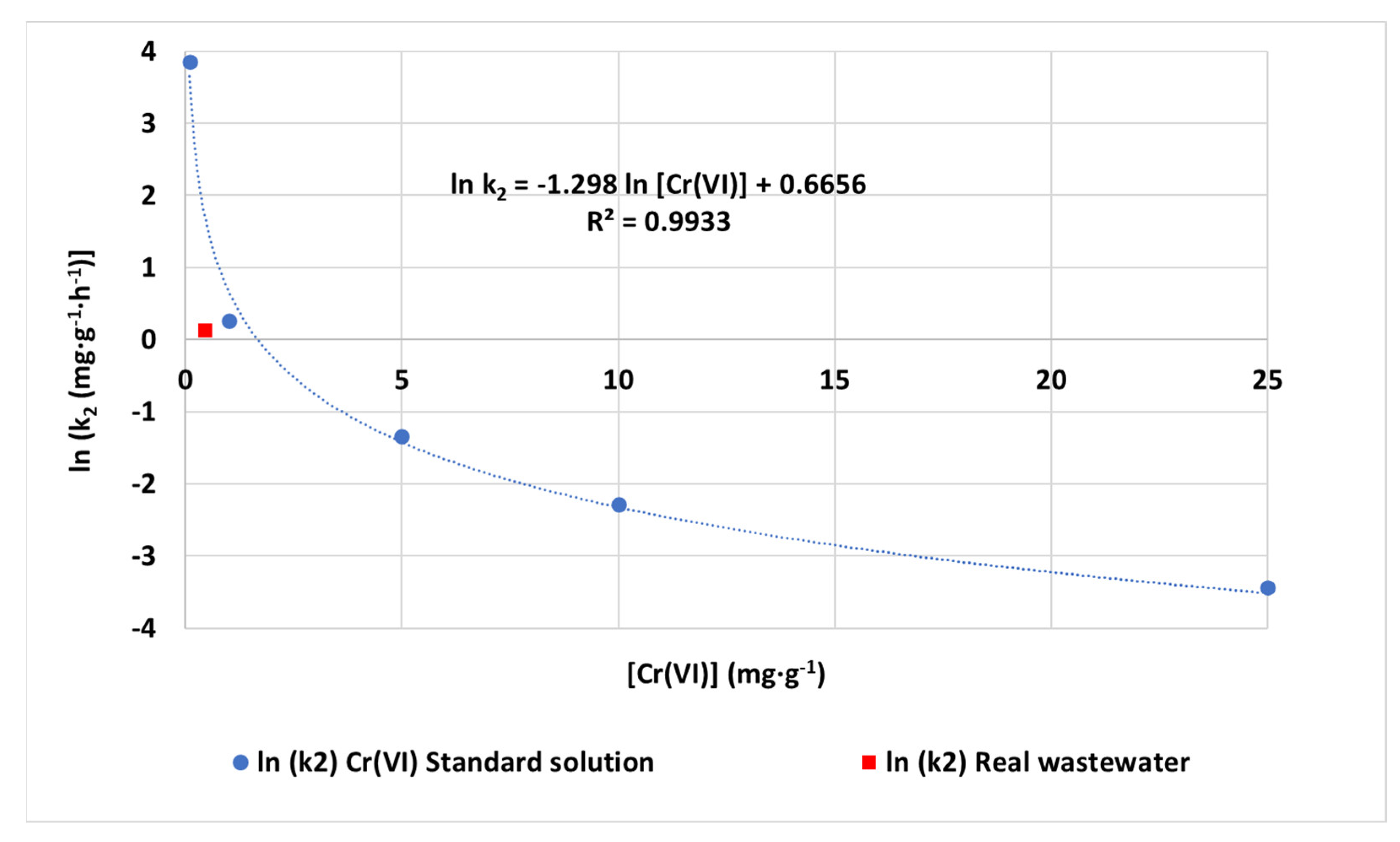
| Model | Parameters | Values |
|---|---|---|
| Langmuir | Isotherm parameters | kL (L·mg−1) = 0.6103 qe,L (mg·g−1) = 42.02 RL (C0 = 0.1 mg·L−1) (-) = 2.29·10−2 RL (C0 = 70 mg·L−1) (-) = 0.9428 |
| Correlation parameters | R2 = 0.9636 RSS = 160.63 | |
| Freundlich | Isotherm parameters | kF (mg(1−1/nF)·L−(1/nF)·g−1) = 19.7944 nF (-) = 8.4674 |
| Correlation parameters | R2 = 0.9648 RSS = 346.55 | |
| D-R | Isotherm parameters | BDR (mol2·J−2) = 8.95·10−7 qmax (mg·g−1) = 33.3948 |
| Thermodynamic parameters | EDR (J·mol−1) = 747.53 | |
| Correlation parameters | R2 = 0.9481 RSS = 275.94 | |
| Temkin | Isotherm parameters | BT (J·mol−1) = 5.7916 bT (J·mol−1) = 416.52 AT (L·g−1) = 23.6462 |
| Correlation parameters | R2 = 0.9441 RSS = 273.18 | |
| Sips | Isotherm parameters | nS (-) = 4.2882 kS (L(1/nS)·mol−(1/nS)) = 0.2408 qe,S (mg·g−1)= 108.54 |
| Correlation parameters | R2 = 0.9787 RSS = 65.87 |
| Parameter | Units | Values |
|---|---|---|
| [Cr(VI)]sol | (mg·L−1) | 0.450 ± 2.8·10−3 |
| CODsol | (mg O2·L−1) | 82.67 ± 4.73 |
| pH | 7.50 ± 0.21 | |
| EC | (mS·cm−1) | 8.88 ± 0.81 |
| Adsorbent | Contact Time (min) | Adsorbent Dosage (mg·L−1) | Initial [Cr(VI)] (mg·L−1) | pH | qmax (mg·g−1) | Maximum Removal Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| EPTMAC-modified CNC | 60 | 1000 | 25 | 2.5 | 22.99 | 96.0 | [55] |
| Black wattle tannin-immobilized CNC | 300 | 500 | 150 | 2 | 104.59 | [52] | |
| Poly(m-phenylenediamine)-modified BC nanoparticles | 240 | 400 | 500 | 3 | 434.78 | [18] | |
| Carboxymethyl NC-stabilized nZVI | 180 | 300 | 15 | 2–3 | 87.71 | 100 | [62] |
