Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation
2.2. Synthesis of MNPs
2.3. Characterization of MNPs
2.4. Flavonoid Content Assay
2.5. Reducing Power Assay
2.6. Phytotoxicity Assessment of MNPs
2.7. Adsorption Study
3. Results
3.1. ‘Hairy’ Root Extract Preparation and Characterization
3.2. Synthesis and Characterization of MNPs
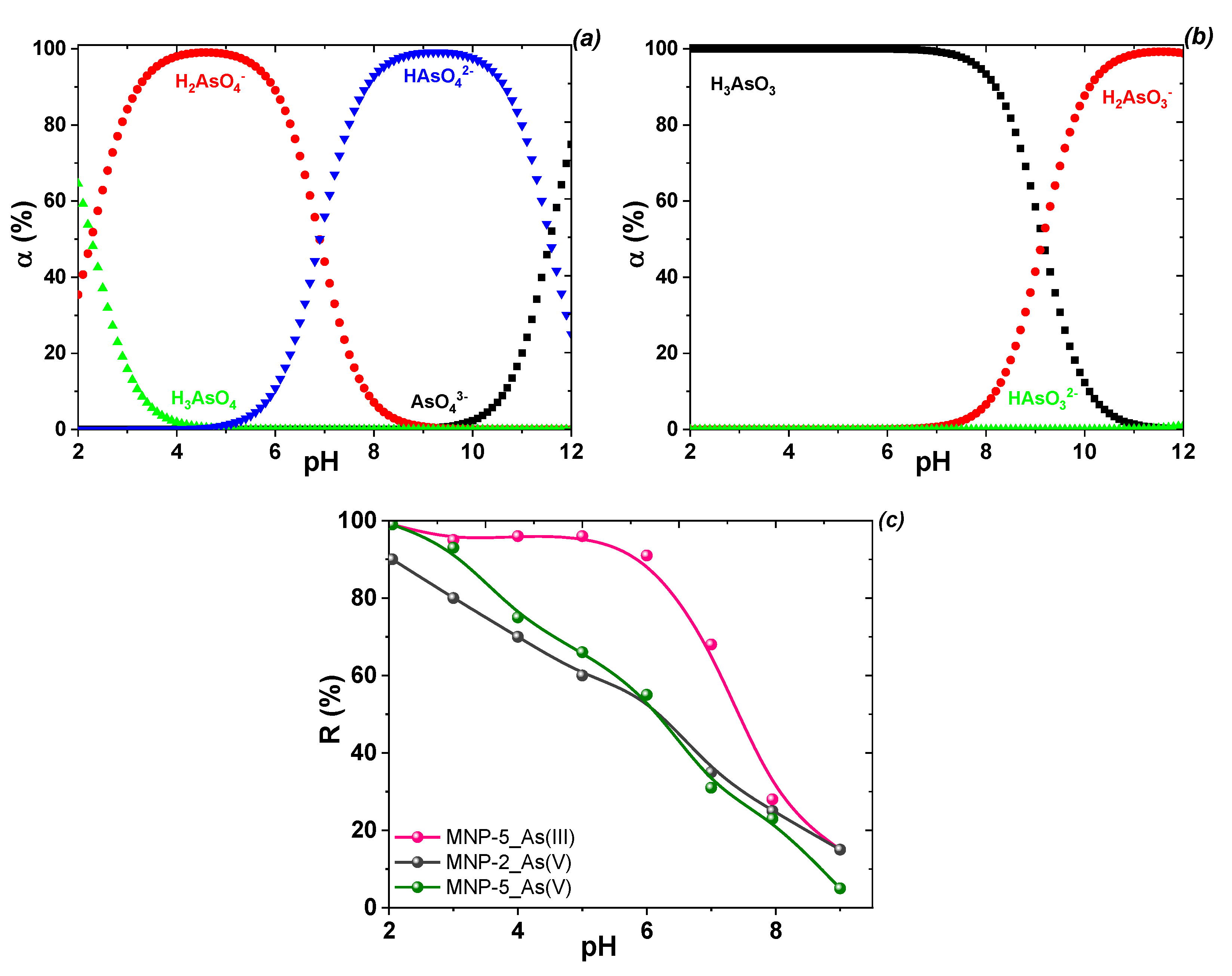
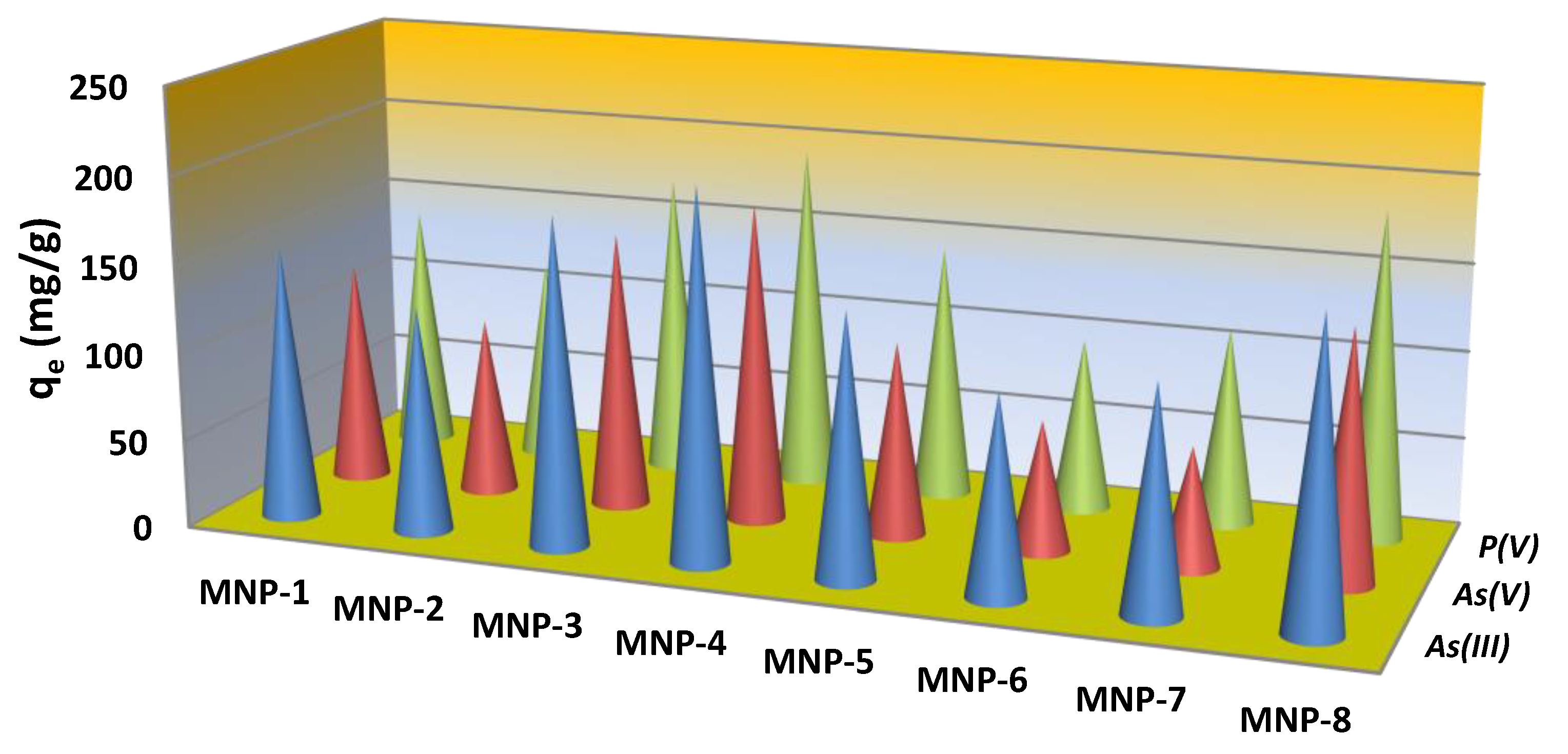
3.3. Adsorption Performance toward Cation and Anion Forms of Toxicants
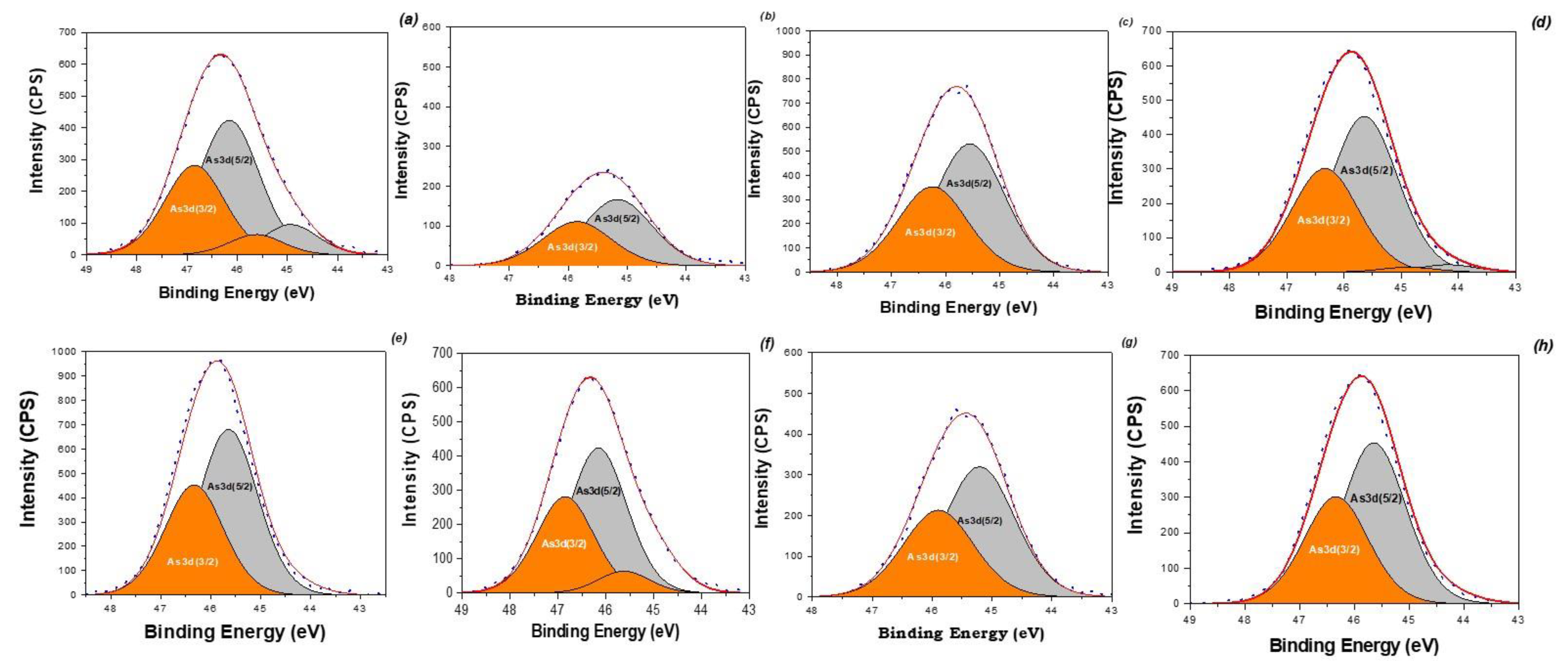
3.4. Adsorptive Performance of the MNPs to Cations and Anions as a Function of (Phase, Element) Speciation, Surface Chemistry and Porous Properties
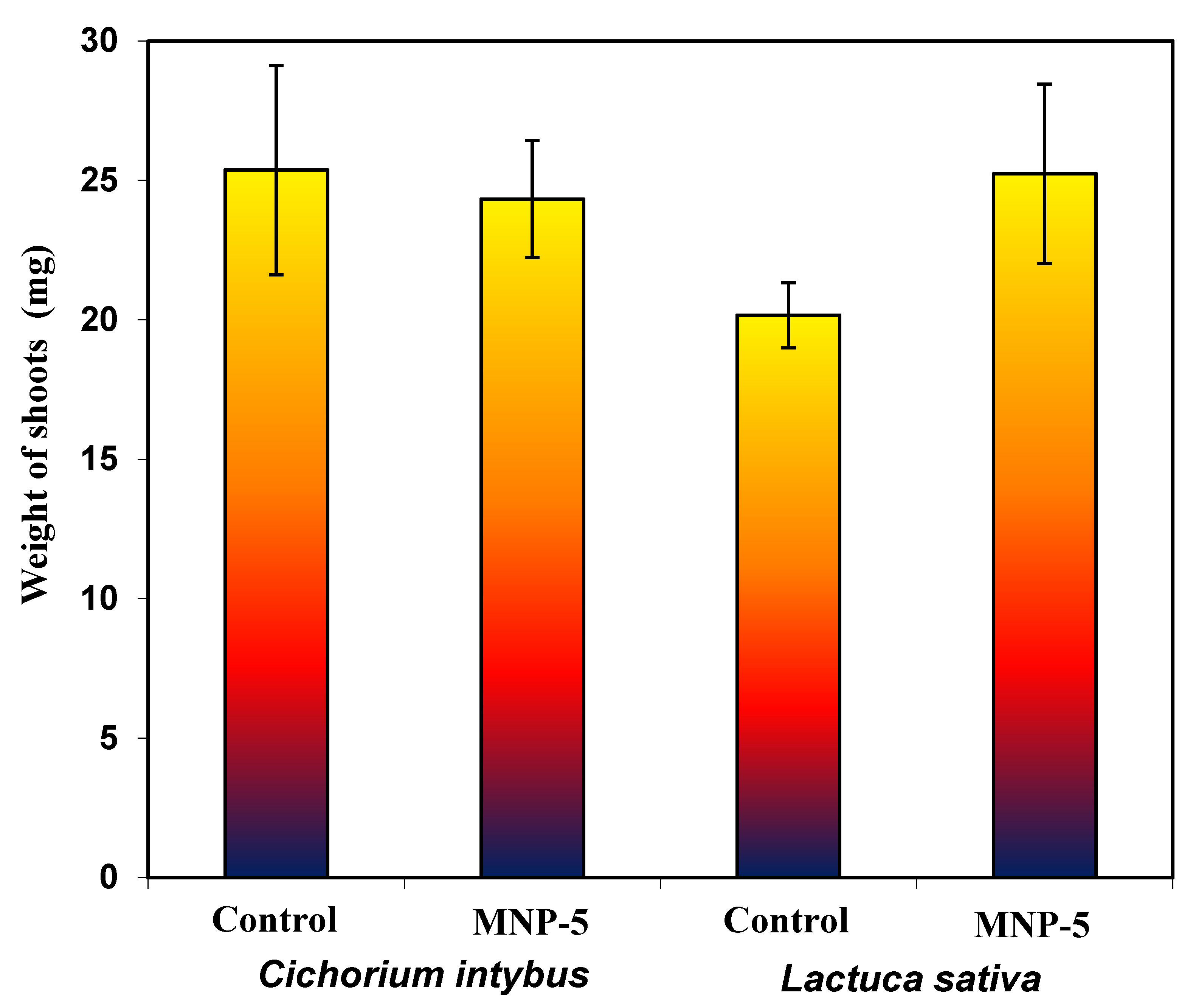
3.5. Safety Evaluation of MNPs through Phytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Smedley, D.G.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Galunin, E.; Ferreti, J.; Zapelini, I.; Vieira, I.; Ricardo Teixeira Tarley, C.; Abrão, T.; Santos, M.J. Cadmium Mobility in Sediments and Soils from a Coal Mining Area on Tibagi River Watershed: Environmental Risk Assessment. J. Hazard. Mater. 2014, 265, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, D.; Saha, R.; Ghosh, T.; Saha, B. A Review on Sources, Toxicity and Remediation Technologies for Removing Arsenic from Drinking Water. Res. Chem. Intermed. 2014, 40, 447–485. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. A Review of Human Carcinogens. Arsenic, Metals, FIbres, and Dusts. In A Review of Human Carcinogens; IARC: Lyon, France, 2012; Volume 100 C. [Google Scholar]
- Kobylinskaya, N.G.; Khainakova, E.A.; Diaz-Garcia, M.E.; Zaitsev, V.N. Nanocomposites based on magnetite modified by chelate groups for a solid-phase concentration of heavy-metal ions from aqueous solutions. Prot. Met. Phys. Chem. Surf. 2017, 53, 675–684. [Google Scholar] [CrossRef]
- Faivre, D. Iron Oxides from Nature to Applications; John Wiley & Sons: New York, NY, USA, 2016; Volume 1999. [Google Scholar]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, Size-and Structure-Controlled Synthesis and Biocompatibility of Iron Oxide Nanoparticles for Magnetic Theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Deb, A.K.; Biswas, B.; Rahman, M.M.; Xi, Y.; Paul, S.K.; Naidu, R. Magnetite Nanoparticles Loaded into Halloysite Nanotubes for Arsenic(V) Removal from Water. ACS Appl. Nano Mater. 2022, 5, 12063–12076. [Google Scholar] [CrossRef]
