Enhanced Adsorption Selectivity of Carbon Dioxide and Ethane on Porous Metal–Organic Framework Functionalized by a Sulfur-Rich Heterocycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Methods
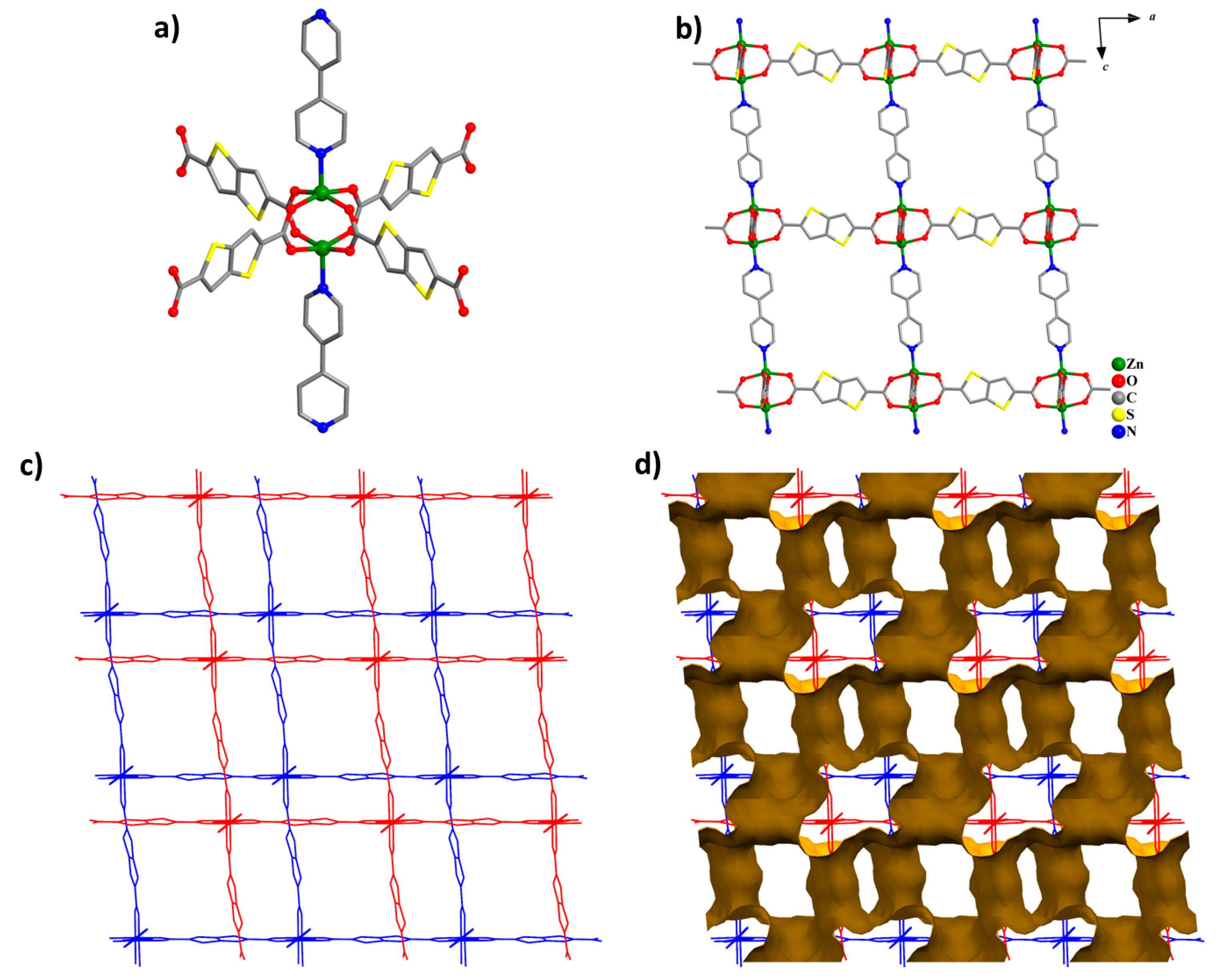
2.2. X-ray Crystallography
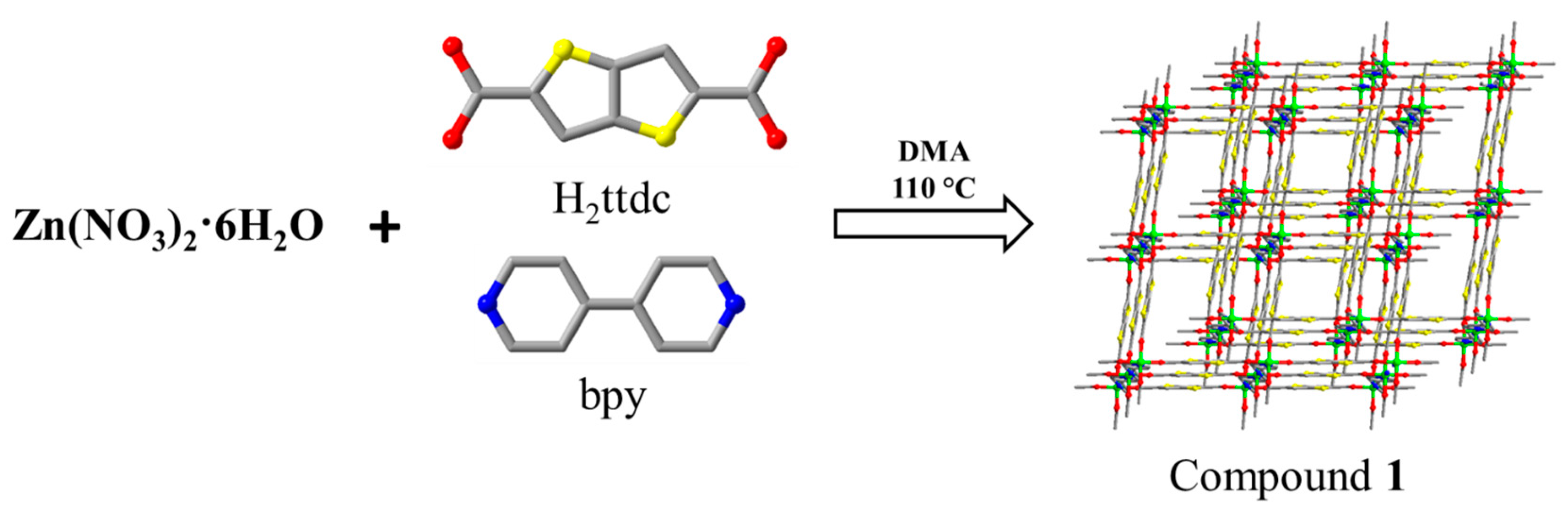
2.3. Synthesis of [Zn2(ttdc)2(bpy)]·3DMA (1)
2.4. Liquid Phase Separation Experiments
2.5. Computational Details
3. Results and Discussion
3.1. Adsorption Studies
3.2. Theoretical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reuters. Available online: https://www.reuters.com/business/energy/germanys-uniper-bring-coal-fired-power-plant-heyden-4-back-onto-electricity-2022-08-22/ (accessed on 25 October 2022).
- Zhu, X.; Xie, W.; Wu, J.; Miao, Y.; Xiang, C.; Chen, C.; Ge, B.; Gan, Z.; Yang, F.; Zhang, M.; et al. Recent advances in direct air capture by adsorption. Chem. Soc. Rev. 2022, 51, 6574–6651. [Google Scholar] [CrossRef] [PubMed]
- Amooghin, A.E.; Sanaeepur, H.; Luque, R.; Garcia, H.; Chen, B. Fluorinated metal—Organic frameworks for gas separation. Chem. Soc. Rev. 2022, 51, 7427–7508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Maddock, J.; Nenoff, T.M.; Denecke, M.A.; Yang, S.; Schröder, M. Adsorption of iodine in metal–organic framework materials. Chem. Soc. Rev. 2022, 51, 3243–3262. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mousavi, S.H.; Singh, R.; Snurr, R.Q.; Li, G.; Webley, P.A. Gating effect for gas adsorption in microporous materials—Mechanisms and applications. Chem. Soc. Rev. 2022, 51, 1139–1166. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, K.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. A novel Zn-based heterocycle metal-organic framework for high C2H2/C2H4, CO2/CH4 and CO2/N2 separations. J. Solid State Chem. 2017, 255, 102–107. [Google Scholar] [CrossRef]
- Chakraborty, G.; Das, P.; Mandal, S.K. Efficient and highly selective CO2 capture, separation, and chemical conversion under ambient conditions by a polar-group appended copper(II) metal–organic framework. Inorg. Chem. 2021, 60, 5071–5080. [Google Scholar] [CrossRef]
- Gandhi, S.; Das, P.; Mandal, S.K. A microporous, amino acid functionalized Zn(II)-organic framework nanoflower for selective CO2 capture and solvent encapsulation. Mater. Adv. 2020, 1, 1455–1463. [Google Scholar] [CrossRef]
- Bolotov, V.A.; Kovalenko, K.A.; Samsonenko, D.G.; Han, X.; Zhang, X.; Smith, G.L.; McCormick, L.J.; Teat, S.J.; Yang, S.; Lennox, M.J.; et al. Enhancement of CO2 uptake and selectivity in a metal–organic framework by the incorporation of thiophene functionality. Inorg. Chem. 2018, 57, 5074–5082. [Google Scholar] [CrossRef]
