High Power Factor of Ag2Se/Ag/Nylon Composite Films for Wearable Thermoelectric Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Se NWs
2.3. Synthesis of Ag2Se Nanostructures with or without Ag Nanoparticles
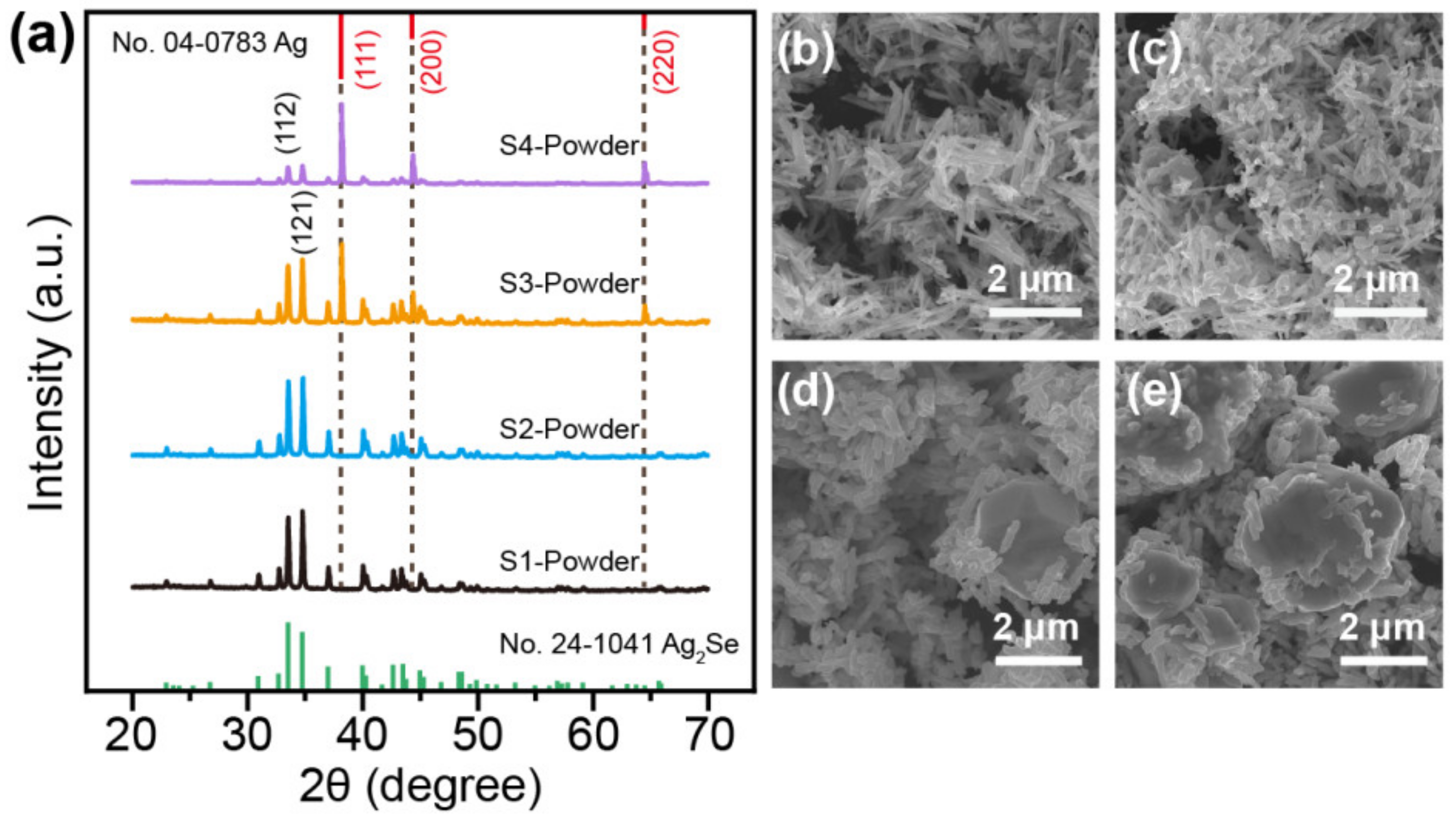
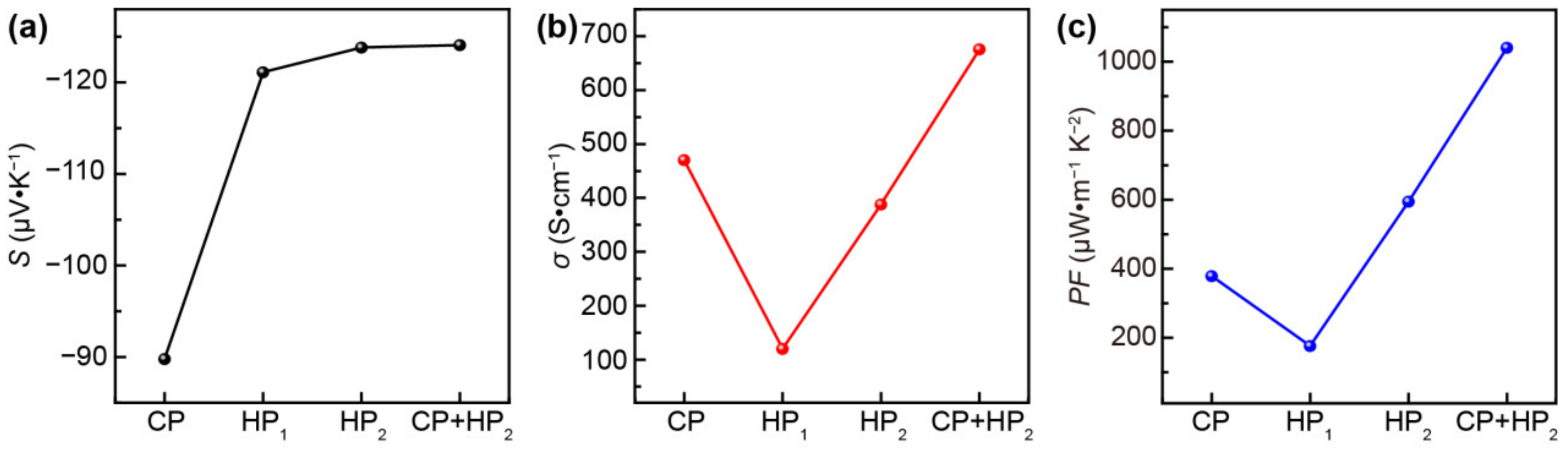
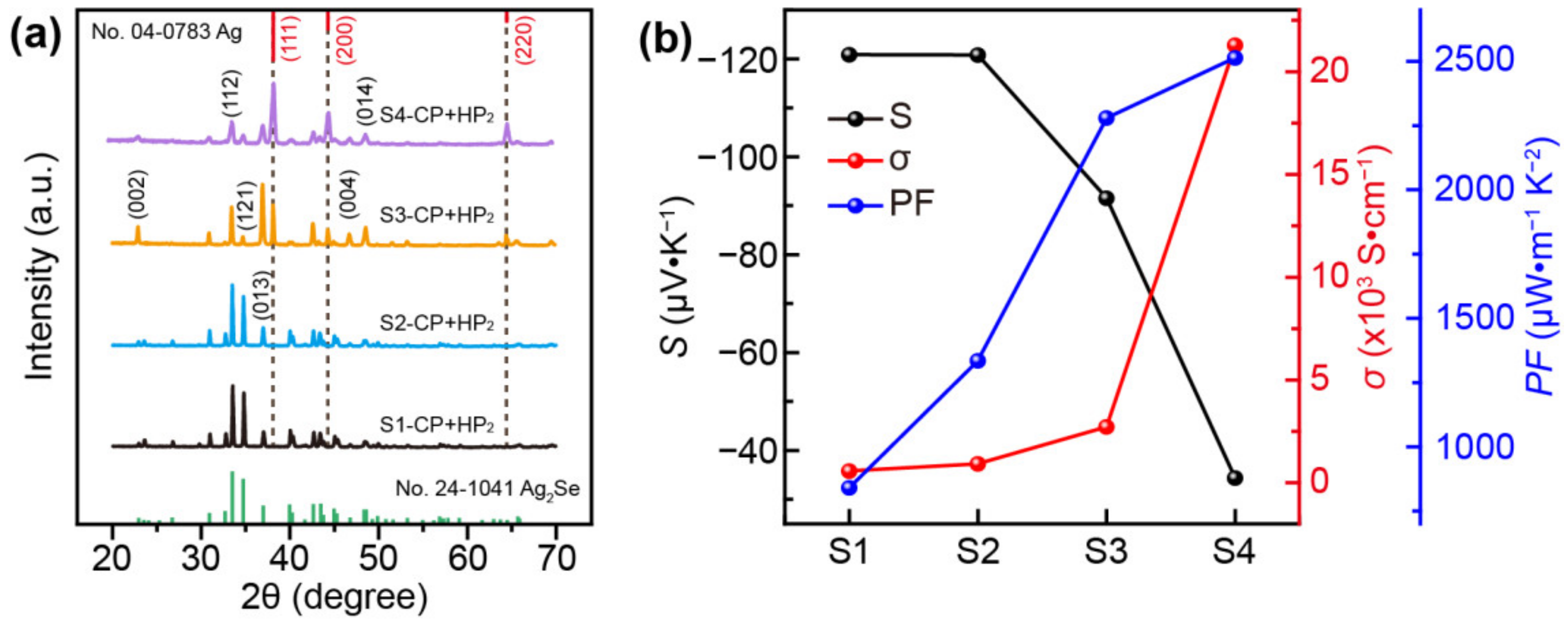
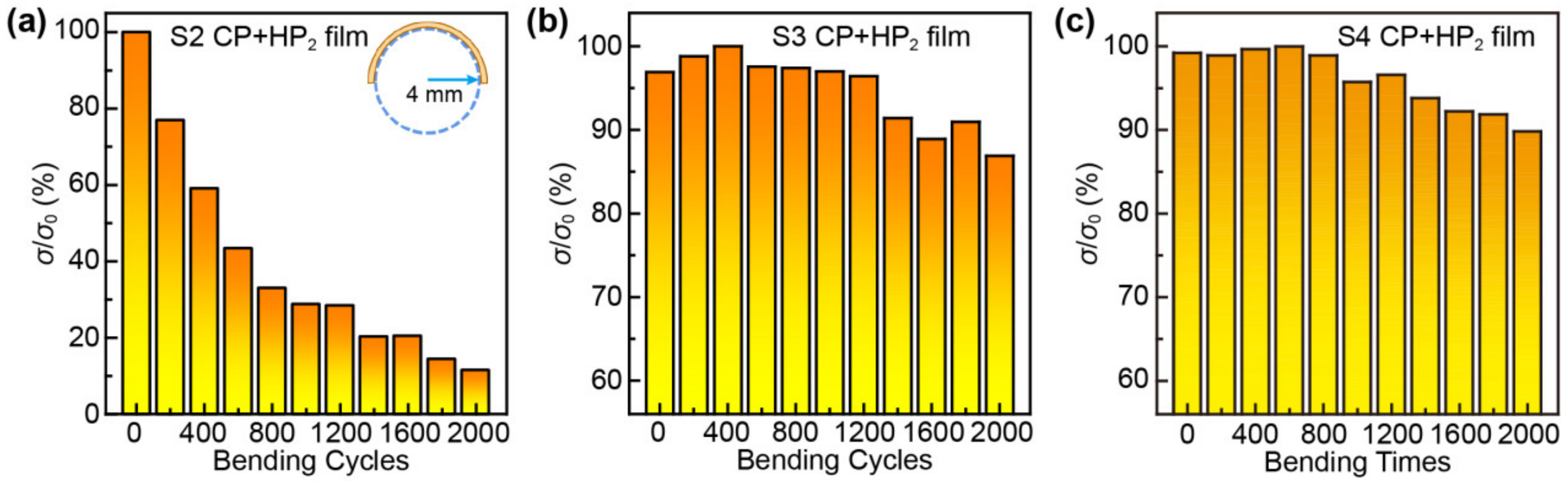
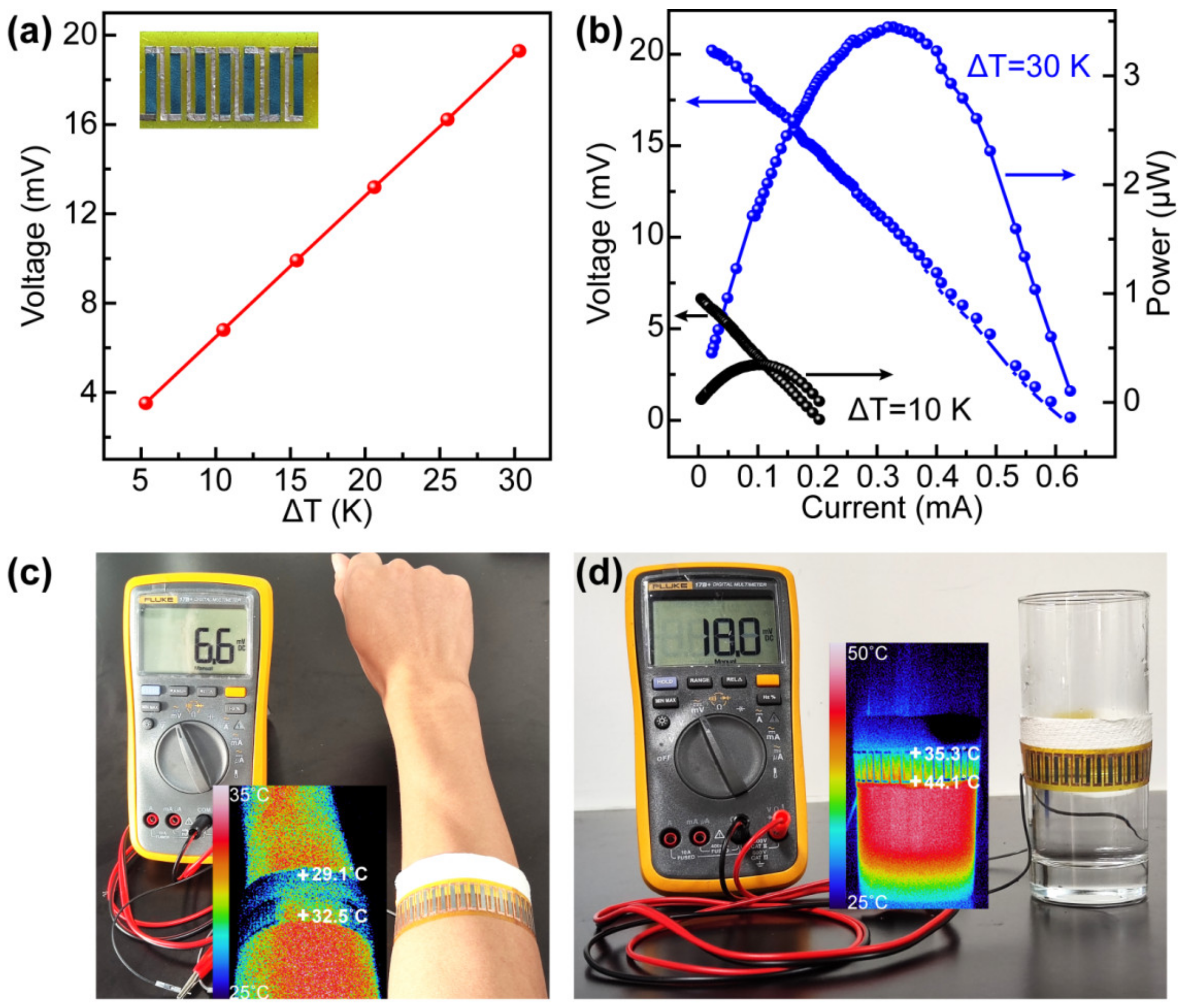
3. Results and Discussion
| Material | S (μV·K−1) | σ (S·cm−1) | PF (μW·m−1 K−2) | PDmax l/ΔT2 (μW m−1 K−2) | Ref. |
|---|---|---|---|---|---|
| Ag2Se/Nylon | −140 | 497 | 987 | 51.1 | [28] |
| Ag2Se/Nylon | −143 | 920 | 1882 | 488.9 | [43] |
| Ag2Se/Ag/CuAgSe/Nylon | −45.5 | 10770 | 2232 | 75.57 | [39] |
| Ag2Se/Ag/PEDOT/Nylon | −49.2 | 5957.3 | 1443 | 204 | [44] |
| Ag2Se/SWCNTs/Nylon | −121 | 704 | 1031 | / | [45] |
| Ag2Se/Ag/Nylon | −70 | 3958 | 1861 | 239.8 | [29] |
| Ag2Se/Ag/Nylon | −91.5 | 2720.1 | 2277.3 | 591.7 | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ates, H.C.; Yetisen, A.K.; Güder, F.; Dincer, C. Wearable devices for the detection of COVID-19. Nat. Electron. 2021, 4, 13–14. [Google Scholar] [CrossRef]
- Gao, J.; Fan, Y.; Zhang, Q.; Luo, L.; Hu, X.; Li, Y.; Song, J.; Jiang, H.; Gao, X.; Zheng, L.; et al. Ultra-robust and extensible fibrous mechanical sensors for wearable smart healthcare. Adv. Mater. 2022, 34, 2107511. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, X.; Liu, J.; Xu, H.; Wen, F.; Li, T.; Cui, J.; Liu, P.; Shen, L.; Cui, Y.; et al. Multiwalled carbon nanotube/graphite powder film for wearable pressure sensors with high sensing performance. Nanomaterials 2022, 12, 2637. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Fu, X.; Zhang, D.; Zhang, Q.; Zhuo, K.; Yuan, Z.; Ma, R. Recent progress of flexible/wearable self-charging power units based on triboelectric nanogenerators. Nano Energy 2021, 84, 105880. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z. Recent advances in triboelectric nanogenerator based self-charging power systems. Energy Storage Mater. 2019, 23, 617–628. [Google Scholar] [CrossRef]
- Lan, B.; Wu, F.; Cheng, Y.; Zhou, Y.; Hossain, G.; Grabher, G.; Shi, L.; Wang, R.; Sun, J. Scalable, stretchable and washable triboelectric fibers for self-powering human-machine interaction and cardiopulmonary resuscitation training. Nano Energy 2022, 102, 107737. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Huang, F.; Tao, X.; Ouyang, Y.; Zhou, Y.; Mo, X. High-output lotus-leaf-bionic triboelectric nanogenerators based on 2D MXene for health monitoring of human feet. Nanomaterials 2022, 12, 3217. [Google Scholar]
- Sun, Q.; Wang, L.; Ren, G.; Zhang, L.; Sheng, H.; Zhu, Y.; Wang, H.; Lu, G.; Yu, H.; Huang, W. Smart band-aid: Multifunctional and wearable electronic device for self-powered motion monitoring and human-machine interaction. Nano Energy 2022, 92, 106840. [Google Scholar] [CrossRef]
- Du, Y.; Deng, J.; Li, P.; Wen, Y. Energy transfer and redistribution: An approach for unifying vibrational energy harvesting and vibration attenuation. Nano Energy 2020, 78, 105245. [Google Scholar] [CrossRef]
- Tan, D.; Zhou, J.; Wang, K.; Zhao, X.; Wang, Q.; Xu, D. Bow-type bistable triboelectric nanogenerator for harvesting energy from low-frequency vibration. Nano Energy 2022, 92, 106746. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, S.; Qiu, P.; Peng, L.; Wei, T.; Zhang, Z.; Shi, X.; Chen, L. Flexible thermoelectrics based on ductile semiconductors. Science 2022, 377, 854–858. [Google Scholar] [CrossRef]