- Sudhakar, C.; Mukherjee, S.; Kumar, A.A.; Paramasivam, G.; Meena, P.K.; Nonappa; Pradeep, T. Interference of Phosphate in Adsorption of Arsenate and Arsenite over Confined Metastable Two-Line Ferrihydrite and Magnetite. J. Phys. Chem. C 2021, 125, 22502–22512. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Manquián-Cerda, K.; Pizarro, C.; Maldonado, T.; Suazo-Hernández, J.; Escudey, M.; Bolan, N.; Sarkar, B. Mechanistic Insights into Simultaneous Removal of Copper, Cadmium and Arsenic from Water by Iron Oxide-Functionalized Magnetic Imogolite Nanocomposites. J. Hazard. Mater. 2020, 398, 122940. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, A.A.; Aguirre-Álvarez, G.; Cariño-Cortés, R.; Mendoza-Huizar, L.H.; Jiménez-Alvarado, R. Iron Oxide Nanoparticles: Synthesis, Functionalization, and Applications in Diagnosis and Treatment of Cancer. Chem. Pap. 2020, 74, 3809–3824. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Nasrazadani, S.; Namduri, H. Study of Phase Transformation in Iron Oxides Using Laser Induced Breakdown Spectroscopy. Spectrochim. Acta-Part B At. Spectrosc. 2006, 61, 565–571. [Google Scholar] [CrossRef]
- Azizi, A. Green Synthesis of Fe3O4 Nanoparticles and Its Application in Preparation of Fe3O4/Cellulose Magnetic Nanocomposite: A Suitable Proposal for Drug Delivery Systems. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3552–3561. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-Mediated Green Synthesis of Iron Nanoparticles. J. Nanopart. 2014, 2014, 140614. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-Dispersible Ascorbic-Acid-Coated Magnetite Nanoparticles for Contrast Enhancement in MRI. Appl. Nanosci. 2015, 5, 435–441. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Krishna, R.; Bardhan, N.; Bahadur, D.; Gracio, J. Wet-Chemical Green Synthesis of l-Lysine Amino Acid Stabilized Biocompatible Iron-Oxide Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 6645–6651. [Google Scholar] [CrossRef]