- Yoon, M.; Moon, D. New Zr (IV) based metal-organic framework comprising a sulfur-containing ligand: Enhancement of CO2 and H2 storage capacity. Microporous Mesoporous Mater. 2015, 215, 116–122. [Google Scholar] [CrossRef]
- Shi, Y.-X.; Li, W.-X.; Zhang, W.-H.; Lang, J.-P. Guest-induced switchable breathing behavior in a flexible metal–organic framework with pronounced negative gas pressure. Inorg. Chem. 2018, 57, 8627–8633. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiang, S.; Chen, Y.-S.; Ma, S.; Lee, Y.; Phely-Bobin, T.; Chen, B.A. Robust highly interpenetrated metal-organic framework constructed from pentanuclear clusters for selective sorption of gas molecules. Inorg. Chem. 2010, 49, 8444–8448. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A selenophene-incorporated metal–organic framework for enhanced CO2 uptake and adsorption selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A series of mesoporous metal-organic frameworks with tunable windows sizes and exceptionally high ethane over ethylene adsorption selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef] [PubMed]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the molecular and cationic affinity in a series of multifunctional metal–organic frameworks based on dodecanuclear Zn(II) carboxylate wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-Q.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 2015, 6, e8697. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, R.-B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362, 443–446. [Google Scholar] [CrossRef]
- Shimomura, S.; Horike, S.; Matsuda, R.; Kitagawa, S. Guest-specific function of a flexible undulating channel in a 7,7,8,8-tetracyano-p-quinodimethane dimer-based porous coordination polymer. J. Am. Chem. Soc. 2007, 129, 10990–10991. [Google Scholar] [CrossRef]
- Shimomura, S.; Matsuda, R.; Kitagawa, S. Flexibility of porous coordination polymers strongly linked to selective sorption mechanism. Chem. Mater. 2010, 22, 4129–4131. [Google Scholar] [CrossRef]
- Hijikata, Y.; Horike, S.; Sugimoto, M.; Sato, H.; Matsuda, R.; Kitagawa, S. Relationship between Channel and Sorption Properties in Coordination Polymers with Interdigitated Structures. Chem. Eur. J. 2011, 17, 5138–5144. [Google Scholar] [CrossRef]
- Thermophysical Properties of Fluid Systems, Database of National Institute of Standards and Technology, NIST. Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 29 October 2022).
- Bruker Apex3 Software Suite: Apex3, SADABS-2016/2 and SAINT; Version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, e154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Peng, H.; Yang, Z.-H.; Perdew, J.P.; Sun, J. Versatile van der Waals density functional based on a meta-generalized gradient approximation. Phys. Rev. X 2016, 6, e041005. [Google Scholar] [CrossRef]
