Evaluation of Photocatalytic Performance of Nano-Sized Sr0.9La0.1TiO3 and Sr0.25Ca0.25Na0.25Pr0.25TiO3 Ceramic Powders for Water Purification
Abstract
:1. Introduction
2. Experimental and Computational Details
2.1. Chemicals and Solutions
2.2. Materials Synthesis
2.3. Characterization Methods
2.4. Photocatalytic Activity
2.5. Analytical Procedures
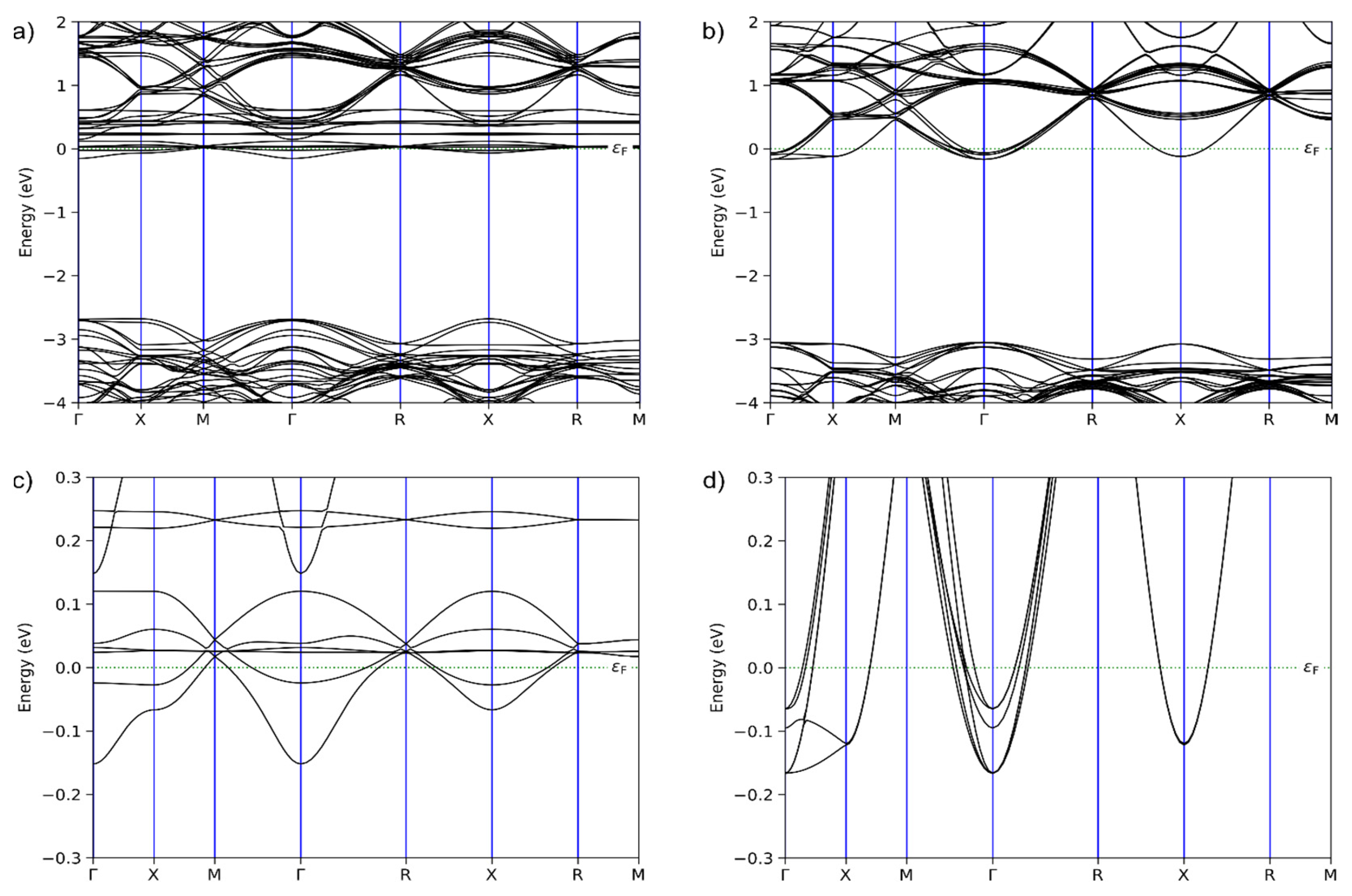
2.6. Computational Methods
3. Results and Discussion
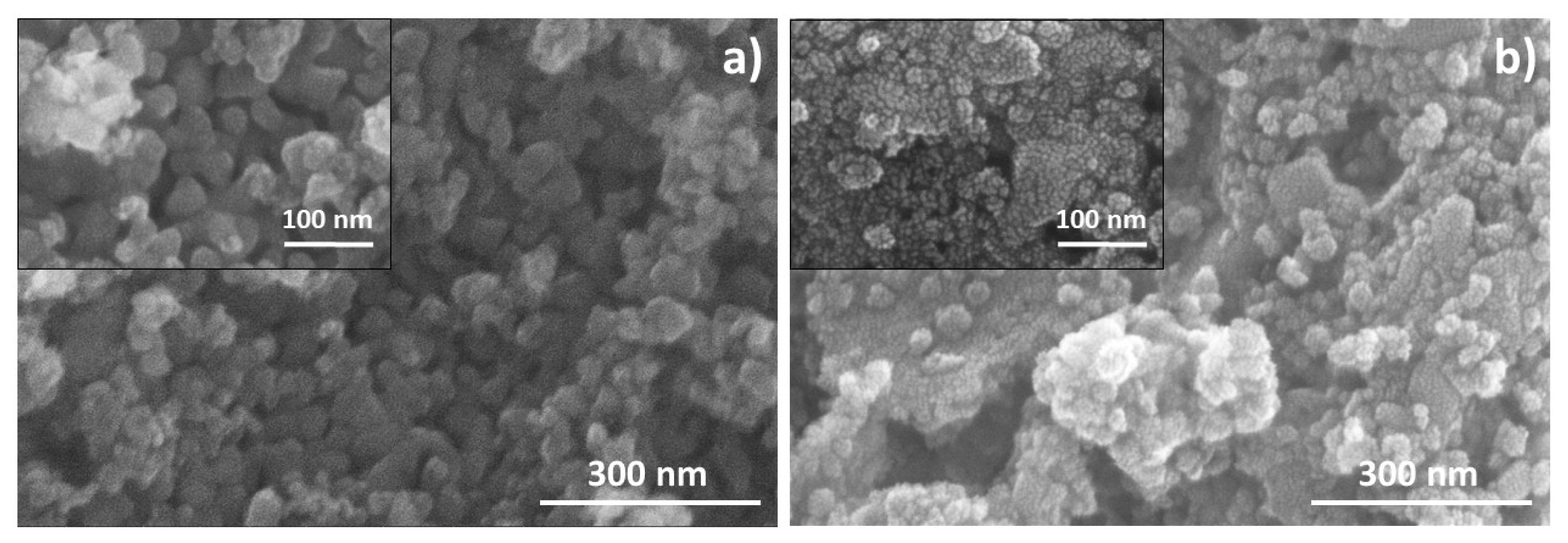
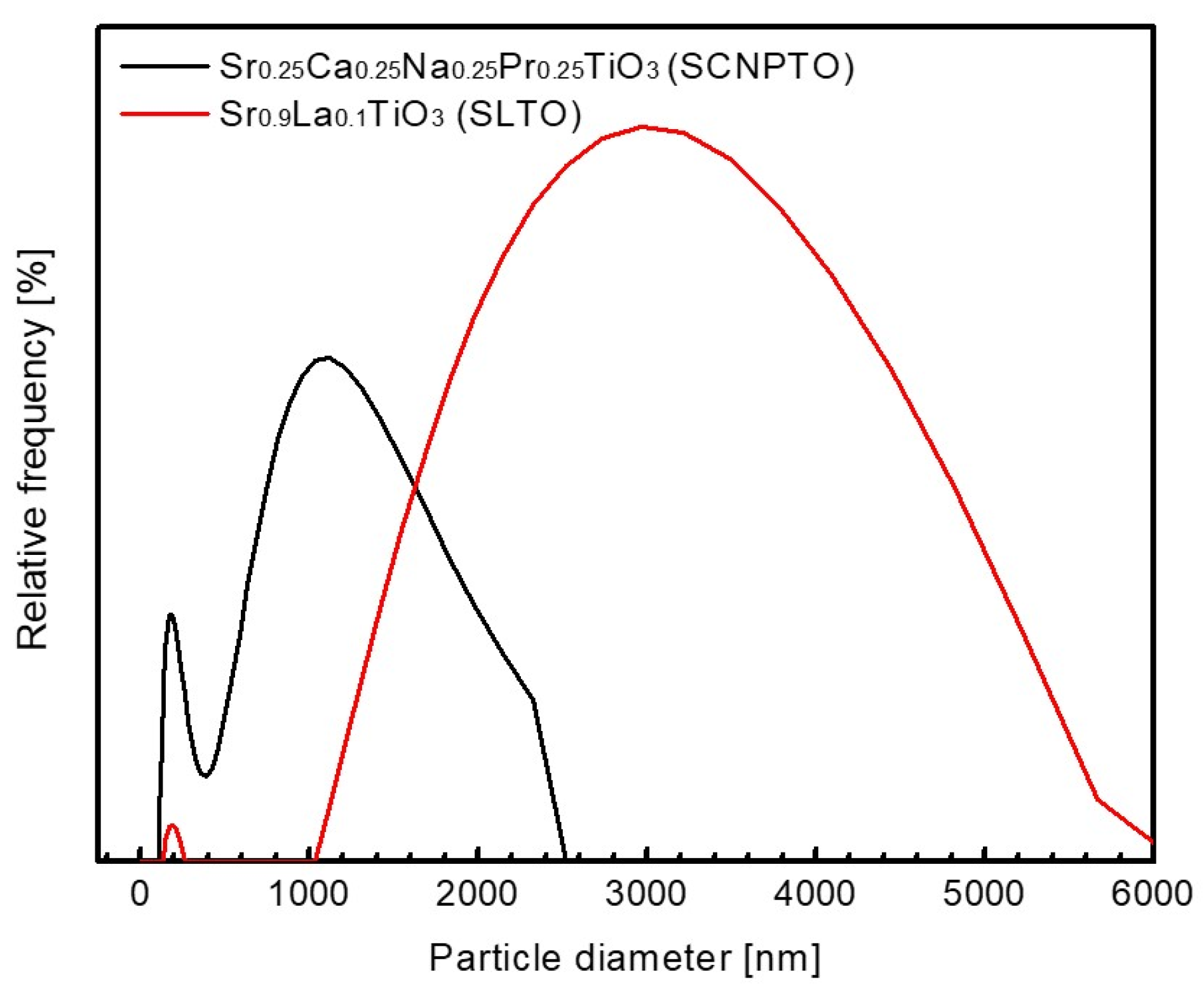
3.1. Powder Characteristics
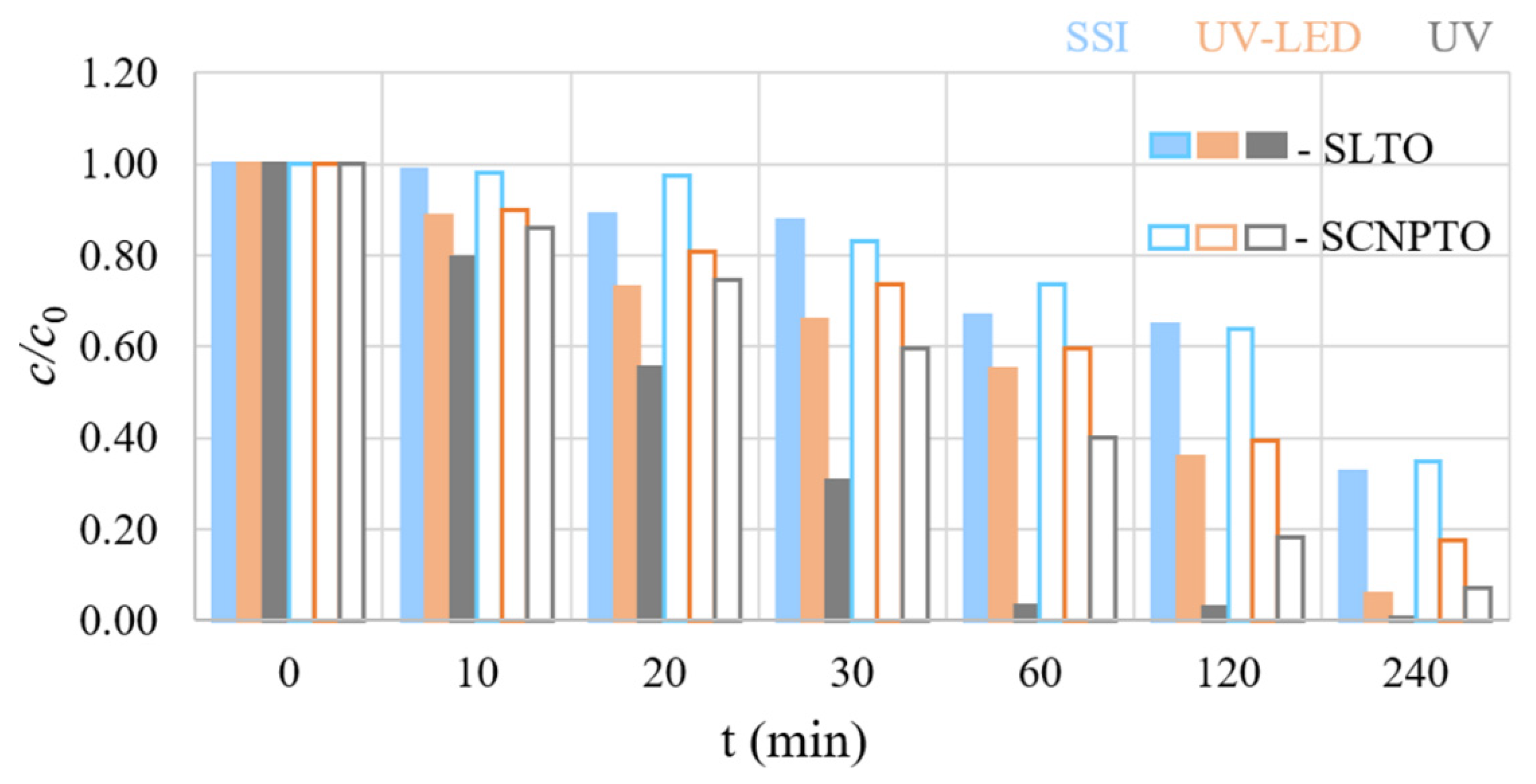
3.2. Degradation Efficiency

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, M.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Simultaneous determination of pindolol, acebutolol and metoprolol in waters by differential-pulse voltammetry using an efficient sensor based on carbon paste electrode modified with amino-functionalized mesostructured silica. Sens. Actuators B Chem. 2019, 283, 434–442. [Google Scholar] [CrossRef]
- Ngqwala, N.P.; Muchesa, P. Occurrence of pharmaceuticals in aquatic environments: A review and potential impacts in South Africa. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhao, M.; Bi, X.; Sun, L.; Yu, X.; Zhao, M.; Zang, W. Novel strategies and underlying protective mechanisms of modulation of vagal activity in cardiovascular diseases. J. Cereb. Blood Flow Metab. 