Photon-Energy-Dependent Reversible Charge Transfer Dynamics of Double Perovskite Nanocrystal-Polymer Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
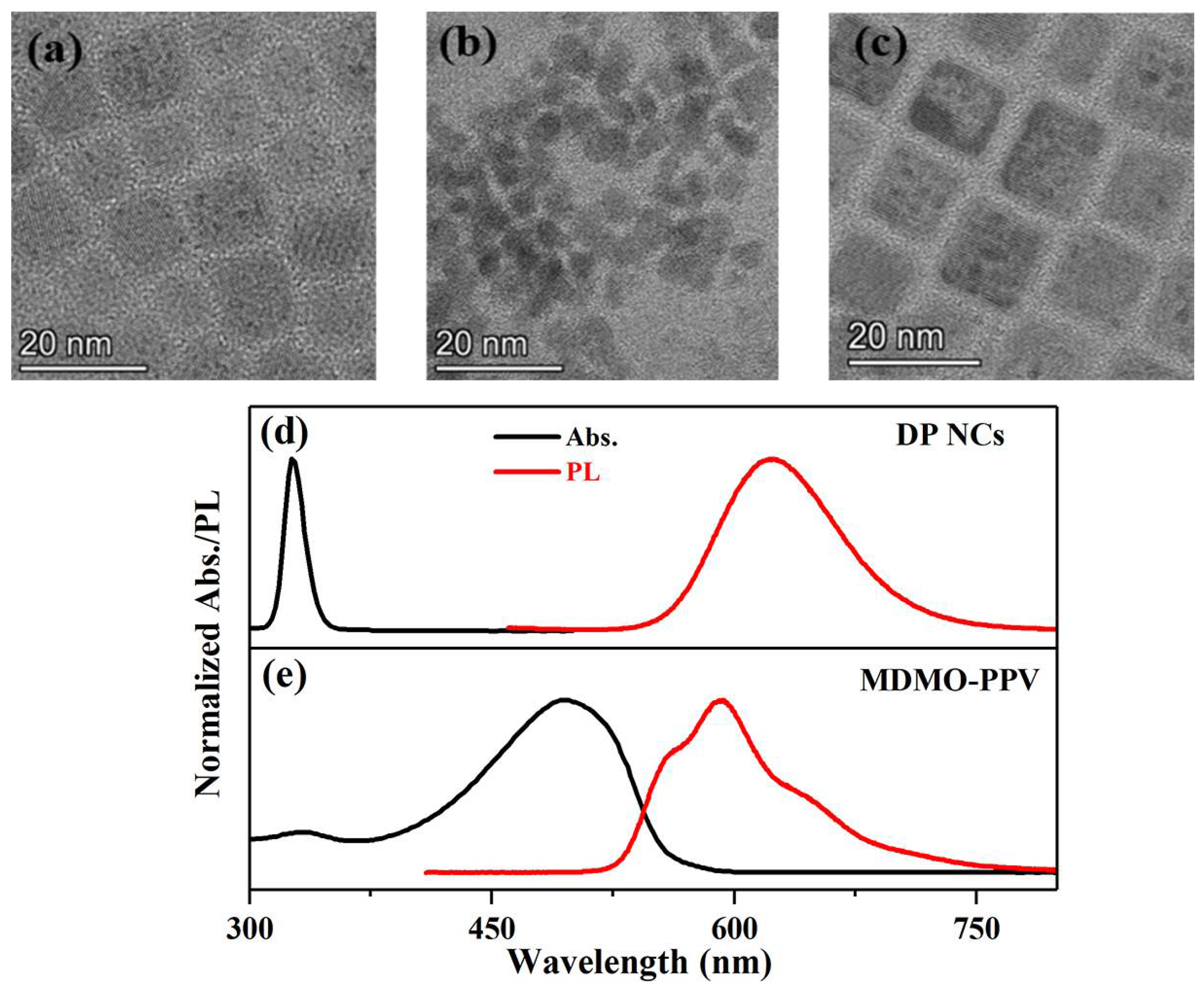
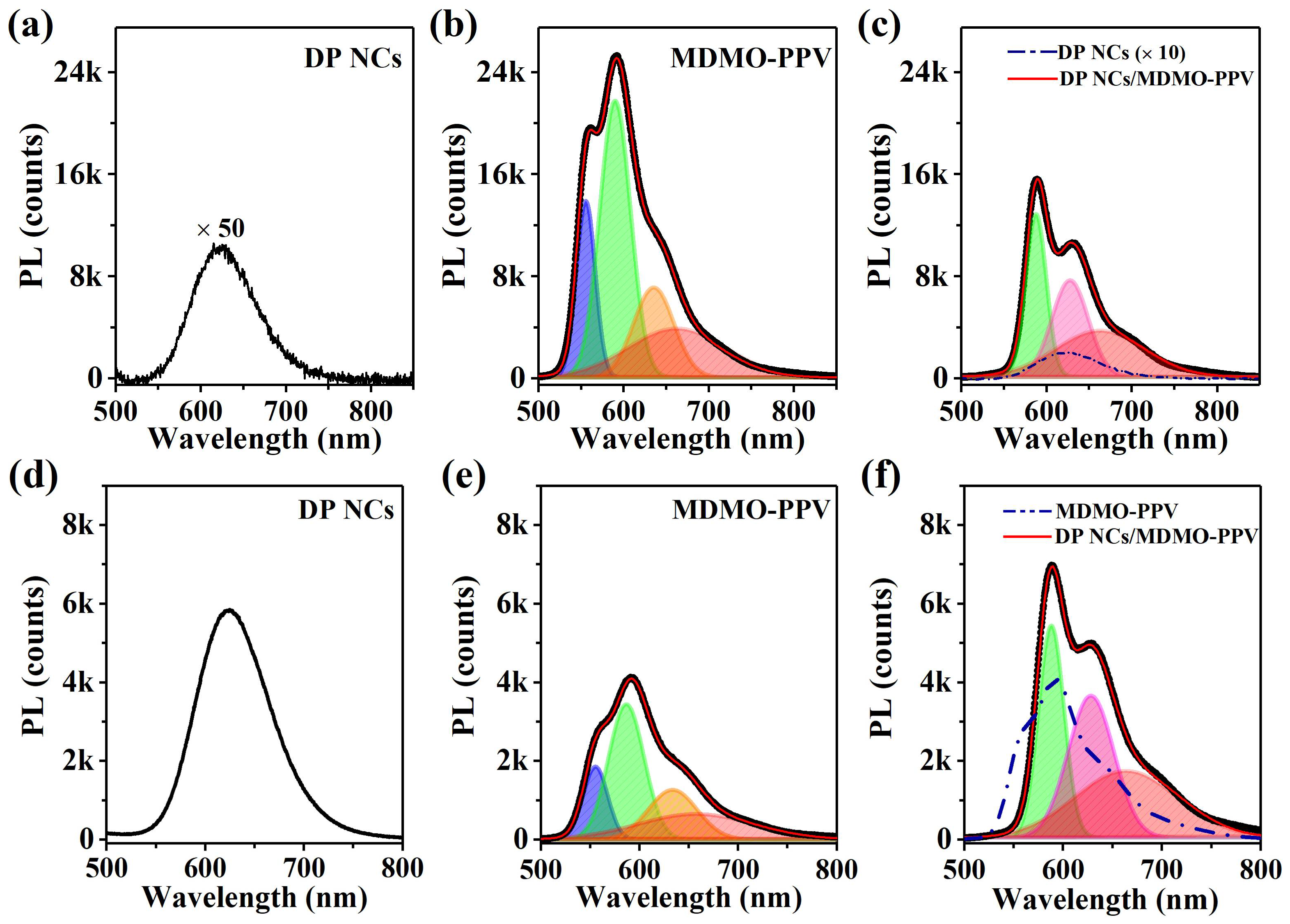
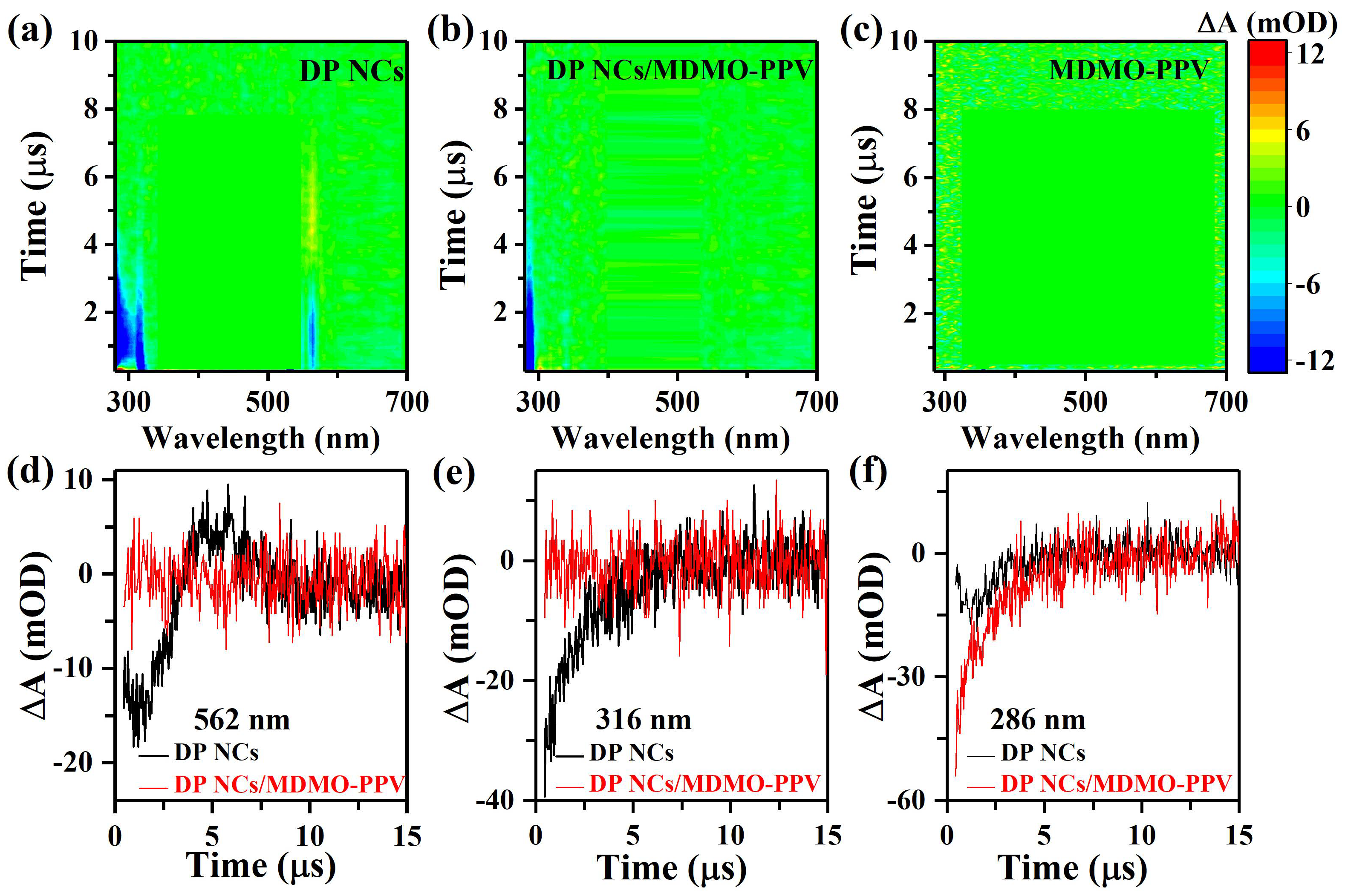
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, M.; Jiang, Y.; He, T.; Yuan, M. Metal halide perovskites for blue light emitting materials. APL Mater. 2020, 8, 040907. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gao, X.; Zhang, X.; Shen, X.; Lu, M.; Wu, J.; Shi, Z.; Colvin, V.L.; Hu, J.; Bai, X.; et al. Lead-free halide perovskites for light emission: Recent advances and perspectives. Adv. Sci. 2021, 8, 2003334. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Zhang, X.; Xie, H.; Cai, J.; Wang, C.; Chen, E.; Xu, S.; Ye, Y.; Sun, J.; Yan, Q.; et al. Perovskite quantum dots for emerging displays: Recent progress and perspectives. Nanomaterials 2022, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cui, M.; Li, S.; Sun, C.; Huang, Y.; Wei, J.; Zhang, L.; Lv, M.; Qin, C.; Liu, Y.; et al. Reducing the impact of Auger recombination in quasi-2D perovskite light-emitting diodes. Nat. Commun. 2021, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jiang, T.; Cao, Y.; Yi, C.; Wang, N.; Huang, W.; Wang, J. Multiple-quantum-well perovskites for high-performance light-emitting diodes. Adv. Mater. 2019, 32, 1904163. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ou, Q.; Wang, C.; Si, G.; Shabbir, B.; Zheng, C.; Wang, Z.; Zhang, Y.; Huang, Y.; Dong, Y.; et al. Capillary-bridge mediated assembly of aligned perovskite quantum dots for high-performance photodetectors. J. Mater. Chem. C 2019, 7, 5954–5961. [Google Scholar] [CrossRef]