- Bultinck, P.; Van Alsenoy, C.; Ayers, P.W.; Carbó Dorca, R. Critical analysis and extension of the Hirshfeld atoms in molecules. J. Chem. Phys. 2007, 126, e144111. [Google Scholar] [CrossRef]
- Tkatchenko, A.; DiStasio, R.A.; Car, R.; Scheffler, M. Accurate and efficient method for many-body van der Waals interactions. Phys. Rev. Lett. 2012, 108, e236402. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, A.; Reilly, A.M.; DiStasio Jr, R.A.; Tkatchenko, A. Long-range correlation energy calculated from coupled atomic response functions. J. Chem. Phys. 2014, 140, 018A508. [Google Scholar] [CrossRef] [PubMed]
- Bucko, T.; Lebègue, S.; Gould, T.; Ángyán, J.G. Many-body dispersion corrections for periodic systems: An efficient reciprocal space implementation. J. Phys. Condens. Matter. 2016, 28, e045201. [Google Scholar] [CrossRef] [PubMed]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Exploration of chemical compound, conformer, and reaction space with meta-dynamics simulations based on tight-binding quantum chemical calculations. J. Chem. Theory Comput. 2019, 15, 2847–2862. [Google Scholar] [CrossRef] [PubMed]
- Spicher, S.; Grimme, S. Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. Int. Ed. 2020, 59, 15665–15673. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized Self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 11, e01493. [Google Scholar] [CrossRef]
- Chun, H.; Dybtsev, D.N.; Kim, H.; Kim, K. Synthesis, X-ray crystal structures, and gas sorption properties of pillared square grid nets based on paddle-wheel motifs: Implications for hydrogen storage in porous materials. Chem. Eur. J. 2005, 11, 3521–3529. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Zhang, Z.; Nijem, N.; Chabal, Y.J.; Zeng, H.; Li, J. The effect of methyl functionalization on microporous metal-organic frameworks’ capacity and binding energy for carbon dioxide adsorption. Adv. Funct. Mater. 2011, 21, 4754–4762. [Google Scholar] [CrossRef]
- Li, G.; Zhu, C.; Xi, X.; Cui, Y. Selective binding and removal of organic molecules in a flexible polymeric material with stretchable metallosalen chains. Chem. Commun. 2009, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Luo, D.; Li, M.; Li, D. Local deprotonation enables cation exchange, porosity modulation and tunable adsorption selectivity in a metal-organic framework. Cryst. Growth Des. 2017, 6, 3387–3394. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kovalenko, K.A.; Samsonenko, D.G.; Barsukova, M.O.; Dybtsev, D.N.; Fedin, V.P. Exceptionally effective benzene/cyclohexane separation using a nitro-decorated metal–organic framework. Chem. Commun. 2020, 56, 8241–8244. [Google Scholar] [CrossRef] [PubMed]