- He, F.; Zhao, D. Preparation and Characterization of a New Class of Starch-Stabilized Bimetallic Nanoparticles for Degradation of Chlorinated Hydrocarbons in Water. Environ. Sci. Technol. 2005, 39, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, C.; Zhang, F.; Yang, Y.; Wu, G.; Yu, A.; Guan, N. Size-Controlled Synthesis of Magnetite (Fe3O4) Nanoparticles Coated with Glucose and Gluconic Acid from a Single Fe(III) Precursor by a Sucrose Bifunctional Hydrothermal Method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In Vitro Biocompatibility of Nanoscale Zerovalent Iron Particles (NZVI) Synthesized Using Tea Polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Makarov, V.V.; Makarova, S.S.; Love, A.J.; Sinitsyna, O.V.; Dudnik, A.O.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. Biosynthesis of Stable Iron Oxide Nanoparticles in Aqueous Extracts of Hordeum Vulgare and Rumex Acetosa Plants. Langmuir 2014, 30, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Bankar, A.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Removal of Hexavalent Chromium Ions by Yarrowia Lipolytica Cells Modified with Phyto-Inspired Fe0/Fe3O4 Nanoparticles. J. Contam. Hydrol. 2013, 146, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Padhi, D.K.; Panigrahi, T.K.; Parida, K.; Singh, S.K.; Mishra, P.M. Green Synthesis of Fe3O4/RGO Nanocomposite with Enhanced Photocatalytic Performance for Cr(VI) Reduction, Phenol Degradation, and Antibacterial Activity. ACS Sustain. Chem. Eng. 2017, 5, 10551–10562. [Google Scholar] [CrossRef]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of Bromothymol Blue by “greener” Nano-Scale Zero-Valent Iron Synthesized Using Tea Polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Weng, X.; Ma, L.; Guo, M.; Su, Y.; Dharmarajan, R.; Chen, Z. Removal of Doxorubicin Hydrochloride Using Fe3O4 Nanoparticles Synthesized by Euphorbia Cochinchinensis Extract. Chem. Eng. J. 2018, 353, 482–489. [Google Scholar] [CrossRef]
