Sensitization of ZnO Photoconductivity in the Visible Range by Colloidal Cesium Lead Halide Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.1.1. Synthesis of Nanocrystalline ZnO
2.1.2. Synthesis of Perovskite Colloidal Nanocrystals
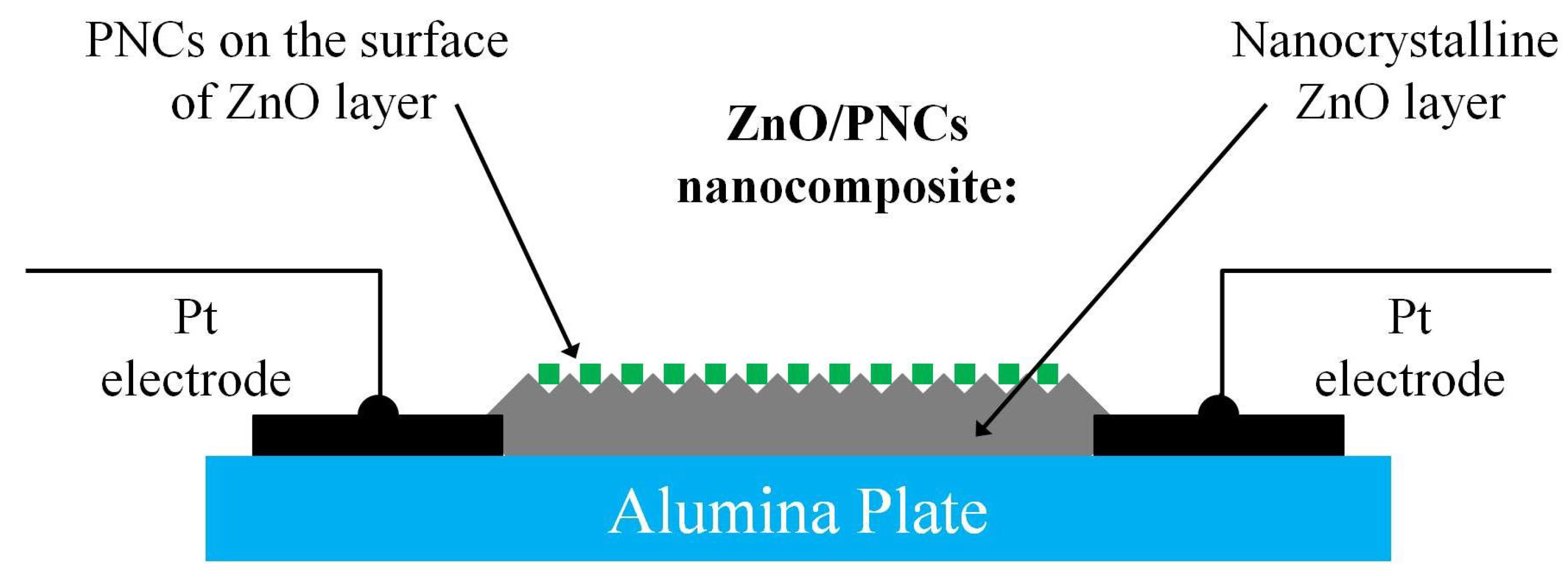
2.1.3. Fabrication of Photoconductive Elements
2.2. Characterization of Materials
2.3. Photoconductivity Measurements
3. Results
3.1. Characterization of Nanocrystals
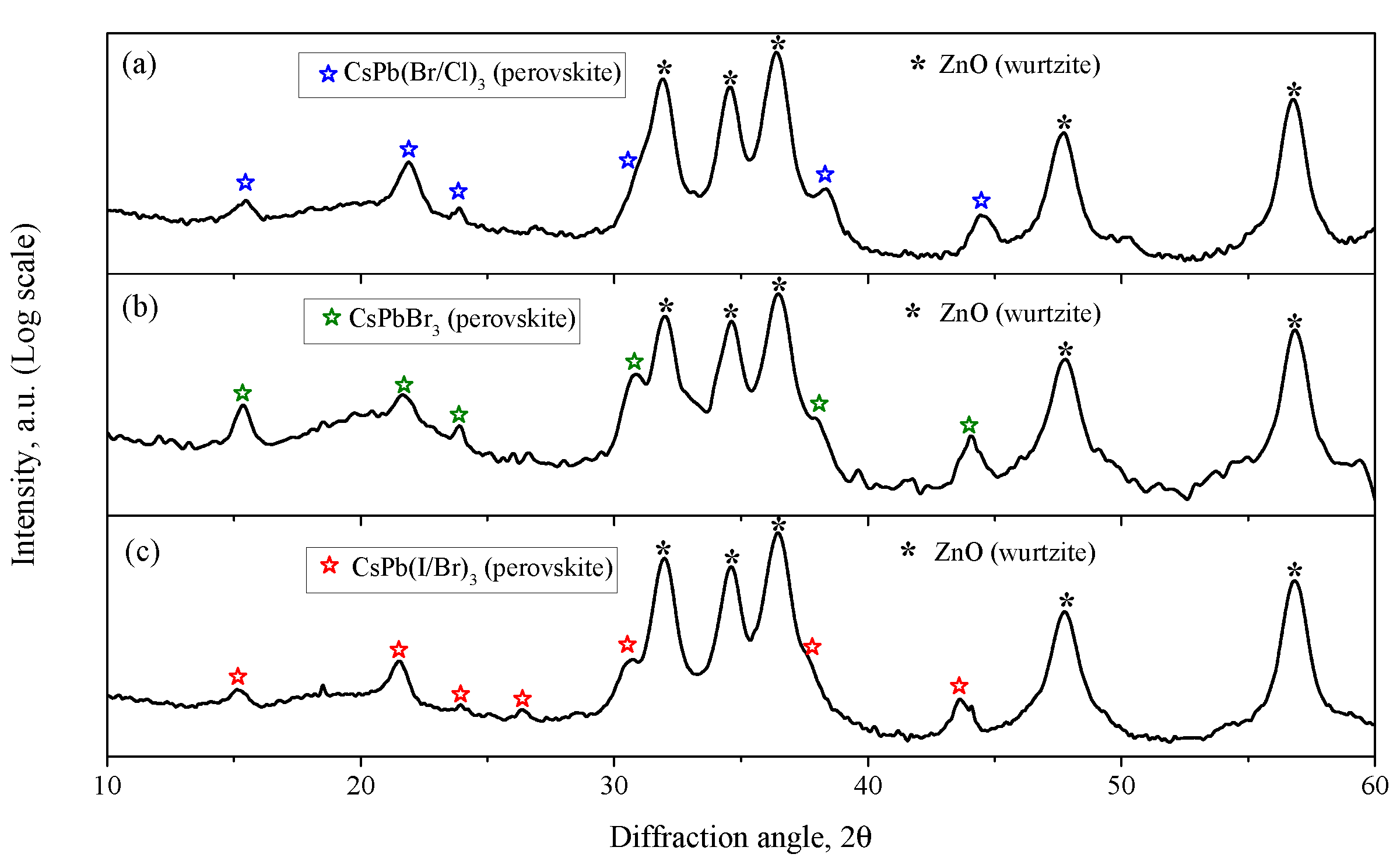
3.1.1. Crystal Structure and Morphology
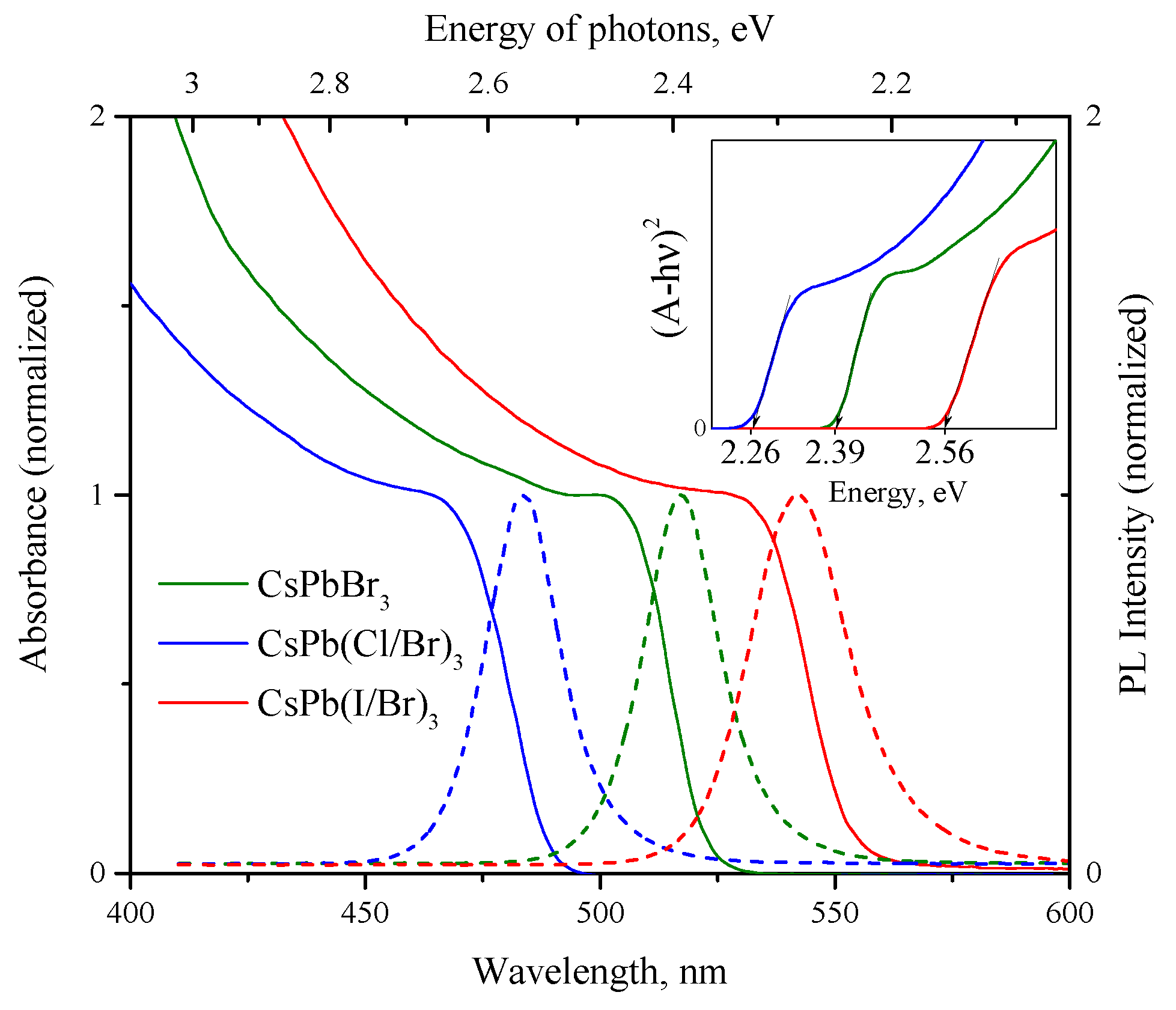
3.1.2. Optical Properties
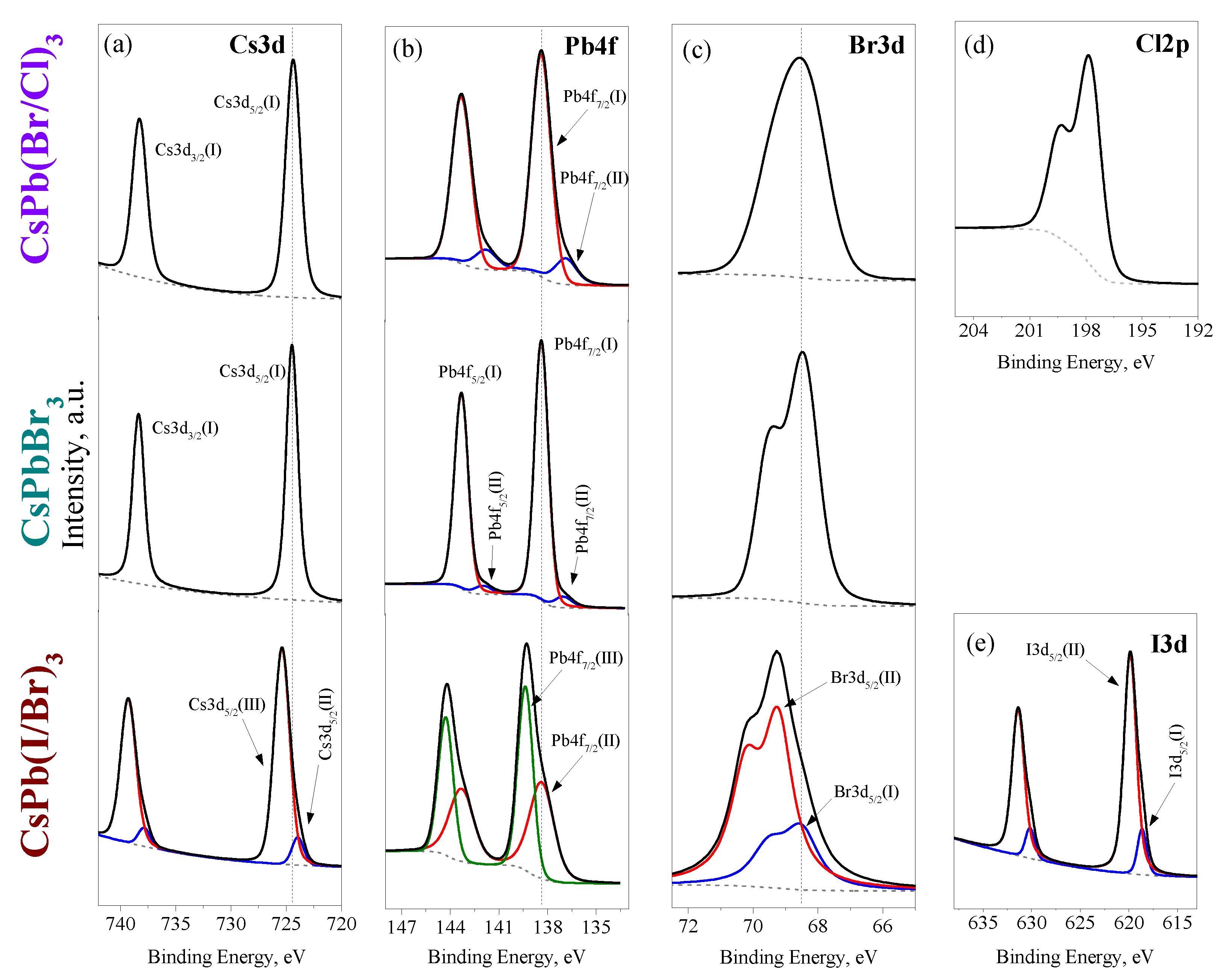
3.1.3. Charge States of Atoms in PNCs
3.1.4. Chemical Composition of PNCs
3.2. Characterization of ZnO/PNCs Nanocomposites
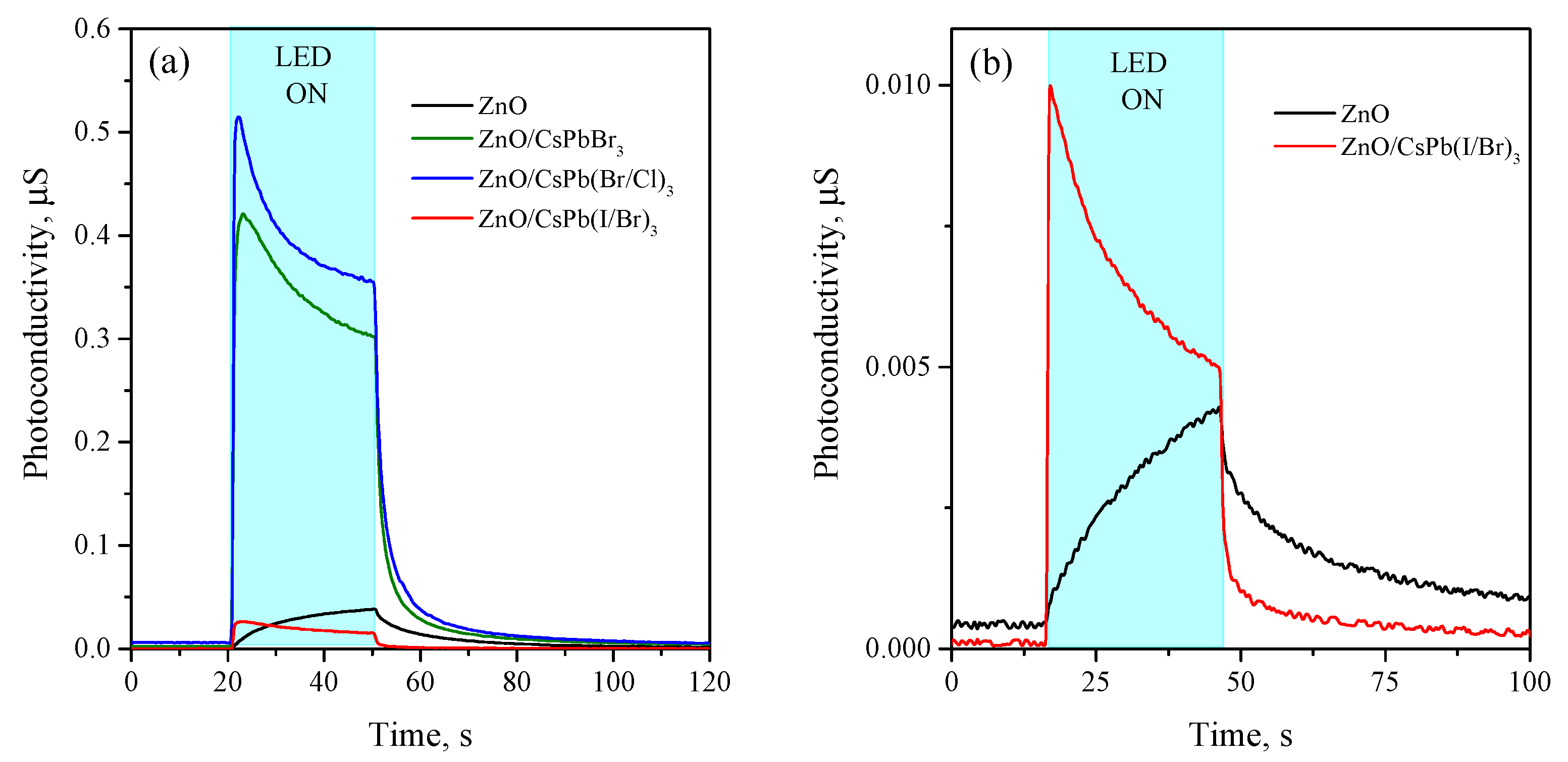
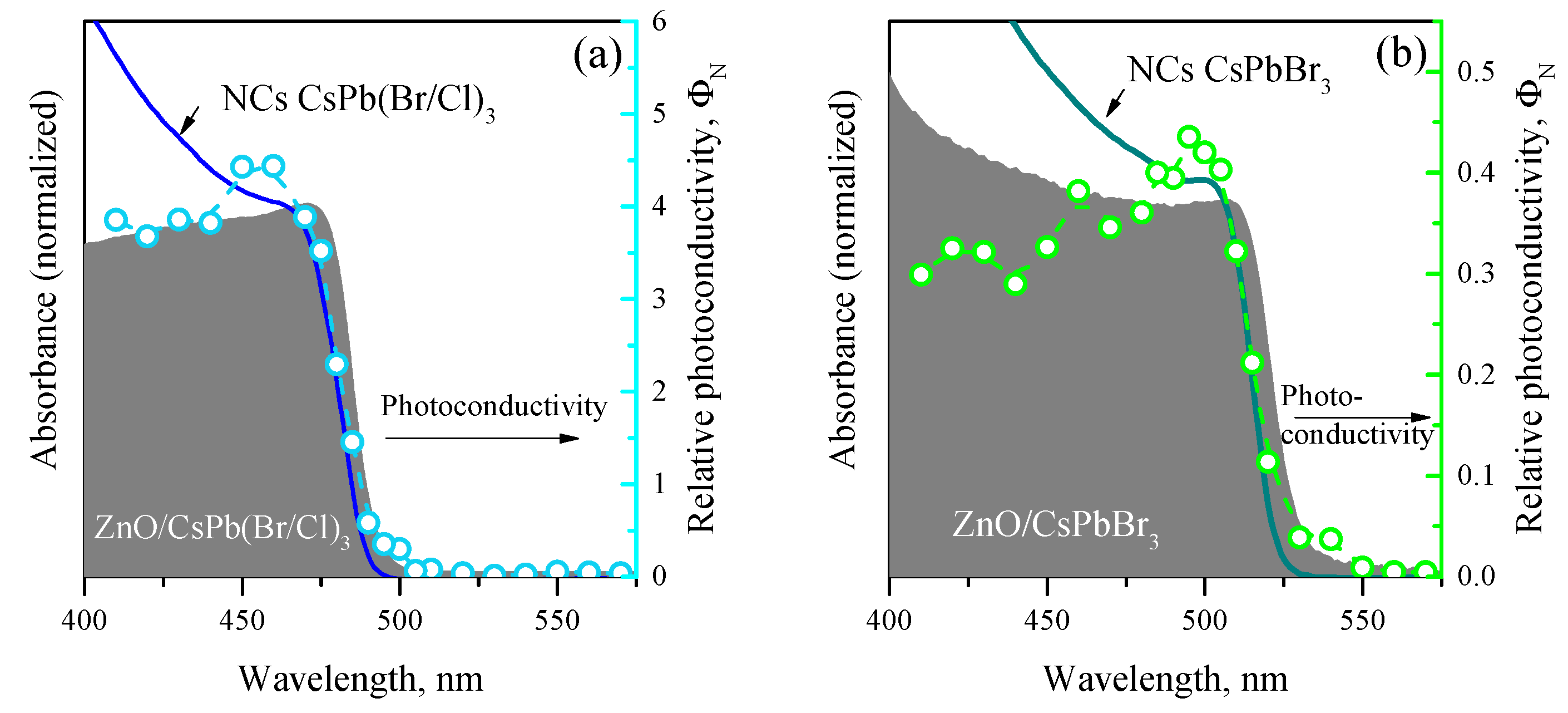
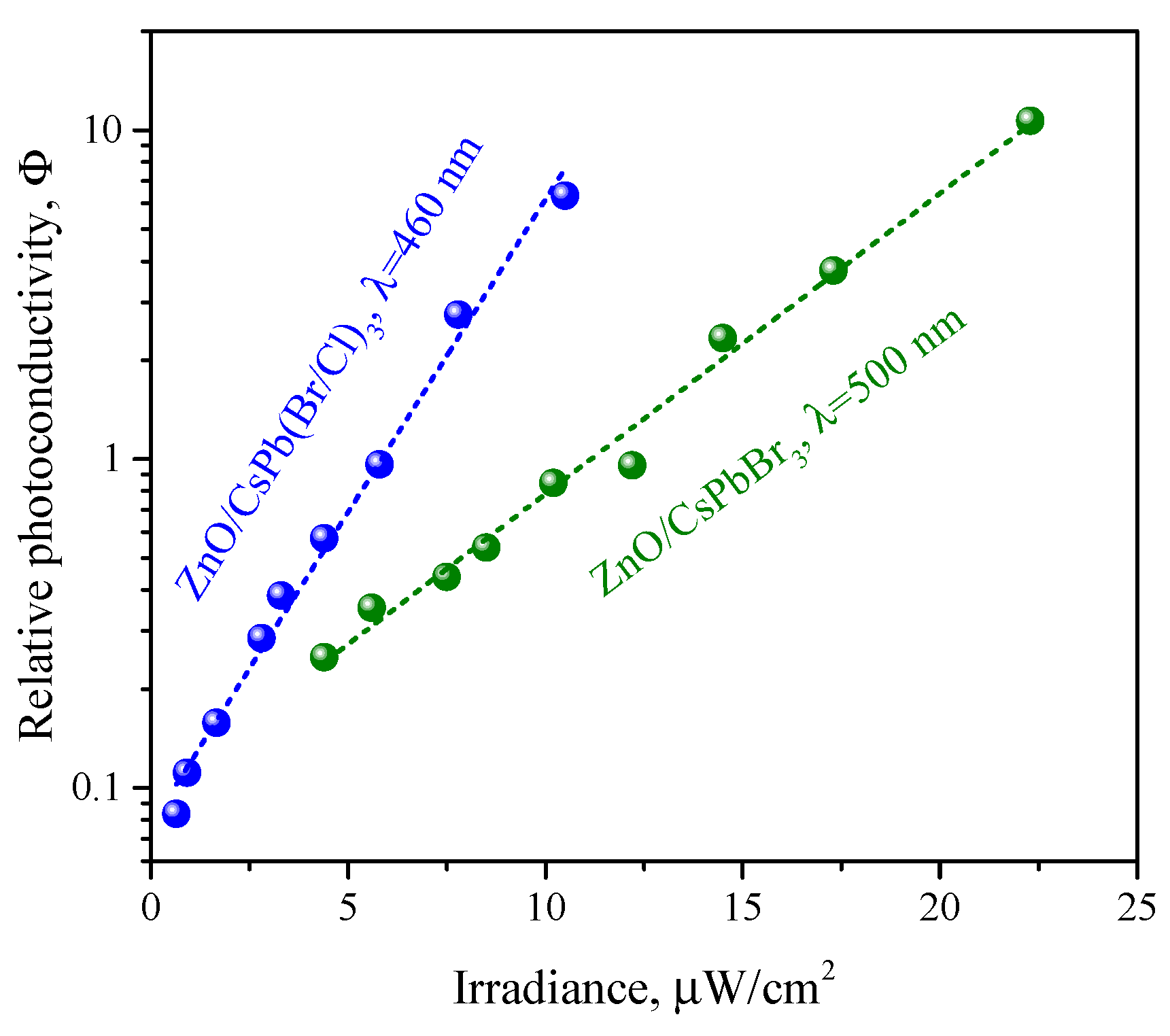
3.3. Photoconductivity of ZnO/PNCs Nanocomposites
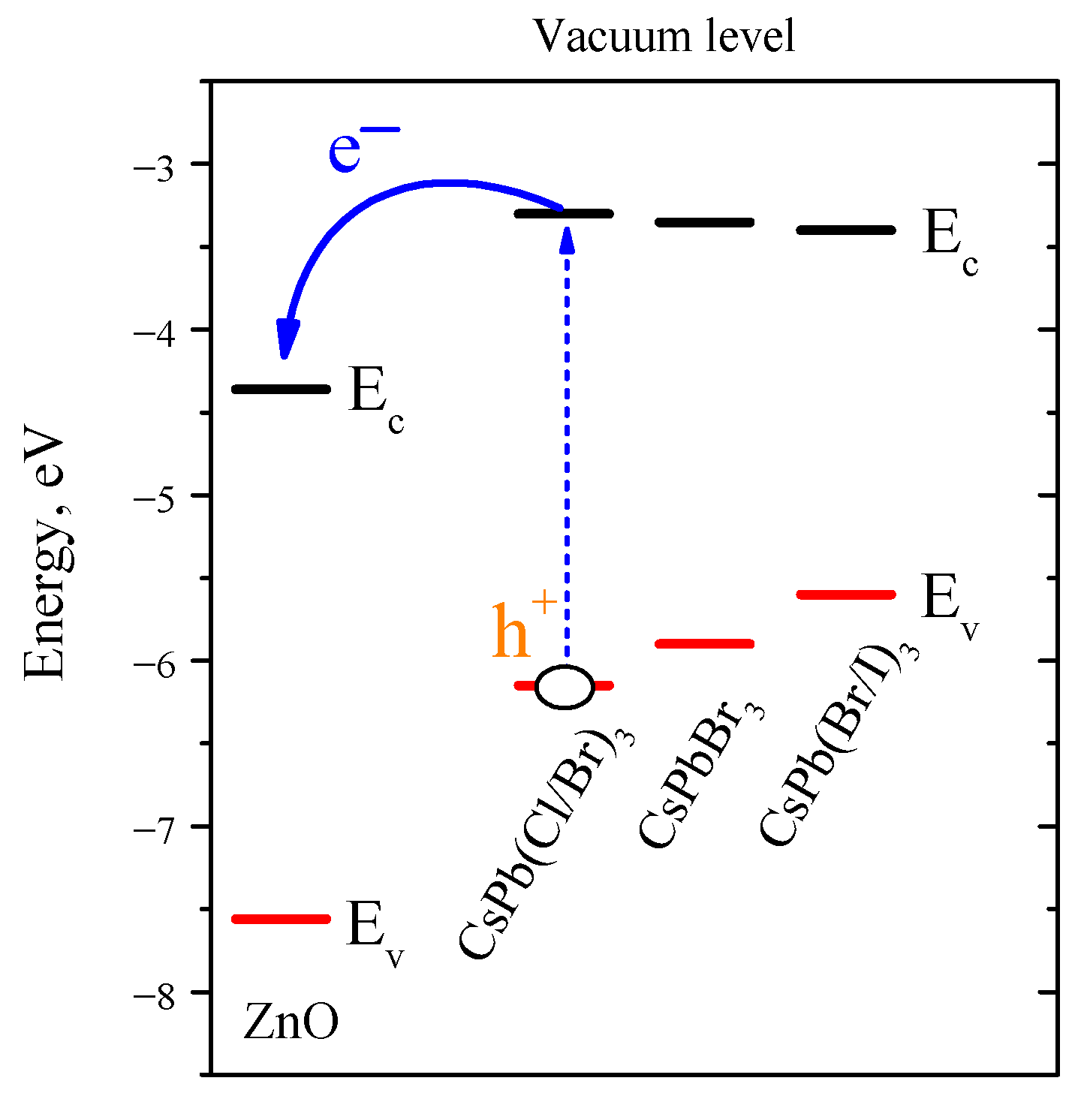
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.J.; Yang, X.; Zang, J.H.; Chen, Y.C.; Lin, C.N.; Liu, Z.X.; Shan, C.X. Ultraviolet irradiation dosimeter based on persistent photoconductivity effect of ZnO. Chin. Phys. 2020, 29, 058504. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.; Aplin, D.; Park, J.; Bao, X.; Lo, Y.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Srivathsa, M.; Kumar, P.; Rajendra, B. Ultraviolet photoconductivity and photoluminescence properties of spray pyrolyzed ZnO nanostructure: Effect of deposition temperature. Opt. Mater. 2022, 131, 112726. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Drozdov, K.; Krylov, I.; Batuk, M.; Hadermann, J.; Filatova, D.; Khmelevsky, N.; Kozlovsky, V.; Maltseva, L.; et al. Photoresistive gas sensor based on nanocrystalline ZnO sensitized with colloidal perovskite CsPbBr3 nanocrystals. Sens. Actuators Chem. 2021, 329, 129035. [Google Scholar] [CrossRef]
- Sha, R.; Basak, A.; Maity, P.C.; Badhulika, S. ZnO nano-structured based devices for chemical and optical sensing applications. Sens. Actuators Rep. 2022, 4, 100098. [Google Scholar] [CrossRef]
- Bagnall, D.; Chen, Y.; Zhu, Z.; Yao, T.; Koyama, S.; Shen, M.; Goto, T. Optically pumped lasing of ZnO at room temperature. Appl. Phys. Lett. 1997, 70, 2230–2232. [Google Scholar] [CrossRef]