- Poryvaev, A.S.; Yazikova, A.A.; Polyukhov, D.M.; Fedin, M.V. Ultrahigh selectivity of benzene/cyclohexane separation by ZIF-8 framework: Insights from spin-probe EPR spectroscopy. Microporous Mesoporous Mater. 2022, 330, 111564. [Google Scholar] [CrossRef]
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.K.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.B.; Yaghi, O.M. A combined experimental–computational investigation of carbon dioxide capture in a series of isoreticular zeolitici frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008. [Google Scholar] [CrossRef]
- Duan, J.; Higuchi, M.; Krishna, R.; Kiyonaga, T.; Tsutsumi, Y.; Sato, Y.; Kubota, Y.; Takata, M.; Kitagawa, S. High CO2/N2/O2/CO separation in a chemically robust porous coordination polymer with low binding energy. Chem. Sci. 2014, 5, 660–666. [Google Scholar] [CrossRef]
- Sahoo, R.; Mondal, S.; Mukherjee, D.; Das, M.C. Metal–Organic Frameworks for CO2 Separation from Flue and Biogas Mixtures. Adv. Funct. Mater. 2022, 32, 2207197. [Google Scholar] [CrossRef]
- Chen, D.-M.; Zhang, X.-P.; Shi, W.; Cheng, P. Microporous metal–organic framework based on a bifunctional linker for selective sorption of CO2 over N2 and CH4. Inorg. Chem. 2015, 54, 5512–5518. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, J.-H.; Meng, D.-X.; Ge, F.-Y.; Wang, L.-F.; Xu, Y.-K.; Liu, X.-G.; Meng, M.-M.; Lu, Z.-Z.; Zheng, H.-G.; et al. Three metal–organic framework isomers of different pore sizes for selective CO2 adsorption and isomerization studies. Dalton Trans. 2020, 49, 5618–5624. [Google Scholar] [CrossRef]
- Nandi, S.; Collins, S.; Chakraborty, D.; Banerjee, D.; Thallapally, P.K.; Woo, T.K.; Vaidhyanathan, R. Ultralow parasitic energy for postcombustion CO2 capture realized in a nickel isonicotinate metal–organic framework with excellent moistures. J. Am. Chem. Soc. 2017, 139, 1734–1737. [Google Scholar] [CrossRef]
- Pal, A.; Chand, S.; Madden, D.G.; Franz, D.; Ritter, L.; Johnson, A.; Space, B.; Curtin, T.; Das, M.C. A Microporous Co-MOF for Highly Selective CO2 Sorption in High Loadings Involving Aryl C–H···O=C=O Interactions: Combined Simulation and Breakthrough Studies. Inorg. Chem. 2019, 58, 11553–11560. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Comm. 2012, 3, 954. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Yao, S.; Krishna, R.; Gu, J.; Li, G.; Liu, Y. A multifunctional double walled zirconium metal–organic framework: High performance for CO2 adsorption and separation and detecting explosives in the aqueous phase. J. Mater. Chem. A 2020, 8, 17106–17112. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, W.; Zhang, M.; Bai, J. Solvents-Dependent Formation of Three MOFs from the Fe3O Cluster and 3,3′,5,5′-Diphenyltetracarboxylic Acid and Their Selective CO2 Adsorption. Inorg. Chem. 2019, 58, 13836–13842. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef]