- Biria, S.; Chen, F.; Pathreeker, S.; Hosein, I. Polymer encapsulants incorporating light-guiding architectures to increase optical energy conversion in solar cells. Adv. Mater. 2018, 30, 1705382. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wu, S.; Zhang, W.; Luo, M. A bioinspired broadband self-powered photodetector based on photo-pyroelectric-thermoelectric effect able to detect human radiation. Nano Energy 2022, 93, 106812. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, P.; Li, G. A multibeam and surface plasmonic clothing with RF energy-localized harvester for powering battery-free wireless sensor. IEEE Internet Things J. 2022, 9, 13955–13964. [Google Scholar] [CrossRef]
- Bu, Z.; Zhang, X.; Hu, Y.; Chen, Z.; Lin, S.; Li, W.; Xiao, C.; Pei, Y. A record thermoelectric efficiency in tellurium-free modules for low-grade waste heat recovery. Nat. Commun. 2022, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Bu, Z.; Zhang, X.; Shan, B.; Tang, J.; Liu, H.; Chen, Z.; Lin, S.; Li, W.; Pei, Y. Realizing a 14% single-leg thermoelectric efficiency in gete alloys. Sci. Adv. 2021, 7, 2738. [Google Scholar] [CrossRef]
- Shi, X.; Zou, J.; Chen, Z. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Sun, H.; Zhao, D.; Zhang, F.; Hu, D.; Jiao, F.; Qin, L.; Linseis, V.; Fabiano, S.; Crispin, X.; et al. High thermoelectric performance in n-type Perylene bisimide induced by the soret effect. Adv. Mater. 2020, 32, 2002752. [Google Scholar] [CrossRef] [PubMed]