2015, 172, 5489–5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part II. Ecotoxicity study. Sci. Total Environ. 2014, 493, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part I. Mobility and hydrolysis study. Sci. Total Environ. 2014, 493, 1112–1121. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Giebułtowicz, J.; Stankiewicz, U.; Wroczyński, P.; Nałęcz-Jawecki, G. Determination of Selected Cardiovascular Active Compounds in Environmental Aquatic Samples–Methods and Results, a Review of Global Publications from the Last 10 Years. Chemosphere 2015, 138, 642–656. [Google Scholar] [CrossRef]
- Kim, K.; Cho, E.; Choi, J.M.; Kim, H.; Jang, A.; Choi, Y.; Lee, I.S.; Yu, J.-H.; Jung, S. Intermolecular complexation of low-molecular-weight succinoglycans directs solubility enhancement of pindolol. Carbohydr. Polym. 2014, 106, 101–108. [Google Scholar] [CrossRef]
- Martinez, D.; Broft, A.; Laruelle, M. Pindolol augmentation of antidepressant treatment: Recent contributions from brain imaging studies. Biol. Psychiatry 2000, 48, 844–853. [Google Scholar] [CrossRef]
- Martinez, D.; Mawlawi, O.; Hwang, D.-R.; Kent, J.; Simpson, N.; Parsey, R.V.; Hashimoto, T.; Slifstein, M.; Huang, Y.; Van Heertum, R.; et al. Positron emission tomography study of pindolol occupancy of 5-HT1A receptors in humans: Preliminary analyses. Nucl. Med. Biol. 2000, 27, 523–527. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- Armaković, S.J.; Armaković, S.; Četojević-Simin, D.D.; Šibul, F.; Abramović, B.F. Photocatalytic degradation of 4-amino-6-chlorobenzene-1,3-disulfonamide stable hydrolysis product of hydrochlorothiazide: Detection of intermediates and their toxicity. Environ. Pollut. 2018, 233, 916–924. [Google Scholar] [CrossRef]
- Du, H.; Wan, T.; Qu, B.; Scott, J.; Lin, X.; Younis, A.; Chu, D. Tailoring the multi-functionalities of one-dimensional ceria nanostructures via oxygen vacancy modulation. J. Colloid Interface Sci. 2017, 504, 305–314. [Google Scholar] [CrossRef]
- Ham, Y.; Hisatomi, T.; Goto, Y.; Moriya, Y.; Sakata, Y.; Yamakata, A.; Kubota, J.; Domen, K. Flux-mediated doping of SrTiO3 photocatalysts for efficient overall water splitting. J. Mater. Chem. A 2015, 4, 3027–3033. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Qiu, X.; Dong, H.; Zhou, X. Trends in non-metal doping of the SrTiO3 surface: A hybrid density functional study. Phys. Chem. Chem. Phys. 2015, 17, 21611–21621. [Google Scholar] [CrossRef]