- Cachoncinlle, C.; Hebert, C.; Perrière, J.; Nistor, M.; Petit, A.; Millon, E. Random lasing of ZnO thin films grown by pulsed-laser deposition. Appl. Surf. Sci. 2015, 336, 103–107. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wu, H.; Yang, J.; Wang, Y.; Zhang, J.; Bao, Q.; Wang, M.; Ma, Z.; Tress, W.; et al. A Transparent Electrode Based on Solution-Processed ZnO for Organic Optoelectronic Devices. Nat. Commun. 2022, 13, 4387. [Google Scholar] [CrossRef]
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, Z.; Chen, J.; Choy, T.H.; Wang, J.; Zhang, N.; Lin, Z.; Yu, S.; Kang, J.; Wong, H.S.P.; et al. Optoelectronic resistive random access memory for neuromorphic vision sensors. Nat. Nanotechnol. 2019, 14, 776–782. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, X.; Jia, R.; Huang, L.; Zhang, X.; Jie, J. Organic molecular crystal-based photosynaptic devices for an artificial visual-perception system. NPG Asia Mater. 2019, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ozgur, U.; Morkoc, H. Chapter 5—Optical Properties of ZnO and Related Alloys. In Zinc Oxide Bulk, Thin Films and Nanostructures; Jagadish, C., Pearton, S., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 175–239. [Google Scholar] [CrossRef]
- Collins, R.J.; Thomas, D.G. Photoconduction and Surface Effects with Zinc Oxide Crystals. Phys. Rev. 1958, 112, 388–395. [Google Scholar] [CrossRef]
- Gurwitz, R.; Cohen, R.; Shalish, I. Interaction of light with the ZnO surface: Photon induced oxygen “breathing”, oxygen vacancies, persistent photoconductivity, and persistent photovoltage. J. Appl. Phys. 2014, 115, 033701. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Zhang, M.; Ding, J.; Liu, A.; Huang, F.; Sheng, M. Advances in colloidal quantum dot-based photodetectors. J. Mater. Chem. C 2022, 10, 7404–7422. [Google Scholar] [CrossRef]
- Heiland, G.; Bauer, W.; Neuhaus, M. Spectrally Sensitized Photoconductivity of Zinc Oxide Crystals*. Photochem. Photobiol. 1972, 16, 315–324. [Google Scholar] [CrossRef]
- Dudkowski, S.; Kepka, A.; Grossweiner, L. Spectral sensitization of ZnO thin films with organic dyes. J. Phys. Chem. Solids 1967, 28, 485–493. [Google Scholar] [CrossRef]