- Zheng, J.; Luo, C.; Shabbir, B.; Wang, C.; Mao, W.; Zhang, Y.; Huang, Y.; Dong, Y.; Jasieniak, J.J.; Pan, C.; et al. Flexible photodetectors based on reticulated SWNTS/perovskite quantum dot heterostructures with ultrahigh durability. Nanoscale 2019, 11, 8020–8026. [Google Scholar] [CrossRef]
- Bi, C.; Kershaw, S.V.; Rogach, A.L.; Tian, J. Improved stability and photodetector performance of CsPbI3 perovskite quantum dots by ligand exchange with Aminoethanethiol. Adv. Funct. Mater. 2019, 29, 1902446. [Google Scholar] [CrossRef]
- Zhuang, B.; Liu, Y.; Yuan, S.; Huang, H.; Chen, J.; Chen, D. Glass stabilized ultra-stable dual-emitting Mn-doped cesium lead halide perovskite quantum dots for cryogenic temperature sensing. Nanoscale 2019, 11, 15010–15016. [Google Scholar] [CrossRef]
- Ding, N.; Zhou, D.; Pan, G.; Xu, W.; Chen, X.; Li, D.; Zhang, X.; Zhu, J.; Ji, Y.; Song, H. Europium-doped lead-free Cs3Bi2Br9 perovskite quantum dots and ultrasensitive Cu2+ detection. ACS Sustain. Chem. Eng. 2019, 7, 8397–8404. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.C.; Kanatzidis, M.G. “Unleaded” perovskites: Status quo and future prospects of Tin-based perovskite solar cells. Adv. Mater. 2019, 31, 1803230. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Qin, C.; Xiao, L.; Chang, Y.; Li, H. Dimethyl sulfoxide and bromide methylamine co-treatment inducing defect healing for effective and stable perovskite solar cells. Mater. Res. Bull. 2019, 112, 165–173. [Google Scholar] [CrossRef]