- Reunchan, P.; Ouyang, S.; Umezawa, N.; Xu, H.; Zhang, Y.; Ye, J. Theoretical design of highly active SrTiO3-based photocatalysts by a codoping scheme towards solar energy utilization for hydrogen production. J. Mater. Chem. A 2013, 1, 4221–4227. [Google Scholar] [CrossRef] [Green Version]
- Le, M.-V.; Vo, N.-Q.-D.; Le, Q.-C.; Tran, V.A.; Phan, T.-Q.-P.; Huang, C.-W.; Nguyen, V.-H. Manipulating the Structure and Characterization of Sr1−Xlaxtio3 Nanocubes toward the Photodegradation of 2-Naphthol under Artificial Solar Light. Catalysts 2021, 11, 564. [Google Scholar] [CrossRef]
- Kuang, Q.; Yang, S. Template Synthesis of Single-Crystal-Like Porous SrTiO3Nanocube Assemblies and Their Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2013, 5, 3683–3690. [Google Scholar] [CrossRef]
- Chang, C.-W.; Hu, C. Graphene oxide-derived carbon-doped SrTiO3 for highly efficient photocatalytic degradation of organic pollutants under visible light irradiation. Chem. Eng. J. 2020, 383, 123116. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Anjaneyulu, O.; Shanker, V. Synthesis of Cr and La-codoped SrTiO3 nanoparticles for enhanced photocatalytic performance under sunlight irradiation. Phys. Chem. Chem. Phys. 2014, 16, 23819–23828. [Google Scholar] [CrossRef]
