Tannic Acid as a Versatile Template for Silica Monoliths Engineering with Catalytic Gold and Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
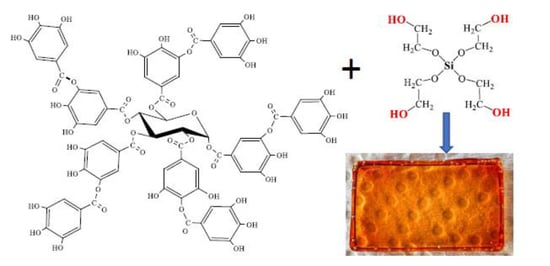
3.1. Sol-Gel Synthesis
3.2. Bionanocomposite Structure
3.3. Synthesis of Gold and Silver Nanoparticles Embedded into Silica
3.4. Catalytic Properties
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, C. Healthy eating—Clarifying advice about fruit and vegetables. Br. Med. J. 1995, 310, 1453–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; de la Luz Cádiz-Gurrea, M.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Duxford, UK, 2018; pp. 393–427. [Google Scholar]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Tannins: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. [Google Scholar]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.J.; Richardson, J.J.; Caruso, F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.S.; Zhou, B.; Wu, D.; Li, J.; Li, B. Supramolecular design and applications of polyphenol-based architecture: A review. Adv. Colloid Interface Sci. 2019, 272, 102019. [Google Scholar] [CrossRef]
- Guo, J.L.; Suma, T.; Richardson, J.J.; Ejima, H. Modular assembly of biomaterials using polyphenols as building blocks. ACS Biomater. Sci. Eng. 2019, 5, 5578–5596. [Google Scholar] [CrossRef]
- Koopmann, A.K.; Schuster, C.; Torres-Rodriguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Huesing, N. Tannin-based hybrid materials and their applications: A review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.; Nam, Y.S. Metal-polyphenol complexes as versatile building blocks for functional biomaterials. Biotechnol. Bioproc. Eng. 2021, 26, 689–707. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q.H. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Coradin, T.; Lopez, P.J. Biogenic silica patterning: Simple chemistry or subtle biology? ChemBioChem. 2003, 3, 251–259. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Karpenko, T.Y.; Krekoten, A.V. Hybrid organic-inorganic nanocomposites fabricated with a novel biocompatible precursor using sol-gel processing. Compos. Interfaces 2005, 11, 587–607. [Google Scholar] [CrossRef] [Green Version]
- Shchipunov, Y.A. Entrapment of biopolymers into sol-gel-derived silica nanocomposites. In Bio-Inorganic Hybrid Nanomaterials; Ruiz-Hitzky, E., Ariga, K., Lvov, Y., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2008; pp. 75–117. [Google Scholar]
- Schnepp, Z. Biopolymers as a flexible resource for nanochemistry. Angew. Chem. Int. Ed. 2013, 52, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Boury, B.; Plumejeau, S. Metal oxides and polysaccharides: An efficient hybrid association for materials chemistry. Green Chem. 2015, 17, 72–88. [Google Scholar] [CrossRef]
- Plumejeau, S.; Alauzun, J.G.; Boury, B. Hybrid metal oxide@biopolymer materials precursors of metal oxides and metal oxide-carbon composites. J. Ceramic. Soc. Jpn. 2015, 123, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Shchipunov, Y.; Postnova, I. Cellulose mineralization as a route for novel functional materials. Adv. Funct. Mater. 2018, 26, 1705042. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted applications: A review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Shchipunov, Y.A. Sol-gel derived biomaterials of silica and carrageenans. J. Colloid Interface Sci. 2003, 268, 68–76. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Karpenko, T.Y. Hybrid polysaccharide-silica nanocomposites prepared by the sol-gel technique. Langmuir 2004, 20, 3882–3887. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Karpenko, T.Y.; Krekoten, A.V.; Postnova, I.V. Gelling of otherwise nongelable polysaccharides. J. Colloid Interface Sci. 2005, 287, 373–378. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Postnova, I.V. One-pot biomimetic synthesis of monolithic titania through mineralization of polysaccharide. Colloid Surf. B. 2009, 74, 172–177. [Google Scholar] [CrossRef]
- Postnova, I.V.; Krekoten, A.V.; Kozlova, E.A.; Tsybulya, S.V.; Rempel, A.A.; Shchipunov, Y.A. Template synthesis of titania on polysaccharides. Russ. Chem. Bull. 2013, 62, 976–983. [Google Scholar] [CrossRef]