| Polypyrrole-modified CNC | 60 | 500 | 10 | 2 | 12.67 | 80 | [49] |
| Hydrophobized CNF | 330 | 500 | 50 | 3 | 70.38 | >97.14 | [20] |
| Humic acid-Fe(II) system structured on V, Ti-bearing magnetite surface | 700 | 100 | 10 | 2 | 3.67 | 90 | [45] |
| Rice husk powder | 60 | 2500 | 25 | 6 | 1.4 | 87.12 | [43] |
| Lignocellulosic substrate extracted from wheat bran | 1440 | 8000 | 20 | 2.5 | 35 | [63] | |
| Activated carbon synthetized from Z. jujuba | 360 | 1000 | 100 | 2 | 62 | 49.6 | [64] |
| CCNC | 5 | 100 | 70 | 3 | 44.36 | 100 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Borja Ojembarrena, F.; Sammaraie, H.; Campano, C.; Blanco, A.; Merayo, N.; Negro, C. Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals. Nanomaterials 2022, 12, 4172. https://doi.org/10.3390/nano12234172
de Borja Ojembarrena F, Sammaraie H, Campano C, Blanco A, Merayo N, Negro C. Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals. Nanomaterials. 2022; 12(23):4172. https://doi.org/10.3390/nano12234172
Chicago/Turabian Stylede Borja Ojembarrena, Francisco, Hassan Sammaraie, Cristina Campano, Angeles Blanco, Noemi Merayo, and Carlos Negro. 2022. "Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals" Nanomaterials 12, no. 23: 4172. https://doi.org/10.3390/nano12234172
APA Stylede Borja Ojembarrena, F., Sammaraie, H., Campano, C., Blanco, A., Merayo, N., & Negro, C. (2022). Hexavalent Chromium Removal from Industrial Wastewater by Adsorption and Reduction onto Cationic Cellulose Nanocrystals. Nanomaterials, 12(23), 4172. https://doi.org/10.3390/nano12234172