- Jiang, J.; Jia, Y.; Wang, Y.; Chong, R.; Xu, L.; Liu, X. Insight into Efficient Photocatalytic Elimination of Tetracycline over SrTiO3 (La, Cr) under Visible-Light Irradiation: The Relationship of Doping and Performance. Appl. Surf. Sci. 2019, 486, 93–101. [Google Scholar] [CrossRef]
- Park, N.-H.; Dang, F.; Wan, C.; Seo, W.-S.; Koumoto, K. Self-Originating Two-Step Synthesis of Core–Shell Structured La-Doped SrTiO3 Nanocubes. J. Asian Ceram. Soc. 2013, 1, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Geneste, G.; Kiat, J.-M. Ground state of Ca-doped strontium titanate: Ferroelectricity versus polar nanoregions. Phys. Rev. B 2008, 77, 174101. [Google Scholar] [CrossRef]
- Jiao, Y.; Song, S.; Chen, F.; Zeng, X.; Wang, X.; Song, C.; Liu, G.; Yan, Y. Energy Storage Performance of 0.55 Bi0.5Na0.5TiO3-0.45 SrTiO3 Ceramics Doped with Lanthanide Elements (Ln = La, Nd, Dy, Sm) Using a Viscous Polymer Processing Route. Ceram. Int. 2022, 48, 10885–10894. [Google Scholar] [CrossRef]
- Dehkordi, A.M.; Bhattacharya, S.; Darroudi, T.; Graff, J.W.; Schwingenschlögl, U.; Alshareef, H.N.; Tritt, T.M. Large Thermoelectric Power Factor in Pr-Doped SrTiO3−δ Ceramics via Grain-Boundary-Induced Mobility Enhancement. Chem. Mater. 2014, 26, 2478–2485. [Google Scholar] [CrossRef]
- MacroModel Schrödinger Release 2022-1; MacroModel, Schrödinger, LLC: New York, NY, USA, 2022.