- Nikić, J.; Tubić, A.; Watson, M.; Maletić, S.; Šolić, M.; Majkić, T.; Agbaba, J. Arsenic Removal from Water by Green Synthesized Magnetic Nanoparticles. Water 2019, 11, 2520. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, Á.d.J.; Reyes-López, S.Y.; Mondragón-Sánchez, M.d.L.; Robles-Cortés, A.I.; Pérez, R. Eco-Friendly Synthesis of Fe3O4 Nanoparticles: Evaluation of Their Catalytic Activity in Methylene Blue Degradation by Kinetic Adsorption Models. Results Phys. 2019, 12, 989–995. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.L.; Leostean, C.; Popa, A.; Stefan, M.; Lazar, M.D.; Opris, O.; Silipas, T.D.; Porav, A.S. Removal of Antibiotics from Aqueous Solutions by Green Synthesized Magnetite Nanoparticles with Selected Agro-Waste Extracts. Process Saf. Environ. Prot. 2017, 107, 357–372. [Google Scholar] [CrossRef]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. A Green Synthetic Route to Phenolics Fabricated Magnetite Nanoparticles from Coconut Husk Extract: Implications to Treat Metal Contaminated Water and Heavy Metal Stress in Oryza sativa L. J. Clean. Prod. 2018, 174, 355–366. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.O.; Ali, A.; Ahmad, I.; Zhao, T.K.; Botha, S.; Maaza, M.; Ezema, F.I. Biosynthesis of Iron Oxide Nanoparticles via a Composite of Psidium Guavaja-Moringa Oleifera and Their Antibacterial and Photocatalytic Study. J. Photochem. Photobiol. B Biol. 2019, 199, 111601. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic Nanoparticles for Environmental and Biomedical Applications: A Review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Dilshad, E.; Cusido, R.M.; Estrada, K.R.; Bonfill, M.; Mirza, B. Genetic Transformation of Artemisia Carvifolia Buch with Rol Genes Enhances Artemisinin Accumulation. PLoS ONE 2015, 10, e0140266. [Google Scholar] [CrossRef] [PubMed]
- Kobylinska, N.; Klymchuk, D.; Shakhovsky, A.; Khainakova, O.; Ratushnyak, Y.; Duplij, V.; Matvieieva, N. Biosynthesis of Magnetite and Cobalt Ferrite Nanoparticles Using Extracts of “Hairy” Roots: Preparation, Characterization, Estimation for Environmental Remediation and Biological Application. RSC Adv. 2021, 11, 26974–26987. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Röntgen Rays. Nachr. Ges. Wiss. Gott. 1918, 2, 96–100. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Chan, S.G.; Murphy, P.A.; Ho, S.C.; Kreiger, N.; Darlington, G.; So, E.K.F.; Chong, P.Y.Y. Isoflavonoid Content of Hong Kong Soy Foods. J. Agric. Food Chem. 2009, 57, 5386–5390. [Google Scholar] [CrossRef]