- Almasoudi, M.; Salah, N.; Alshahrie, A.; Saeed, A.; Aljaghtham, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Koumoto, K. High thermoelectric power generation by SWCNT/PPy core shell nanocomposites. Nanomaterials 2022, 12, 2582. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, J.; Huang, S.; Tan, B.; Luo, J.; Wang, D.; Liu, D.; Wang, L. Cross-conjugated spiro molecules and single-walled carbon nanotubes composite for high-performance organic thermoelectric materials and generators. Chem. Eng. J. 2021, 426, 131859. [Google Scholar] [CrossRef]
- Bao, D.; Chen, J.; Yu, Y.; Liu, W.; Huang, L.; Han, G.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z. Texture-dependent thermoelectric properties of nano-structured Bi2Te3. Chem. Eng. J. 2020, 388, 124295. [Google Scholar] [CrossRef]
- Lou, L.; Yang, J.; Zhu, Y.; Liang, H.; Zhang, Y.; Feng, J.; He, J.; Ge, Z.; Zhao, L. Tunable electrical conductivity and simultaneously enhanced thermoelectric and mechanical properties in n-type Bi2Te3. Adv. Sci. 2022, 9, 2203250. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bo, L.; Zhang, R.; Liu, S.; Zhu, J.; Zuo, M.; Zhao, D. Enhanced thermoelectric properties of Te doped polycrystalline Sn0.94Pb0.01Se. Nanomaterials 2022, 12, 1575. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, H.; Shi, H.; Xu, L.; Zhu, Y.; Qin, Y.; Peng, G.; Zhang, Y.; Ge, Z.; Ding, X.; et al. High-ranged ZT value promotes thermoelectric cooling and power generation in n-type PbTe. Adv. Energy Mater. 2022, 12, 2200204. [Google Scholar] [CrossRef]
- Bo, L.; Li, F.; Hou, Y.; Zuo, M.; Zhao, D. Enhanced thermoelectric performance of Cu2Se via nanostructure and compositional gradient. Nanomaterials 2022, 12, 640. [Google Scholar] [CrossRef]
- Kanno, T.; Tamaki, H.; Yoshiya, M.; Uchiyama, H.; Maki, S.; Takata, M.; Miyazaki, Y. High-density frenkel defects as origin of n-type thermoelectric performance and low thermal conductivity in Mg3Sb2-based materials. Adv. Funct. Mater. 2021, 31, 2008469. [Google Scholar] [CrossRef]