- Beljonne, D.; Pourtois, G.; Silva, C.; Hennebicq, E.; Herz, L.M.; Friend, R.H.; Scholes, G.D.; Setayesh, S.; Müllen, K.; Brédas, J.L. Interchain vs. intrachain energy transfer in acceptor-capped conjugated polymers. Proc. Natl. Acad. Sci. USA 2002, 99, 10982–10987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Du, X.; Zeng, Q.; Yang, B. Recent development and understanding of polymer–nanocrystal hybrid solar cells. Mater. Chem. Front. 2017, 1, 1502–1513. [Google Scholar] [CrossRef]
- Mantela, M.; Lambropoulos, K.; Theodorakou, M.; Simserides, C. Quasi-periodic and fractal polymers: Energy structure and carrier transfer. Materials 2019, 12, 2177. [Google Scholar] [CrossRef] [Green Version]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-j.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Al-Asbahi, B.A.; Ghaithan, H.M.; Aldwayyan, A.S. Tuning the optical properties of MEH–PPV/PFO hybrid thin films via the incorporation of CsPbBr3 quantum dots. Coatings 2021, 11, 154. [Google Scholar] [CrossRef]
- Masi, S.; Colella, S.; Listorti, A.; Roiati, V.; Liscio, A.; Palermo, V.; Rizzo, A.; Gigli, G. Growing perovskite into polymers for easy-processable optoelectronic devices. Sci. Rep. 2015, 5, 7725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikalova-Luzina, O.P.; Aleshin, A.N.; Shcherbakov, I.P.; Vyatkin, V.M.; Matyushkin, L.B. Energy transfer in hybrid optoelectronic structures between perovskite nanocrystals and an organic matrix. Synth. Met. 2018, 246, 230–235. [Google Scholar] [CrossRef]