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Shivakumar, D.; Williams, J.; Wu, Y.; Damm, W.; Shelley, J.; Sherman, W. Prediction of Absolute Solvation Free Energies using Molecular Dynamics Free Energy Perturbation and the OPLS Force Field. J. Chem. Theory Comput. 2010, 6, 1509–1519. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent Molecular-orbital Methods. IX. An Extended Gaussian-type Basis for Molecular-orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput. Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-1; Epik, Schrödinger, LLC: New York, NY, USA, 2022.
- Jacobson, L.D.; Bochevarov, A.D.; Watson, M.A.; Hughes, T.F.; Rinaldo, D.; Ehrlich, S.; Steinbrecher, T.B.; Vaitheeswaran, S.; Philipp, D.M.; Halls, M.D.; et al. Automated Transition State Search and Its Application to Diverse Types of Organic Reactions. J. Chem. Theory Comput. 2017, 13, 5780–5797. [Google Scholar] [CrossRef]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- QuantumATK Version T-2022.03, Synopsys QuantumATK. Available online: Https://Www.Synopsys.Com/Silicon/Quantumatk.Html (accessed on 15 June 2022).
- Schneider, J.; Hamaekers, J.; Chill, S.T.; Smidstrup, S.; Bulin, J.; Thesen, R.; Blom, A.; Stokbro, K. ATK-ForceField: A New Generation Molecular Dynamics Software Package. Model. Simul. Mater. Sci. Eng. 2017, 25, 85007. [Google Scholar] [CrossRef] [Green Version]
- Smidstrup, S.; Stradi, D.; Wellendorff, J.; Khomyakov, P.A.; Vej-Hansen, U.G.; Lee, M.-E.; Ghosh, T.; Jónsson, E.; Jónsson, H.; Stokbro, K. First-principles Green's-function method for surface calculations: A pseudopotential localized basis set approach. Phys. Rev. B 2017, 96, 195309. [Google Scholar] [CrossRef] [Green Version]
- Smidstrup, S.; Markussen, T.; Vancraeyveld, P.; Wellendorff, J.; Schneider, J.; Gunst, T.; Verstichel, B.; Stradi, D.; Khomyakov, P.A.; Vej-Hansen, U.G.; et al. QuantumATK: An integrated platform of electronic and atomic-scale modelling tools. J. Physics: Condens. Matter 2019, 32, 015901. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar]
- Fuchs, M.; Scheffler, M. Ab initio pseudopotentials for electronic structure calculations of poly-atomic systems using density-functional theory. Comput. Phys. Commun. 1999, 119, 67–98. [Google Scholar] [CrossRef]
- van Setten, M.; Giantomassi, M.; Bousquet, E.; Verstraete, M.; Hamann, D.; Gonze, X.; Rignanese, G.-M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Al-Najar, B.; Younis, A.; Hazeem, L.; Sehar, S.; Rashdan, S.; Shaikh, M.N.; Albuflasa, H.; Hankins, N.P. Thermally induced oxygen related defects in eco-friendly ZnFe2O4 nanoparticles for enhanced wastewater treatment efficiencies. Chemosphere 2021, 288, 132525. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, G.; An, T.; Gao, Y.; Fu, J. Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: A case of sulfa drugs. Catal. Today 2010, 153, 200–207. [Google Scholar] [CrossRef]
- Romero, V.; De la Cruz, N.; Dantas, R.F.; Marco, P.; Giménez, J.; Esplugas, S. Photocatalytic treatment of metoprolol and propranolol. Catal. Today 2011, 161, 115–120. [Google Scholar] [CrossRef]