- Liu, Z.; Sato, N.; Gao, W.; Yubuta, K.; Kawamoto, N.; Mitome, M.; Kurashima, K.; Owada, Y.; Nagase, K.; Lee, C.-H.; et al. Demonstration of ultrahigh thermoelectric efficiency of ~7.3% in Mg3Sb2/MgAgSb module for low-temperature energy harvesting. Joule 2021, 5, 1196–1208. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, W.; Lu, Y.; Cai, K.; Li, Y.; Wang, Z.; Wu, M.; Huang, C.; He, J. High power factor Ag/Ag2Se composite films for flexible thermoelectric generators. ACS Appl. Mater. Interfaces 2021, 13, 14327–14333. [Google Scholar] [CrossRef] [PubMed]
- Rama, V.; Edward, S.; Thomas, C.; Brooks, O.Q. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar]
- Zhu, Y.; Sun, Y.; Zhu, J.; Song, K.; Liu, Z.; Liu, M.; Guo, M.; Dong, X.; Guo, F.; Tan, X.; et al. Mediating point defects endows n-type Bi2 Te3 with high thermoelectric performance and superior mechanical robustness for power generation application. Small 2022, 18, 2201352. [Google Scholar] [CrossRef]
- Huang, S.; Wei, T.; Chen, H.; Xiao, J.; Zhu, M.; Zhao, K.; Shi, X. Thermoelectric Ag2Se: Imperfection, homogeneity, and reproducibility. ACS Appl. Mater. Interfaces 2021, 13, 60192–60199. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Guo, R.; Zhang, Z.; Liu, D.; Xue, C. Effect of L-ascorbic acid solution concentration on the thermoelectric properties of silver selenide flexible films prepared by vacuum-assisted filtration. Nanomaterials 2022, 12, 624. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Sun, C.; Deng, H.; Fu, Q. Biomimetic approach to facilitate the high filler content in free-standing and flexible thermoelectric polymer composite films based on PVDF and Ag2Se nanowires. ACS Appl. Mater. Interfaces 2020, 12, 51506–51516. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.; Nagao, J. Thermoelectric and transport properties of β-Ag2Se compounds. J. Appl. Phys. 2000, 88, 813–816. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Q.; Bao, D.; Liu, T.; Liu, W.; Liu, C.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z. Hierarchical structures advance thermoelectric properties of porous n-type β-Ag2Se. ACS Appl. Mater. Interfaces 2020, 12, 51523–51529. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kojda, D.; Mitdank, R.; Handwerg, M.; Mogilatenko, A.; Albrecht, M.; Wang, Z.; Ruhhammer, J.; Kroener, M.; Woias, P.; Fischer, S.F. Temperature-dependent thermoelectric properties of individual silver nanowires. Phys. Rev. B 2015, 91, 024302. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Ding, Y.; Wang, M.; Jiang, C.; Yao, Q.; Huang, C.; Chen, L.; He, J. Ultrahigh power factor and flexible silver selenide-based composite film for thermoelectric devices. Energy Environ. Sci. 2020, 13, 1240–1249. [Google Scholar] [CrossRef]
- Faleev, S.V.; Léonard, F. Theory of enhancement of thermoelectric properties of materials with nanoinclusions. Phys. Rev. B 2008, 77, 214304. [Google Scholar] [CrossRef]
- Chhatrasal, G.; Yaron, A. Energy filtering of charge carriers: Current trends, challenges, and prospects for thermoelectric materials. Adv. Funct. Mater. 2019, 4, 1901789. [Google Scholar]