- Balena, A.; Cretí, A.; Lomascolo, M.; Anni, M. Investigation of the exciton relaxation processes in poly(9,9-dioctylfluorene-co-benzothiadiazole):CsPbI1.5Br1.5 nanocrystal hybrid polymer–perovskite nanocrystal blend. RSC Adv. 2021, 11, 33531–33539. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Mao, X.; Yang, S.; Zhang, F.; Yang, B.; Wei, D.; Deng, W.; Han, K. Lead-free sodium-indium double perovskite nanocrystals through doping silver cations for bright yellow emission. Angew. Chem. Int. Ed. 2019, 58, 17231–17235. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, X.; Mao, X.; Yang, B.; Yang, S.; Feng, Z.; Wei, D.; Deng, W.; Pullerits, T.; Han, K. Size effect of lead-free halide double perovskite on luminescence property. Sci. China Chem. 2019, 62, 1405–1413. [Google Scholar] [CrossRef]
- Han, P.; Zhang, X.; Luo, C.; Zhou, W.; Yang, S.; Zhao, J.; Deng, W.; Han, K. Manganese-doped, lead-free double perovskite nanocrystals for bright orange-red emission. ACS Cent. Sci. 2020, 6, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Han, K. Recent advances in all-inorganic lead-free three-dimensional halide double perovskite nanocrystals. Energy Fuels 2021, 35, 18871–18887. [Google Scholar] [CrossRef]