- McDonald, T.M.; D’Alessandro, D.M.; Krishna, R.; Long, J.R. Enhanced carbon dioxide capture upon incorporation of N,N′-dimethylethylenediamine in the metal–organic framework CuBTTri. Chem. Sci. 2011, 2, 2022–2028. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]



| Gas | 273 K | 298 K | ||||
|---|---|---|---|---|---|---|
| cm3 (STP)·g–1 | mmol·g–1 | wt. % | cm3 (STP)·g–1 | mmol·g–1 | wt. % | |
| CO2 | 22.3 | 0.99 | 4.2 | 12.1 | 0.54 | 2.3 |
| CH4 | 5.7 | 0.25 | 0.4 | 3.1 | 0.14 | 0.2 |
| N2 | 1.3 | 0.06 | 0.2 | 0.7 | 0.03 | 0.1 |
| C2H2 | 22.1 | 0.99 | 2.5 | 14.8 | 0.66 | 1.7 |
| C2H4 | 20.8 | 0.93 | 2.5 | 13.3 | 0.59 | 1.6 |
| C2H6 | 21.1 | 0.94 | 2.7 | 12.8 | 0.57 | 1.7 |
| CO | 2.4 | 0.11 | 0.3 | 1.5 | 0.07 | 0.2 |
| O2 | 1.7 | 0.07 | 0.2 | 1.1 | 0.05 | 0.2 |
| Gas Mixtures | 273 K | 298 K | ||||
|---|---|---|---|---|---|---|
| V1/V2 | KH1/KH2 | IAST | V1/V2 | KH1/KH2 | IAST | |
| CO2/N2 | 17.2 | 93.8 | 34.0 (73.6 a) | 17.3 | 58.8 | 27.2 (56.4 a) |
| CO2/CH4 | 3.9 | 15.8 | 14.4 (38.3 b, 143.9 c) | 3.9 | 10.6 | 11.7 (29.1 b, 93.7 c) |
| C2H2/C2H4 | 1.06 | 1.48 | 1.12 | 1.11 | 1.27 | 1.12 |
| C2H6/C2H2 | 0.95 | 1.09 | 2.08 | 0.86 | 1.13 | 3.50 |
| C2H6/CH4 | 3.7 | 30.5 | 12.0 (28.4 b) | 4.1 | 17.6 | 14.8 (39.1 b) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubskikh, V.A.; Kovalenko, K.A.; Nizovtsev, A.S.; Lysova, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Enhanced Adsorption Selectivity of Carbon Dioxide and Ethane on Porous Metal–Organic Framework Functionalized by a Sulfur-Rich Heterocycle. Nanomaterials 2022, 12, 4281. https://doi.org/10.3390/nano12234281
Dubskikh VA, Kovalenko KA, Nizovtsev AS, Lysova AA, Samsonenko DG, Dybtsev DN, Fedin VP. Enhanced Adsorption Selectivity of Carbon Dioxide and Ethane on Porous Metal–Organic Framework Functionalized by a Sulfur-Rich Heterocycle. Nanomaterials. 2022; 12(23):4281. https://doi.org/10.3390/nano12234281
Chicago/Turabian StyleDubskikh, Vadim A., Konstantin A. Kovalenko, Anton S. Nizovtsev, Anna A. Lysova, Denis G. Samsonenko, Danil N. Dybtsev, and Vladimir P. Fedin. 2022. "Enhanced Adsorption Selectivity of Carbon Dioxide and Ethane on Porous Metal–Organic Framework Functionalized by a Sulfur-Rich Heterocycle" Nanomaterials 12, no. 23: 4281. https://doi.org/10.3390/nano12234281
APA StyleDubskikh, V. A., Kovalenko, K. A., Nizovtsev, A. S., Lysova, A. A., Samsonenko, D. G., Dybtsev, D. N., & Fedin, V. P. (2022). Enhanced Adsorption Selectivity of Carbon Dioxide and Ethane on Porous Metal–Organic Framework Functionalized by a Sulfur-Rich Heterocycle. Nanomaterials, 12(23), 4281. https://doi.org/10.3390/nano12234281