- Gao, Z.; Zharov, I. Large pore mesoporous silica nanoparticles by templating with a nonsurfactant molecule, Tannic acid. Chem. Mater. 2014, 26, 2030–2037. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation and characterization of monodisperse, mesoporous natural poly(tannic acid)-silica nanoparticle composites with antioxidant properties. Micropor. Mesopor. Mater. 2016, 226, 316–324. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.J.; Wang, B.F.; Lv, M.J.; Zhu, Y.Y.; Gao, J.K. Fabrication and characterization of novel shapestabilized stearic acid composite phase change materials with tannic-acid-templated mesoporous silica nanoparticles for thermal energy storage. RSC Adv. 2017, 7, 15625–15631. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.L.; Panzarasa, G.; Osypova, A.; Sorin, F.; Spano, F.; Rossi, R.M.; Sadeghpour, A.; Boesel, L.F. Polyphenols as morphogenetic agents for the controlled synthesis of mesoporous silica nanoparticles. Chem. Mater. 2019, 31, 3192–3200. [Google Scholar] [CrossRef]
- Xu, L.Q.; Neoh, K.G.; Kang, E.T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Tescione, F.; Tammaro, O.; Bifulco, A.; Del Monaco, G.; Esposito, S.; Pansini, M.; Silvestri, B.; Costantini, A. Silica meets tannic acid: Designing green nanoplatforms for environment preservation. Molecules 2022, 27, 1944. [Google Scholar] [CrossRef]
- Dos Santos, C.; Vargas, A.; Fronza, N.; dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloid Surf. B 2017, 151, 26–33. [Google Scholar] [CrossRef]
- Gao, J.; Yu, H.; Zhou, L.Y.; He, Y.; Ma, L.; Jiang, Y.J. Formation of cross-linked nitrile hydratase aggregates in the pores of tannic-acid-templated magnetic mesoporous silica: Characterization and catalytic application. Biochem. Eng. J. 2017, 117, 92–101. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Shi, C.; Sun, M.; Yang, C.; Li, H.; Chen, F.; Chang, Z.; Zheng, X.; Wang, Z.; et al. Tannic acid-assisted synthesis of biodegradable and antibacterial mesoporous organosilica nanoparticles decorated with nanosilver. ACS Sustain. Chem. Eng. 2020, 8, 1695–1702. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Liao, X.P.; Shi, B. Preparation of platinum nanoparticles supported on bayberry tannin grafted silica bead and its catalytic properties in hydrogenation. J. Mol. Catal. A-Chem. 2010, 320, 40–46. [Google Scholar] [CrossRef]
- Feiz, A.; Amini, M.M.; Bazgir, A. Tannic acid grafted SBA-15 decorated with palladium and its catalytic activity in synthesis of aromatic ketones and biaryls. Mol. Catal. 2017, 438, 159–166. [Google Scholar] [CrossRef]
- Binaeian, E.; Seghatoleslami, N.; Chaichi, M.J. Synthesis of oak gall tannin-immobilized hexagonal mesoporous silicate (OGT-HMS) as a new super adsorbent for the removal of anionic dye from aqueous solution. Desalin. Water Treat. 2016, 57, 8420–8436. [Google Scholar] [CrossRef]
- Bazzaz, F.; Binaeian, E.; Heydarinasab, A.; Ghadi, A. Adsorption of BSA onto hexagonal mesoporous silicate loaded by APTES and tannin: Isotherm, thermodynamic and kinetic studies. Adv. Powder Techn. 2018, 29, 1664–1675. [Google Scholar] [CrossRef]
- Pierre, A.C. Introduction to Sol-Gel Processing; Kluwer: Boston, MA, USA, 1998. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science. The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Shilova, O.A. Synthesis and structure features of composite silicate and hybrid TEOS-derived thin films doped by inorganic and organic additives. J. Sol-Gel Sci. Techn. 2013, 68, 387–410. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Beland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Sattler, K.; Gradzielski, M.; Mortensen, K.; Hoffmann, H. Influence of surfactant on the gelation of novel ethylene glycol esters of silicic acid. Ber. Bunsenges. Phys. Chem. 1998, 102, 1544–1547. [Google Scholar] [CrossRef]
- Sattler, K.; Hoffmann, H. A novel glycol silicate and its interaction with surfactant for the synthesis of mesoporous silicate. Prog. Colloid Polym. Sci. 1999, 112, 40–44. [Google Scholar]