- Vodenicharova, M.; Jensen, G. Photosensitization of ZnO single crystals by means of dyes. J. Phys. Chem. Solids 1975, 36, 1241–1247. [Google Scholar] [CrossRef]
- Rani, M.; Tripathi, S. Effect of eosin Y dye on electrical properties of ZnO film synthesized by sol–gel technique. J. Electron. Mater. 2014, 43, 426–434. [Google Scholar] [CrossRef]
- Sivalingam, Y.; Martinelli, E.; Catini, A.; Magna, G.; Pomarico, G.; Basoli, F.; Paolesse, R.; Di Natale, C. Gas-Sensitive Photoconductivity of Porphyrin-Functionalized ZnO Nanorods. J. Phys. Chem. 2012, 116, 9151–9157. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Tokarev, S.; Platonov, V.; Botezzatu, A.; Fedorova, O.; Rumyantseva, M.; Fedorov, Y. Heterobimetallic Ru(II)/M (M = Ag+, Cu2+, Pb2+) Complexes as Photosensitizers for Room-Temperature Gas Sensing. Molecules 2022, 27, 5058. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Tokarev, S.; Fedorova, O.; Bozhev, I.; Rumyantseva, M. In2O3 Based Hybrid Materials: Interplay between Microstructure, Photoelectrical and Light Activated NO2 Sensor Properties. Chemosensors 2022, 10, 135. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Babynina, A.V.; Maslova, O.A.; Rumyantseva, M.N.; Ryabova, L.I.; Dobrovolsky, A.A.; Drozdov, K.A.; Khokhlov, D.R.; Abakumov, A.M.; Gaskov, A.M. Photoconductivity of nanocrystalline SnO2 sensitized with colloidal CdSe quantum dots. J. Mater. Chem. C 2013, 1, 1005–1010. [Google Scholar] [CrossRef]
- Drozdov, K.A.; Kochnev, V.I.; Dobrovolsky, A.A.; Popelo, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Ryabova, L.I.; Khokhlov, D.R.; Vasiliev, R.B. Photoconductivity of structures based on the SnO2 porous matrix coupled with core-shell CdSe/CdS quantum dots. Appl. Phys. Lett. 2013, 103, 4823549. [Google Scholar] [CrossRef]
- Aga, R.S.; Jowhar, D.; Ueda, A.; Pan, Z.; Collins, W.E.; Mu, R.; Singer, K.D.; Shen, J. Enhanced photoresponse in ZnO nanowires decorated with CdTe quantum dot. Appl. Phys. Lett. 2007, 91, 232108. [Google Scholar] [CrossRef] [Green Version]
- White, M.A.; Dempsey, J.L.; Carroll, G.M.; Gallagher, J.D.; Gamelin, D.R. Photoconductive ZnO films with embedded quantum dot or ruthenium dye sensitizers. APL Mater. 2013, 1, 032107. [Google Scholar] [CrossRef]