- Han, P.; Luo, C.; Zhou, W.; Hou, J.; Li, C.; Zheng, D.; Han, K. Band-gap engineering of lead-free Iron-based halide double-perovskite single crystals and nanocrystals by an alloying or doping strategy. J. Phys. Chem. C 2021, 125, 11743–11749. [Google Scholar] [CrossRef]
- Wu, R.; Han, P.; Zheng, D.; Zhang, J.; Yang, S.; Zhao, Y.; Miao, X.; Han, K. All-inorganic rare-earth-based double perovskite nanocrystals with near-infrared emission. Laser Photonics Rev. 2021, 15, 2100218. [Google Scholar] [CrossRef]
- Zheng, M.; Bai, F.; Zhu, D. Photophysical process of MEH-PPV solution. J. Photochem. Photobiol. A Chem. 1998, 116, 143–145. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Doan, V.; Schwartz, B.J. Conjugated polymer aggregates in solution: Control of interchain interactions. J. Chem. Phys. 1999, 110, 4068–4078. [Google Scholar] [CrossRef]
- Potai, R.; Traiphol, R. Controlling chain organization and photophysical properties of conjugated polymer nanoparticles prepared by reprecipitation method: The effect of initial solvent. J. Colloid Interface Sci. 2013, 403, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ryan, J.W.; Singh, A.; Beirne, J.G.; Palomares, E.; Redmond, G. Encapsulation of MEH-PPV:PCBM hybrids in the cores of block copolymer micellar assemblies: Photoinduced electron transfer in a nanoscale donor-acceptor system. Langmuir 2016, 32, 329–337. [Google Scholar] [CrossRef]
- Mohan, S.R.; Joshi, M.P.; Dhami, T.S.; Awasthi, V.; Shalu, C.; Singh, B.; Singh, V. Charge transport in thin films of MDMO PPV dispersed with lead sulfide nanoparticles. Synth. Met. 2017, 224, 80–85. [Google Scholar] [CrossRef]