- Kobylinska, N.; Shakhovsky, A.; Khainakova, O.; Klymchuk, D.; Avdeeva, L.; Ratushnyak, Y.; Duplij, V.; Matvieieva, N. “Hairy” Root Extracts as Source for “Green” Synthesis of Silver Nanoparticles and Medical Applications. RSC Adv. 2020, 10, 39434–39446. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, M.; Yang, B.; Liu, Z.; Huang, J.; Sun, D. Plant-Mediated Synthesis of Highly Active Iron Nanoparticles for Cr(VI) Removal: Investigation of the Leading Biomolecules. Chemosphere 2016, 150, 357–364. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. Characterization of Bimetallic Fe/Pd Nanoparticles by Grape Leaf Aqueous Extract and Identification of Active Biomolecules Involved in the Synthesis. Sci. Total Environ. 2016, 562, 526–532. [Google Scholar] [CrossRef]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D Appl. Phys. 2009, 42, 031001. [Google Scholar] [CrossRef]
- Abraime, B.; El Maalam, K.; Fkhar, L.; Mahmoud, A.; Boschini, F.; Ait Tamerd, M.; Benyoussef, A.; Hamedoun, M.; Hlil, E.K.; Ait Ali, M.; et al. Influence of Synthesis Methods with Low Annealing Temperature on the Structural and Magnetic Properties of CoFe2O4 Nanopowders for Permanent Magnet Application. J. Magn. Magn. Mater. 2020, 500, 166416. [Google Scholar] [CrossRef]
- Sarkar, N.N.; Tirpude, S.A.; Sawadh, P.S.; Rewatkar, K.G. Effect of Cobalt and Nickel Substitution on Structural and Magnetic Properties of Spinel Ferrite. Integr. Ferroelectr. 2019, 203, 61–66. [Google Scholar] [CrossRef]
- Shan, J.; Wang, L.; Yu, H.; Ji, J.; Amer, W.A.; Chen, Y.; Jing, G.; Khalid, H.; Akram, M.; Abbasi, N.M. Recent Progress in Fe3O4 Based Magnetic Nanoparticles: From Synthesis to Application. Mater. Sci. Technol. 2016, 32, 602–614. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.F.; Cohen-Jonathan, S.; Soucé, M.; Marchais, H.; Dubois, P. Molecular Composition of Iron Oxide Nanoparticles, Precursors for Magnetic Drug Targeting, as Characterized by Confocal Raman Microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Liang, X.; Lin, X.; Wei, G.; Ma, L.; He, H.; Santos-Carballal, D.; Zhu, J.; Zhu, R.; De Leeuw, N.H. Competitive Adsorption Geometries for the Arsenate As(V) and Phosphate P(V) Oxyanions on Magnetite Surfaces: Experiments and Theory. Am. Mineral. 2021, 106, 374–388. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of Arsenic from Water by Supported Nano Zero-Valent Iron on Activated Carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Iron Oxide-Loaded Slag for Arsenic Removal from Aqueous System. Chemosphere 2005, 60, 319–325. [Google Scholar] [CrossRef]
- Matsunaga, H.; Yokoyama, T.; Eldridge, R.J.; Bolto, B.A. Adsorption Characteristics of Arsenic(III) and Arsenic(V) on Iron(III)-Loaded Chelating Resin Having Lysine-Nα,Nα-Diacetic Acid Moiety. React. Funct. Polym. 1996, 29, 167–174. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F. Removal of Arsenic by Bead Cellulose Loaded with Iron Oxyhydroxide from Groundwater. Environ. Sci. Technol. 2005, 39, 6808–6818. [Google Scholar] [CrossRef]
- Hlavay, J.; Polyák, K. Determination of Surface Properties of Iron Hydroxide-Coated Alumina Adsorbent Prepared for Removal of Arsenic from Drinking Water. J. Colloid Interface Sci. 2005, 284, 71–77. [Google Scholar] [CrossRef]
- Chen, W.; Parette, R.; Zou, J.; Cannon, F.S.; Dempsey, B.A. Arsenic Removal by Iron-Modified Activated Carbon. Water Res. 2007, 41, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chauhan, V.S.; Sankararamakrishnan, N. Preparation and Evaluation of Iron-Chitosan Composites for Removal of As(III) and As(V) from Arsenic Contaminated Real Life Groundwater. Water Res. 2009, 43, 3862–3870. [Google Scholar] [CrossRef]
- Giménez, J.; Martínez, M.; de Pablo, J.; Rovira, M.; Duro, L. Arsenic Sorption onto Natural Hematite, Magnetite, and Goethite. J. Hazard. Mater. 2007, 141, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lian, C.; Xu, M.; Zhang, W.; Liu, L.; Lin, K. Study on Competitive Adsorption Mechanism among Oxyacid-Type Heavy Metals in Co-Existing System: Removal of Aqueous As(V), Cr(III) and As(III) Using Magnetic Iron Oxide Nanoparticles (MIONPs) as Adsorbents. Appl. Surf. Sci. 2017, 422, 675–681. [Google Scholar] [CrossRef]