- Cammi, D.; Zimmermann, K.; Gorny, R.; Vogt, A.; Dissinger, F.; Gad, A.; Markiewcz, N.; Waag, A.; Prades, J.D.; Ronning, C.; et al. Enhancement of the Sub-Band-Gap Photoconductivity in ZnO Nanowires through Surface Functionalization with Carbon Nanodots. J. Phys. Chem. C 2018, 122, 1852–1859. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Dev, A.; Frank, K.; Rosenauer, A.; Voss, T. Oxygen-Controlled Photoconductivity in ZnO Nanowires Functionalized with Colloidal CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 19604–19610. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Mordvinova, N.E.; Rumyantsevaa, M.N.; Krylov, I.V.; Drozdov, K.A.; Li, X.; Gaskov, A.M. The Effect of CdSe and InP Quantum Dots on the Interaction of ZnO with NO2 under Visible Light Irradiation. Russ. J. Inorg. Chem. 2018, 63, 512–518. [Google Scholar] [CrossRef]
- Chizhov, A.; Vasiliev, R.; Rumyantseva, M.; Krylov, I.; Drozdov, K.; Batuk, M.; Hadermann, J.; Abakumov, A.; Gaskov, A. Light-Activated Sub-ppm NO2 Detection by Hybrid ZnO/QD Nanomaterials vs. Charge Localization in Core-Shell QD. Front. Mater. 2019, 6, 231. [Google Scholar] [CrossRef]
- Jasieniak, J.; Smith, L.; van Embden, J.; Mulvaney, P.; Califano, M. Re-examination of the Size-Dependent Absorption Properties of CdSe Quantum Dots. J. Phys. Chem. C 2009, 113, 19468–19474. [Google Scholar] [CrossRef]
- Bley, S.; Diez, M.; Albrecht, F.; Resch, S.; Waldvogel, S.R.; Menzel, A.; Zacharias, M.; Gutowski, J.; Voss, T. Electron Tunneling from Colloidal CdSe Quantum Dots to ZnO Nanowires Studied by Time-Resolved Luminescence and Photoconductivity Experiments. J. Phys. Chem. C 2015, 119, 15627–15635. [Google Scholar] [CrossRef] [Green Version]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Han, S. Intrinsic Carrier Mobility of Cesium Lead Halide Perovskites. Phys. Rev. Appl. 2018, 10, 044013. [Google Scholar] [CrossRef] [Green Version]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Guo, Y.; Que, M.; Yin, X.; Liu, J.; Xie, H.; Zhang, C.; Que, W. Metal halide perovskite nanocrystals: Application in high-performance photodetectors. Mater. Adv. 2021, 2, 856–879. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Jung, U.; Xian, Z.; Jung, B.; Park, J. Enhanced device performance of Cs2AgBiBr6 double perovskite photodetector by SnO2/ZnO double electron transport layer. J. Alloys Compd. 2022, 929, 167329. [Google Scholar] [CrossRef]