- Ton-That, C.; Phillips, M.R.; Nguyen, T.-P. Blue shift in the luminescence spectra of MEH-PPV films containing ZnO nanoparticles. J. Lumin. 2008, 128, 2031–2034. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, A.; Uriarte, L.M.; Mutoh, K.; Fron, E.; Hofkens, J.; Sliwa, M.; Abe, J. Photochromic reaction by red light via triplet fusion upconversion. J. Am. Chem. Soc. 2019, 141, 17744–17753. [Google Scholar] [CrossRef]
- Ruckebusch, C.; Sliwa, M.; Pernot, P.; de Juan, A.; Tauler, R. Comprehensive data analysis of femtosecond transient absorption spectra: A review. J. Photochem. Photobiol. C 2012, 13, 1–27. [Google Scholar] [CrossRef]
- Rossi, A.; Price, M.B.; Hardy, J.; Gorman, J.; Schmidt, T.W.; Davis, N.J.L.K. Energy transfer between perylene diimide based ligands and cesium lead bromide perovskite nanocrystals. J. Phys. Chem. C 2020, 124, 3306–3313. [Google Scholar] [CrossRef]
- Wu, R.; Guo, X.; Luo, J.; Miao, X.; Zhang, J. Manipulating exciton transfer between colloidal quantum dots and graphene oxide. J. Phys. Chem. C 2020, 124, 25038–25042. [Google Scholar] [CrossRef]
- Wu, K.; Liang, G.; Shang, Q.; Ren, Y.; Kong, D.; Lian, T. Ultrafast interfacial electron and hole transfer from CsPbBr3 perovskite quantum dots. J. Am. Chem. Soc. 2015, 137, 12792–12795. [Google Scholar] [CrossRef]
- Yan, Q.-Q.; Wu, D.-X.; Chu, S.-Q.; Chen, Z.-Q.; Lin, Y.; Chen, M.-X.; Zhang, J.; Wu, X.-J.; Liang, H.-W. Reversing the charge transfer between platinum and sulfur-doped carbon support for electrocatalytic hydrogen evolution. Nat. Commun. 2019, 10, 4977. [Google Scholar] [CrossRef] [PubMed]