- Guo, H.; Ren, Y.; Liu, Q.; Zhao, K.; Li, Y. Enhancement of Arsenic Adsorption during Mineral Transformation from Siderite to Goethite: Mechanism and Application. Environ. Sci. Technol. 2013, 47, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Tian, X.; Ma, L.; Yang, C.; Wang, Y.; Wang, Z.; Zhang, L. Well-Dispersed Magnetic Iron Oxide Nanocrystals on Sepiolite Nanofibers for Arsenic Removal. RSC Adv. 2015, 5, 25236–25243. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Goldberg, S. Surface Structures and Stability of Arsenic(III) on Goethite: Spectroscopic Evidence for Inner-Sphere Complexes. Environ. Sci. Technol. 1998, 32, 2383–2388. [Google Scholar] [CrossRef]
- Stern, S.T.; McNeil, S.E. Nanotechnology Safety Concerns Revisited. Toxicol. Sci. 2008, 101, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in Vitro and in Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Zhang, H.; Lal, R. Effects of Stabilized Nanoparticles of Copper, Zinc, Manganese, and Iron Oxides in Low Concentrations on Lettuce (Lactuca Sativa) Seed Germination: Nanotoxicants or Nanonutrients. Water. Air. Soil Pollut. 2016, 227, 42. [Google Scholar] [CrossRef]


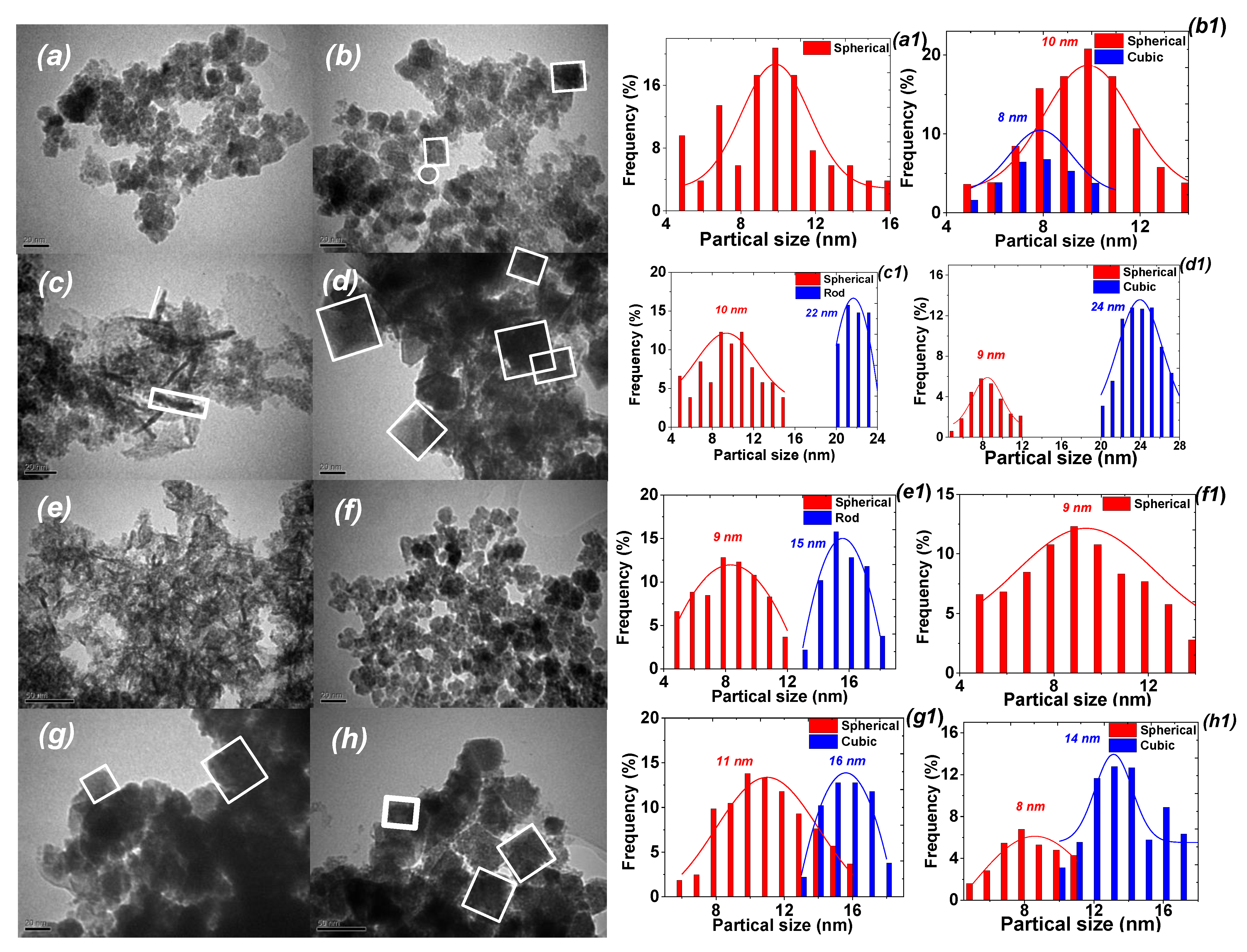

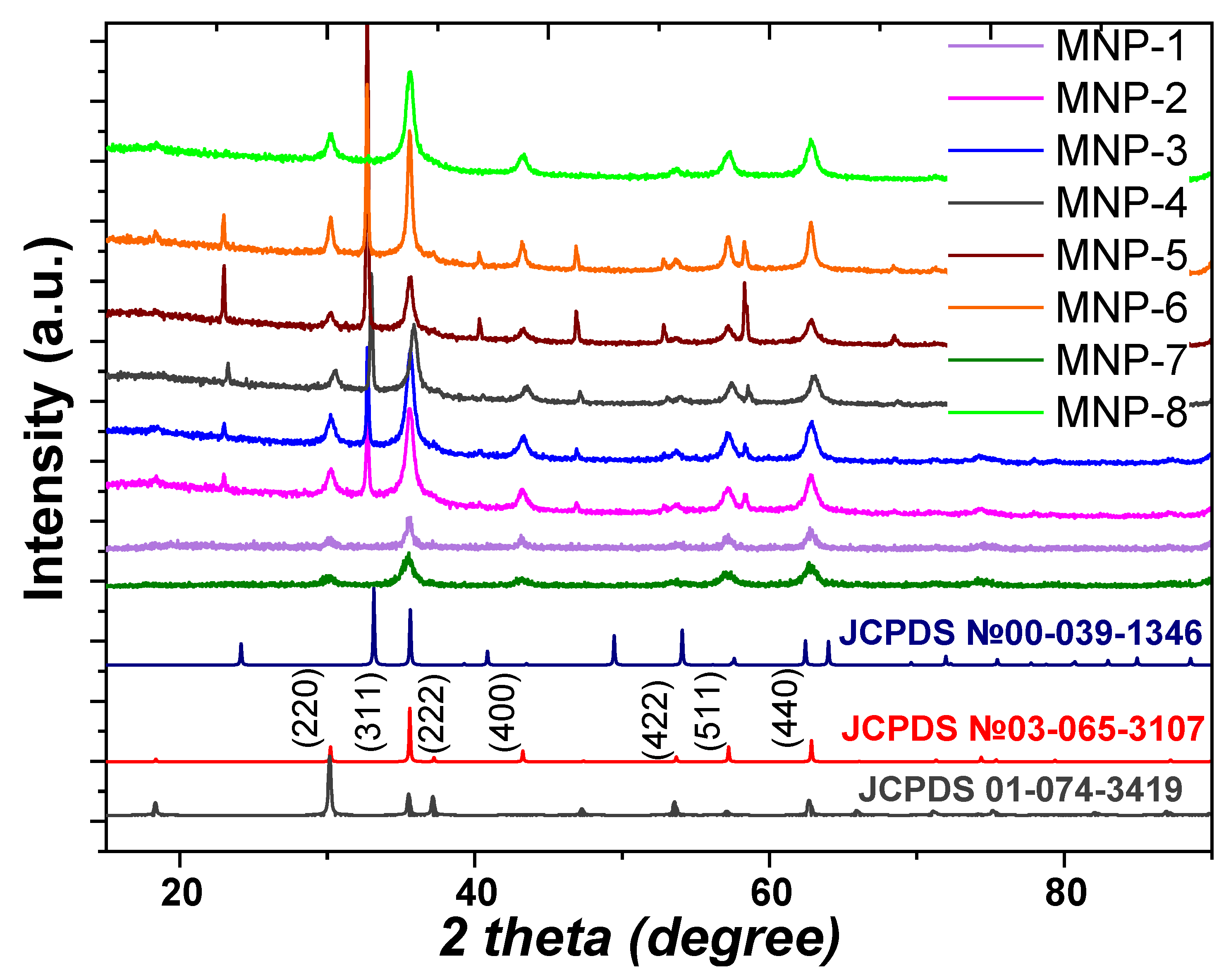
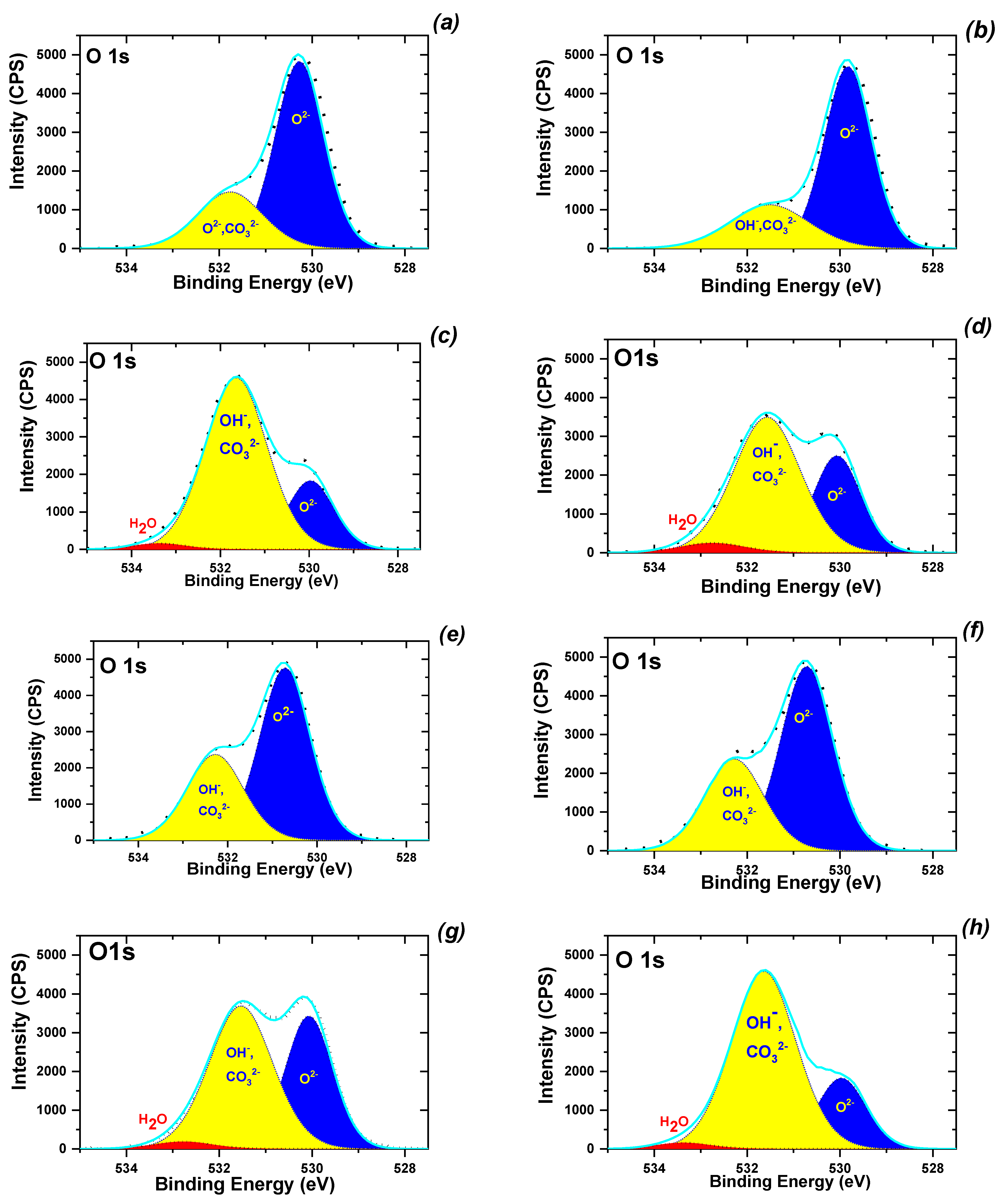



| Sample | d1, nm | d2, nm | SBET, m2/g | dpor, nm/ Vtot, cm3/g | MS, emu/g | HC, Oe | |
|---|---|---|---|---|---|---|---|
| Spherical | Other Shapes | ||||||
| * Alfa Aesar® | - | 50–100 | – | 20–50 | - | 83.0 | 18.53 |
| MNP-1 | 12.1 | 10.5 ± 3.0 | – | 23 | 0.4/0.42 | 42.1 | 0.18 |
| MNP-2 | 10.21 | 10.1 ± 3.2 | 8.5 ± 2.0 | 35 | 0.3/0.61 | 45.8 | 0.85 |
| MNP-3 | 16.11 | 10.0 ± 2.9 | 22.1 ± 1.0 | 51 | 0.6/0.84 | 49.4 | 0.02 |
| MNP-4 | 17.23 | 9.5 ± 1.5 | 24.5 ± 1.6 | 105 | 2.04/0.61 | 67.4 | 0.29 |
| MNP-5 | 10.6 | 9.0 ± 2.0 | 15.2 ± 1.9 | 90 | 1.0/0.42 | 72.9 | 3.72 |
| MNP-6 | 16.11 | 9.1 ± 1.9 | – | 85 | 1.6/0.74 | 67.4 | 3.70 |
| MNP-7 | 17.23 | 11.2 ± 3.2 | 16.0 ± 2.2 | 115 | 2.12/0.41 | 42.0 | 0.02 |
| MNP-8 | 12.6 | 8.1 ± 2.1 | 14.1 ± 2.5 | 90 | 1.8/0.32 | 46.6 | 0.27 |