- Tang, J.F.; Sie, Y.D.; Tseng, Z.L.; Lin, J.H.; Chen, L.C.; Hsu, C.L. Perovskite Quantum Dot-ZnO Nanowire Composites for Ultraviolet-Visible Photodetectors. ACS Appl. Nano Mater. 2022, 5, 7237–7245. [Google Scholar] [CrossRef]
- Ge, S.; Huang, F.; He, J.; Xu, Z.; Sun, Z.; Han, X.; Wang, C.; Huang, L.B.; Pan, C. Bidirectional Photoresponse in Perovskite-ZnO Heterostructure for Fully Optical-Controlled Artificial Synapse. Adv. Opt. Mater. 2022, 10, 409. [Google Scholar] [CrossRef]
- Yin, L.; Huang, W.; Xiao, R.; Peng, W.; Zhu, Y.; Zhang, Y.; Pi, X.; Yang, D. Optically stimulated synaptic devices based on the hybrid structure of silicon nanomembrane and perovskite. Nano Lett. 2020, 20, 3378–3387. [Google Scholar] [CrossRef]
- Gong, J.; Yu, H.; Zhou, X.; Wei, H.; Ma, M.; Han, H.; Zhang, S.; Ni, Y.; Li, Y.; Xu, W. Lateral Artificial Synapses on Hybrid Perovskite Platelets with Modulated Neuroplasticity. Adv. Funct. Mater. 2020, 30, 5413. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Singh, A.P.; Upadhyay, D.; Ratan, S.; Kumar, C.; Jit, S. High-Performance Photodetector Based on Organic-Inorganic Perovskite CH3NH3PbI3/ZnO Heterostructure. IEEE Photonics Technol. Lett. 2019, 31, 1151–1154. [Google Scholar] [CrossRef]
- Cao, Y.; Sha, X.; Bai, X.; Shao, Y.; Gao, Y.; Wei, Y.M.; Meng, L.; Zhou, N.; Liu, J.; Li, B.; et al. Ultralow Light-Power Consuming Photonic Synapses Based on Ultrasensitive Perovskite/Indium-Gallium-Zinc-Oxide Heterojunction Phototransistors. Adv. Electron. Mater. 2022, 8, 902. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Wang, Y.; Zhou, H.; Xue, M.; Xu, Y.; Li, Z.; Ye, C.; Zhang, J.; Van Aken, P.A.; et al. A high-performance self-powered broadband photodetector based on a CH3NH3PbI3 perovskite/ZnO nanorod array heterostructure. J. Mater. Chem. C 2016, 4, 7302–7308. [Google Scholar] [CrossRef] [Green Version]