- Shchipunov, Y.A.; Shipunova, N.Y. Regulation of silica morphology by proteins serving as a template for mineralization. Colloid Surf. B 2008, 63, 7–11. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Karpenko, T.Y.; Bakunina, I.Y.; Burtseva, Y.; Zvyagintseva, T.N. A new precursor for the immobilization of enzymes inside sol-gel derived hybrid silica nanocomposites containing polysaccharides. J. Biochem. Biophys. Methods 2004, 58, 25–38. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Burtseva, Y.V.; Karpenko, T.Y.; Shevchenko, N.M.; Zvyagintseva, T.N. Highly efficient immobilization of endo-1,3--d-glucanases (laminarinases) from marine mollusks in novel hybrid polysaccharidesilica nanocomposites with regulated composition. J. Mol. Catal. B. Enzym. 2006, 40, 16–23. [Google Scholar] [CrossRef]
- Postnova, I.V.; Sarin, S.A.; Karpenko, T.Y.; Shchipunov, Y.A. Formation of photocatalytically active titania on mesoporous silica with silver nanoparticles synthesized using tannin as a template and a reductant. Doklady Chem. 2020, 495, 191–194. [Google Scholar] [CrossRef]
- Mehrotra, R.C.; Narain, R.P. Reactions of tetramethoxy- and triethoxysilanes with glycols. Indian J. Chem. 1967, 5, 444–448. [Google Scholar]
- Stoeber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Pedroso, M.A.S.; Dias, M.L.; Azuma, C.; Mothe, C.G. Hydrocarbon dispersion of nanospherical silica by a sol-gel process. 1. Tetraethoxysilane homopolymerization. Colloid Polym. Sci. 2000, 278, 1180–1186. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Krekoten, A.V.; Kuryavyi, V.G.; Topchieva, I.N. Microporous nanocomposite material synthesized by sol-gel processing in the presence of cyclodextrins. Colloid J. 2005, 67, 380–384. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Fulcrand, H.; Roumeas, L.; Billerach, G.; Aouf, C.; Dubreucq, E. Advances in biobased thermosetting polymers. In Recent Advances in Polyphenol Research; Halbwirth, H., Stich, K., Cheynier, V., Quideau, S., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 285–334. [Google Scholar]
- Lea, M.C. LXI. Allotropic silver. Part III. Blue silver, soluble and insoluble forms. Phil. Mag. Ser. 1891, 31, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Pelton, M.; Aizpurua, J.; Bryant, G. Metal-nanoparticle plasmonics. Laser Photonics Rev. 2008, 2, 136–159. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.Z.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Mashaly, H.M.; Mowafi, S.; Abou El-Kheir, A.; Emam, H.E. Multi-functional textile design using in-situ Ag NPs incorporation into natural fabric matrix. Dyes Pigments. 2015, 118, 9–17. [Google Scholar] [CrossRef]
- Bensebaa, F. Nanoparticle Technologies. From Lab to Market; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.Y.; Bantz, K.C.; Haynes, C.L. Stabilization of silver and gold nanoparticles: Preservation and improvement of plasmonic functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Edgar, J.A.; Cortie, M.B. Nanotechnological applications of gold. In Gold: Science and Applications; Corti, C., Holliday, R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 369–397. [Google Scholar]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, K.; Masubuchi, Y.; Shchipunov, Y.; Zinchenko, A. DNA-chitosan hydrogels: Formation, properties, and functionalization with catalytic nanoparticles. ACS Appl. Biomater. 2021, 4, 1823–1832. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postnova, I.; Shchipunov, Y. Tannic Acid as a Versatile Template for Silica Monoliths Engineering with Catalytic Gold and Silver Nanoparticles. Nanomaterials 2022, 12, 4320. https://doi.org/10.3390/nano12234320
Postnova I, Shchipunov Y. Tannic Acid as a Versatile Template for Silica Monoliths Engineering with Catalytic Gold and Silver Nanoparticles. Nanomaterials. 2022; 12(23):4320. https://doi.org/10.3390/nano12234320
Chicago/Turabian StylePostnova, Irina, and Yury Shchipunov. 2022. "Tannic Acid as a Versatile Template for Silica Monoliths Engineering with Catalytic Gold and Silver Nanoparticles" Nanomaterials 12, no. 23: 4320. https://doi.org/10.3390/nano12234320