- Armaković, S.J.; Armaković, S.; Šibul, F.; Četojević-Simin, D.D.; Tubić, A.; Abramović, B.F. Kinetics, mechanism and toxicity of intermediates of solar light induced photocatalytic degradation of pindolol: Experimental and computational modeling approach. J. Hazard. Mater. 2020, 393, 122490. [Google Scholar] [CrossRef]
- Nundy, S.; Tatar, D.; Kojčinović, J.; Ullah, H.; Ghosh, A.; Mallick, T.K.; Meinusch, R.; Smarsly, B.M.; Tahir, A.A.; Djerdj, I. Bandgap Engineering in Novel Fluorite-Type Rare Earth High-Entropy Oxides (RE-HEOs) with Computational and Experimental Validation for Photocatalytic Water Splitting Applications. Adv. Sustain. Syst. 2022, 6, 2200067. [Google Scholar] [CrossRef]
- Theurich, J.; Lindner, A.M.; Bahnemann, D.W. Photocatalytic Degradation of 4-Chlorophenol in Aerated Aqueous Titanium Dioxide Suspensions: A Kinetic and Mechanistic Study. Langmuir 1996, 12, 6368–6376. [Google Scholar] [CrossRef]
- Shalaeva, M.; Kenseth, J.; Lombardo, F.; Bastin, A. Measurement of Dissociation Constants (PKa Values) of Organic Compounds by Multiplexed Capillary Electrophoresis Using Aqueous and Cosolvent Buffers. J. Pharm. Sci. 2008, 97, 2581–2606. [Google Scholar] [CrossRef]
- Chen, A.-N.; Wu, J.-M.; Cheng, L.-J.; Liu, S.-J.; Ma, Y.-X.; Li, H.; Liu, F.; Chen, S.; Shi, Y.-S.; Li, C.-H. Enhanced densification and dielectric properties of CaTiO3-0.3NdAlO3 ceramics fabricated by direct coagulation casting. J. Eur. Ceram. Soc. 2020, 40, 1174–1180. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Çelik, E.; Polat, M.; Deliormanli, A.M. The Isoelectric Point of Lead Magnesium Niobate. J. Am. Ceram. Soc. 2007, 90, 3314–3317. [Google Scholar] [CrossRef]
- López, M.B.; Rand, B.; Riley, F. The isoelectric point of BaTiO3. J. Eur. Ceram. Soc. 2000, 20, 107–118. [Google Scholar] [CrossRef]
- Younis, A.; Loucif, A. Defects mediated enhanced catalytic and humidity sensing performance in ceria nanorods. Ceram. Int. 2021, 47, 15500–15507. [Google Scholar] [CrossRef]



| Suspension | COD (mg O2 dm−3) | Mineralization (%) |
|---|---|---|
| SSI | ||
| SLTO | 73.79 | 22.5 |
| SCNPTO | 79.13 | 16.9 |
| UV-LED | ||
| SLTO | 48.81 | 48.7 |
| SCNPTO | 54.16 | 43.1 |
| UV | ||
| SLTO | 18.48 | 80.6 |
| SCNPTO | 25.61 | 73.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanoski Kostić, A.; Kanas, N.; Rajić, V.; Sharma, A.; Bhattacharya, S.S.; Armaković, S.; Savanović, M.M.; Armaković, S.J. Evaluation of Photocatalytic Performance of Nano-Sized Sr0.9La0.1TiO3 and Sr0.25Ca0.25Na0.25Pr0.25TiO3 Ceramic Powders for Water Purification. Nanomaterials 2022, 12, 4193. https://doi.org/10.3390/nano12234193
Jovanoski Kostić A, Kanas N, Rajić V, Sharma A, Bhattacharya SS, Armaković S, Savanović MM, Armaković SJ. Evaluation of Photocatalytic Performance of Nano-Sized Sr0.9La0.1TiO3 and Sr0.25Ca0.25Na0.25Pr0.25TiO3 Ceramic Powders for Water Purification. Nanomaterials. 2022; 12(23):4193. https://doi.org/10.3390/nano12234193
Chicago/Turabian StyleJovanoski Kostić, Aleksandra, Nikola Kanas, Vladimir Rajić, Annu Sharma, Subramshu S. Bhattacharya, Stevan Armaković, Maria M. Savanović, and Sanja J. Armaković. 2022. "Evaluation of Photocatalytic Performance of Nano-Sized Sr0.9La0.1TiO3 and Sr0.25Ca0.25Na0.25Pr0.25TiO3 Ceramic Powders for Water Purification" Nanomaterials 12, no. 23: 4193. https://doi.org/10.3390/nano12234193