- Alwadai, N.; Haque, M.A.; Mitra, S.; Flemban, T.; Pak, Y.; Wu, T.; Roqan, I. High-Performance Ultraviolet-to-Infrared Broadband Perovskite Photodetectors Achieved via Inter-/Intraband Transitions. ACS Appl. Mater. Interfaces 2017, 9, 37832–37838. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, Z.; Chen, J.; Cui, Q.; Jian, A.; Zhu, Y.; Xu, Q.; Lou, Z.; Xu, C. Dynamics of interfacial carriers and negative photoconductance in CH3NH3PbBr3-ZnO heterostructure. Appl. Phys. Lett. 2021, 118, 171901. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, F.; Zhang, Z.; Chen, J.; Tang, X.; Shi, Z.; Cui, Q.; Xu, C. Ag nanowires assisted CH3NH3PbBr3-ZnO heterostructure with fast negative photoconductive response. Appl. Phys. Lett. 2022, 121, 061902. [Google Scholar] [CrossRef]
- Han, J.; Liang, Z.; Guo, S.; Wang, S.; Qiao, S. Photoresponse improvement of a MAPbI3 p-i-n heterojunction photodetector by modifying with a PCBM layer and optimizing ZnO layer thickness. Surf. Interfaces 2022, 34, 102315. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, L.; Yin, Z.; Wang, D.; Meng, J.; Jiang, Q.; Wang, Y.; You, J. A high-performance photodetector based on an inorganic perovskite-ZnO heterostructure. J. Mater. Chem. C 2017, 5, 6115–6122. [Google Scholar] [CrossRef]
- Yueyue, L.; Siqi, S.; Yilin, W.; Fengmin, L.; Hongtao, W.; Jihao, B.; Min, L.; Geyu, L. CsPbBr3 quantum dots enhanced ZnO sensing to NO2 at room temperature. Sens. Actuators B Chem. 2022, 368, 132189. [Google Scholar] [CrossRef]
- An, J.; Chen, G.; Zhu, X.; Lv, X.; Bao, J.; Xu, X. Ambipolar Photoresponse of CsPbX3-ZnO (X = Cl, Br, and I) Heterojunctions. ACS Appl. Electron. Mater. 2022, 4, 1525–1532. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Zhu, Z.; Wang, S.; Gu, Y.; Shan, F.; Zou, Y. Improved flexible ZnO/CsPbBr3/Graphene UV photodetectors with interface optimization by solution process. Mater. Res. Bull. 2020, 130, 110956. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Zang, Z. High performance CsPbBr3 quantum dots photodetectors by using zinc oxide nanorods arrays as an electron-transport layer. Appl. Phys. Lett. 2020, 116, 162103. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Li, T.; Zhu, Y. A vertical CsPbBr3/ZnO heterojunction for photo-sensing lights from UV to green band. Opt. Express 2022, 30, 23330–23340. [Google Scholar] [CrossRef]