| Sample | Ratio in Reaction Mixture Fe3+/Fe2+/Co2+/Extract | Chemical Composition in Final Solids | Ratio Fen+/Con+ | Composition | |||
|---|---|---|---|---|---|---|---|
| Fe, % | Co, % | C, % | H, % | ||||
| MNP-1 | 2.0 mL (5.4%)/2.0 mL (1.9%)/0.2 mL (2%)/1.0 mL | 12.86 | 66.8 | 0.19 | 0.33 | 1:2.07 | CoFe2O4 |
| MNP-2 | 15.91 | 57.47 | 0.13 | 0.50 | 1:1.44 | CoFe2O4 | |
| MNP-3 | 55.55 | - | 2.52 | 1.40 | - | Fe3O4 | |
| MNP-4 | 61.02 | - | 0.35 | 0.62 | - | Fe3O4 | |
| MNP-5 | 20.77 | 40.07 | 0.63 | 1.30:1 | CoFe2O4 | ||
| MNP-6 | 25.81 | 37.67 | - | 1.69:1 | CoFe2O4 | ||
| MNP-7 | 61.41 | - | 2.89 | 0.70 | - | Fe3O4 | |
| MNP-8 | 79.96 | - | - | 0.44 | - | Fe3O4 | |
| Adsorbent | Adsorption Conditions | Adsorption Capacity (mg/g) | Reference | |
|---|---|---|---|---|
| As(III) | As(V) | |||
| Fe0/C | pH 7.0 | 18.9 | 12.02 | [50] |
| Iron oxide-loaded slag | pH 10 (As(III)), pH 2.5 (As(V)) | 2.9–30 | 18.8–78.5 | [51] |
| Fe(III)/lysine-Nα,Nα-diacetic acid | pH 9 (As(III)), pH 3.5 (As(V)) | 62.93 | 55.44 | [52] |
| Bead cellulose-loaded iron-oxy-OH | pH 7.0 | 99.6 | 33.2 | [53] |
| Fe(OH)3-coated Al2O3 | pH 6.6–7.2 | 7.64 | 36.64 | [54] |
| Iron-modified activated carbon | pH 8 (As(III)), pH 6.0 As(V)) | 39.2 | 51.3 | [55] |
| Iron oxide-doped chitosan composite | pH 7.0 | 22.47 | 16.1 | [56] |
| Natural goethite hematite magnetite | pH 7.5 | 10.1 | 12.1 | [57] |
| pH 7.3 | 10 | 31.3 | ||
| pH 6.5 | 10.0 | 12.0 | ||
| Magnetite and cobalt ferrite | pH 6.7 | 2.9–30 | 18.8–78.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobylinska, N.; Klymchuk, D.; Khaynakova, O.; Duplij, V.; Matvieieva, N. Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation. Nanomaterials 2022, 12, 4231. https://doi.org/10.3390/nano12234231
Kobylinska N, Klymchuk D, Khaynakova O, Duplij V, Matvieieva N. Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation. Nanomaterials. 2022; 12(23):4231. https://doi.org/10.3390/nano12234231
Chicago/Turabian StyleKobylinska, Natalia, Dmytro Klymchuk, Olena Khaynakova, Volodymyr Duplij, and Nadiia Matvieieva. 2022. "Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation" Nanomaterials 12, no. 23: 4231. https://doi.org/10.3390/nano12234231