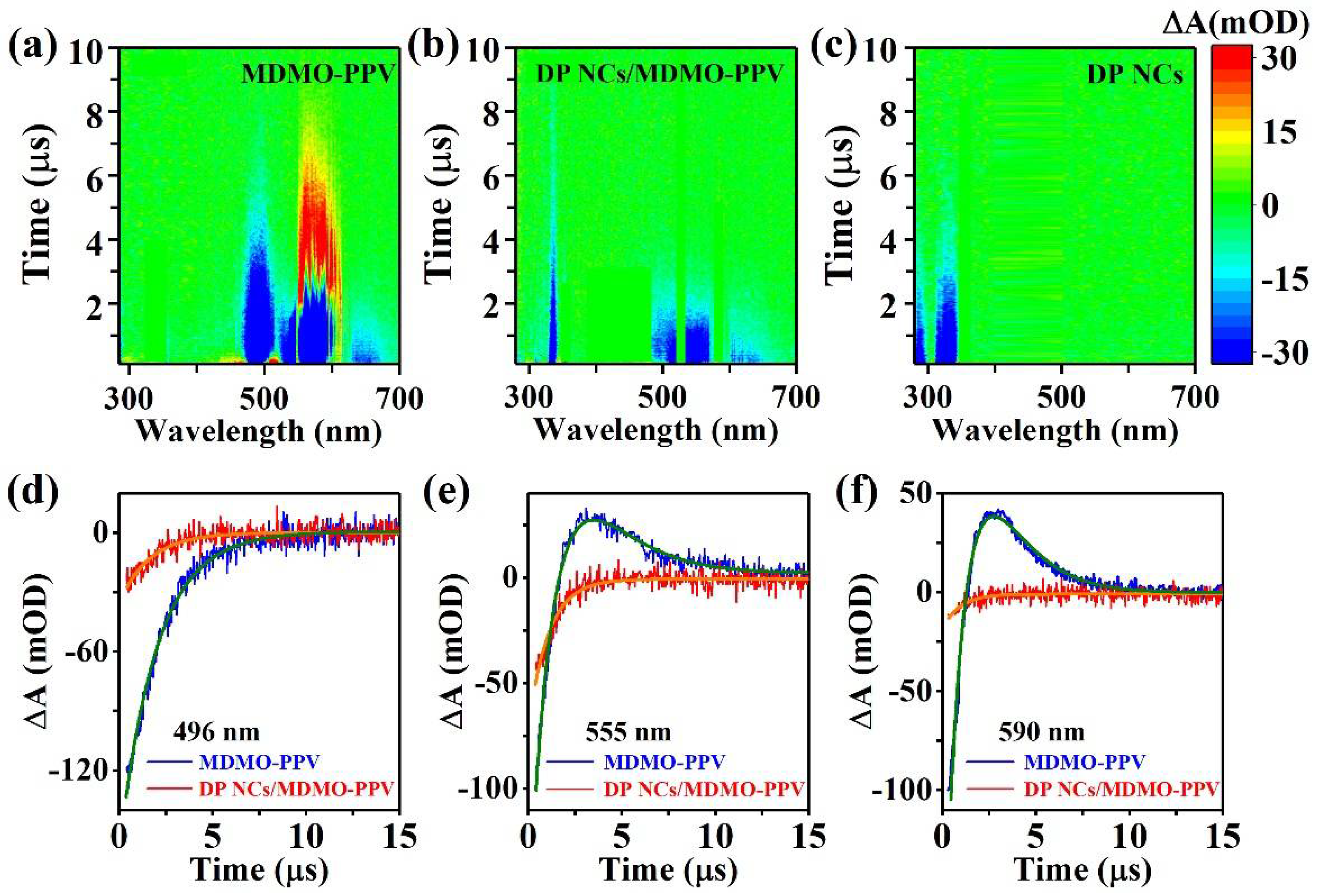
| λ (nm) | MDMO-PPV (µs) | DP NCs/MDMO-PPV (µs) |
|---|---|---|
| 496 | τGSB = 2.00 | τGSB = 1.58 |
| 555 | τPIA = 1.30, τSE = 1.22 | τSE = 1.08 |
| 590 | τPIA = 1.48, τSE = 1.45 | τSE = 0.92 |
| 635/660 | τSE = 1.32 | τSE = 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Wang, X.; Luo, J.; Liu, X.; Guo, F.; Li, B.; Wang, S.; Han, P.; Miao, X. Photon-Energy-Dependent Reversible Charge Transfer Dynamics of Double Perovskite Nanocrystal-Polymer Nanocomposites. Nanomaterials 2022, 12, 4300. https://doi.org/10.3390/nano12234300
Wu R, Wang X, Luo J, Liu X, Guo F, Li B, Wang S, Han P, Miao X. Photon-Energy-Dependent Reversible Charge Transfer Dynamics of Double Perovskite Nanocrystal-Polymer Nanocomposites. Nanomaterials. 2022; 12(23):4300. https://doi.org/10.3390/nano12234300
Chicago/Turabian StyleWu, Ruixiang, Xiaoshuai Wang, Jingjing Luo, Xin Liu, Fengjie Guo, Bin Li, Shengzhi Wang, Peigeng Han, and Xiangyang Miao. 2022. "Photon-Energy-Dependent Reversible Charge Transfer Dynamics of Double Perovskite Nanocrystal-Polymer Nanocomposites" Nanomaterials 12, no. 23: 4300. https://doi.org/10.3390/nano12234300
APA StyleWu, R., Wang, X., Luo, J., Liu, X., Guo, F., Li, B., Wang, S., Han, P., & Miao, X. (2022). Photon-Energy-Dependent Reversible Charge Transfer Dynamics of Double Perovskite Nanocrystal-Polymer Nanocomposites. Nanomaterials, 12(23), 4300. https://doi.org/10.3390/nano12234300