- Yang, B.; Guo, P.; Hao, D.; Wang, Y.; Li, L.; Dai, S.; Huang, J. Self-powered photodetectors based on CsPbBr3 quantum dots/organic semiconductors/SnO2 heterojunction for weak light detection. Sci. China Mater. 2022, 1–8. [Google Scholar] [CrossRef]
- Yan, S.; Li, Q.; Zhang, X.; Tang, S.; Lei, W.; Chen, J. A vertical structure photodetector based on all-inorganic perovskite quantum dots. J. Soc. Inf. Disp. 2020, 28, 9–15. [Google Scholar] [CrossRef]
- Na, H.J.; Cho, N.K.; Park, J.; Lee, S.E.; Lee, E.G.; Im, C.; Kim, Y.S. A visible light detector based on a heterojunction phototransistor with a highly stable inorganic CsPbIxBr3-x perovskite and In-Ga-Zn-O semiconductor double-layer. J. Mater. Chem. C 2019, 7, 14223–14231. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, T.; Xu, T.; Ye, S.; Liu, R.; Sun, Z.; Tan, C.; Lv, X.; Yang, J.; et al. Unencapsulated CsPbClBr2 Film Photodetectors Grown by Thermal Vacuum Deposition Exhibit Exceptional Environmental Stability in High-Humidity Air. ACS Appl. Energy Mater. 2022, 5, 8709–8716. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, G.; Ren, D.; Huang, J.; Gao, Z.; Duan, J.; Wang, H. Solution-Processing of CsPbClxBr3-x Perovskite Micro/Nanostructure Near-Ultraviolet Photodetectors with High Performance. Adv. Opt. Mater. 2022, 10, 2201270. [Google Scholar] [CrossRef]
- Chizhov, A.; Kutukov, P.; Gulin, A.; Astafiev, A.; Rumyantseva, M. UV-Activated NO2 Gas Sensing by Nanocrystalline ZnO: Mechanistic Insights from Mass Spectrometry Investigations. Chemosensors 2022, 10, 147. [Google Scholar] [CrossRef]
- Eliseev, E.A.; Filatova, D.G.; Chizhov, A.S.; Rumyantseva, M.N.; Gaskov, A.M. Simple in situ analysis of metal halide perovskite-based sensor materials using micro X-ray fluorescence and inductively coupled plasma mass spectrometry. Mendeleev Commun. 2021, 31, 462–464. [Google Scholar] [CrossRef]
- Cottingham, P.; Brutchey, R.L. On the crystal structure of colloidally prepared CsPbBr3 quantum dots. Chem. Commun. 2016, 52, 5246–5249. [Google Scholar] [CrossRef] [PubMed]
- Alaei, A.; Circelli, A.; Yuan, Y.; Yang, Y.; Lee, S.S. Polymorphism in metal halide perovskites. Mater. Adv. 2021, 2, 47–63. [Google Scholar] [CrossRef]
- McGettrick, J.D.; Hooper, K.; Pockett, A.; Baker, J.; Troughton, J.; Carnie, M.; Watson, T. Sources of Pb(0) artefacts during XPS analysis of lead halide perovskites. Mater. Lett. 2019, 251, 98–101. [Google Scholar] [CrossRef]