- Lee, B.; Cho, H.; Park, K.T.; Kim, J.S.; Park, M.; Kim, H.; Hong, Y.; Chung, S. High-performance compliant thermoelectric generators with magnetically self-assembled soft heat conductors for self-powered wearable electronics. Nat. Commun. 2020, 11, 5948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ding, Y.; Cai, K.; Tong, L.; Lu, Y.; Zhao, W.; Wei, P. Ultrahigh performance of n-type Ag2Se films for flexible thermoelectric power generators. ACS Appl. Mater. Interfaces 2020, 12, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, Q.; Wang, W.; Lu, Y.; Cai, K.; Li, Y.; Wu, M.; He, J. High performance Ag2Se/Ag/PEDOT composite films for wearable thermoelectric power generators. Mater. Today Phys. 2021, 21, 100553. [Google Scholar] [CrossRef]
- Geng, J.; Wu, B.; Guo, Y.; Hou, C.; Li, Y.; Wang, H.; Zhang, Q. High power factor n-type Ag2Se/SWCNTs hybrid film for flexible thermoelectric generator. J. Phys. D Appl. Phys. 2021, 54, 434004. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Liang, Z.; Jia, M.; Li, Y.; Guan, X.; Zhan, Y.; Wen, J.; Luo, J. High Power Factor of Ag2Se/Ag/Nylon Composite Films for Wearable Thermoelectric Devices. Nanomaterials 2022, 12, 4238. https://doi.org/10.3390/nano12234238
Wu W, Liang Z, Jia M, Li Y, Guan X, Zhan Y, Wen J, Luo J. High Power Factor of Ag2Se/Ag/Nylon Composite Films for Wearable Thermoelectric Devices. Nanomaterials. 2022; 12(23):4238. https://doi.org/10.3390/nano12234238
Chicago/Turabian StyleWu, Wenhang, Zheng Liang, Meng Jia, Yuwei Li, Xiongcong Guan, Yunfeng Zhan, Jinxiu Wen, and Jianyi Luo. 2022. "High Power Factor of Ag2Se/Ag/Nylon Composite Films for Wearable Thermoelectric Devices" Nanomaterials 12, no. 23: 4238. https://doi.org/10.3390/nano12234238
APA StyleWu, W., Liang, Z., Jia, M., Li, Y., Guan, X., Zhan, Y., Wen, J., & Luo, J. (2022). High Power Factor of Ag2Se/Ag/Nylon Composite Films for Wearable Thermoelectric Devices. Nanomaterials, 12(23), 4238. https://doi.org/10.3390/nano12234238