- Ravi, V.K.; Markad, G.B.; Nag, A. Band Edge Energies and Excitonic Transition Probabilities of Colloidal CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals. ACS Energy Lett. 2016, 1, 665–671. [Google Scholar] [CrossRef]




| Sample | , eV | , eV | , eV | , eV | , eV |
|---|---|---|---|---|---|
| CsPbBr3 | 724.4(I) | 138.4(I) | 68.5(I) | - | - |
| 136.9(II) | |||||
| CsPb(Br/Cl)3 | 724.4(I) | 138.4(I) | 68.5(I) | 197.9 | - |
| 136.9(II) | |||||
| CsPb(I/Br)3 | 724.0(II) | 138.4(I) | 68.5(I) | - | 618.7(I) |
| 725.4(III) | 139.4(III) | 69.3(II) | 619.9(II) |
| Sample | Cs | Pb | Br | Cl | I |
|---|---|---|---|---|---|
| CsPbBr3 | 18.1(I) | 19.1(I) | 60.6(I) | - | - |
| 2.2(II) | |||||
| CsPb(Br/Cl)3 | 14.4(I) | 18.9(I) | 43.4(I) | 18.5 | - |
| 4.8(II) | |||||
| CsPb(I/Br)3 | 4.4(II) | 9.2(I) | 14.8(I) | - | 3.6(I) |
| 10.3(III) | 12.5(III) | 38.4(II) | 6.8(II) |
| Sample | , S, | On/Off Ratio | R, mA/W | , s |
|---|---|---|---|---|
| ZnO | 0.01 | 2500 | 0.7 | 115 |
| CsPb(Br/Cl)3 | 0.3 | 1800 | 20 | 18 |
| CsPbBr3 | 0.2 | 2090 | 16 | 25 |
| CsPb(I/Br)3 | 0.03 | 560 | 0.5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chizhov, A.; Rumyantseva, M.; Khmelevsky, N.; Grunin, A. Sensitization of ZnO Photoconductivity in the Visible Range by Colloidal Cesium Lead Halide Nanocrystals. Nanomaterials 2022, 12, 4316. https://doi.org/10.3390/nano12234316
Chizhov A, Rumyantseva M, Khmelevsky N, Grunin A. Sensitization of ZnO Photoconductivity in the Visible Range by Colloidal Cesium Lead Halide Nanocrystals. Nanomaterials. 2022; 12(23):4316. https://doi.org/10.3390/nano12234316
Chicago/Turabian StyleChizhov, Artem, Marina Rumyantseva, Nikolay Khmelevsky, and Andrey Grunin. 2022. "Sensitization of ZnO Photoconductivity in the Visible Range by Colloidal Cesium Lead Halide Nanocrystals" Nanomaterials 12, no. 23: 4316. https://doi.org/10.3390/nano12234316
APA StyleChizhov, A., Rumyantseva, M., Khmelevsky, N., & Grunin, A. (2022). Sensitization of ZnO Photoconductivity in the Visible Range by Colloidal Cesium Lead Halide Nanocrystals. Nanomaterials, 12(23), 4316. https://doi.org/10.3390/nano12234316








