Modeling of BN-Doped Carbon Nanotube as High-Performance Thermoelectric Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
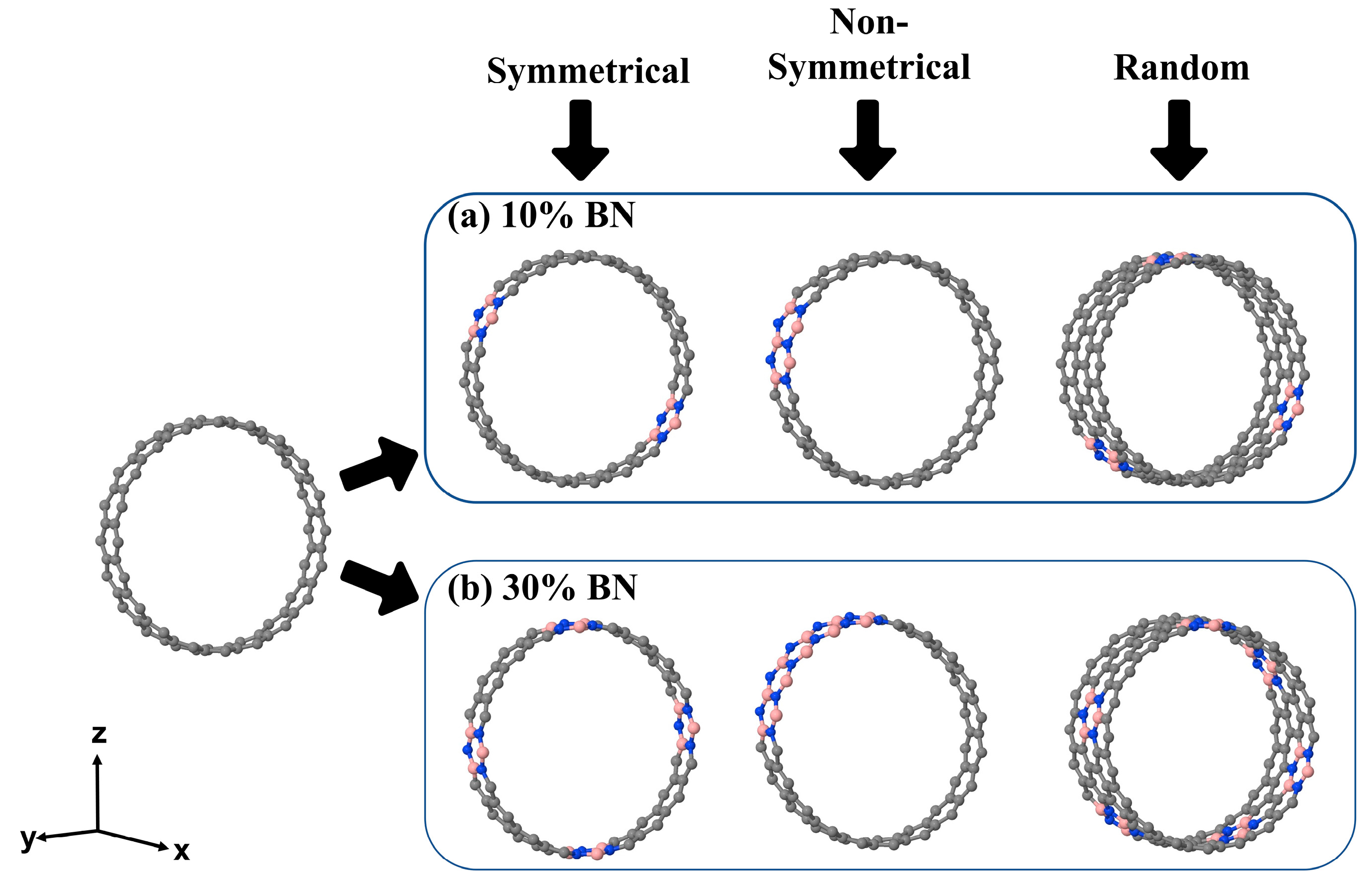
3.1. Structural Properties of C@(BN)1−xCx
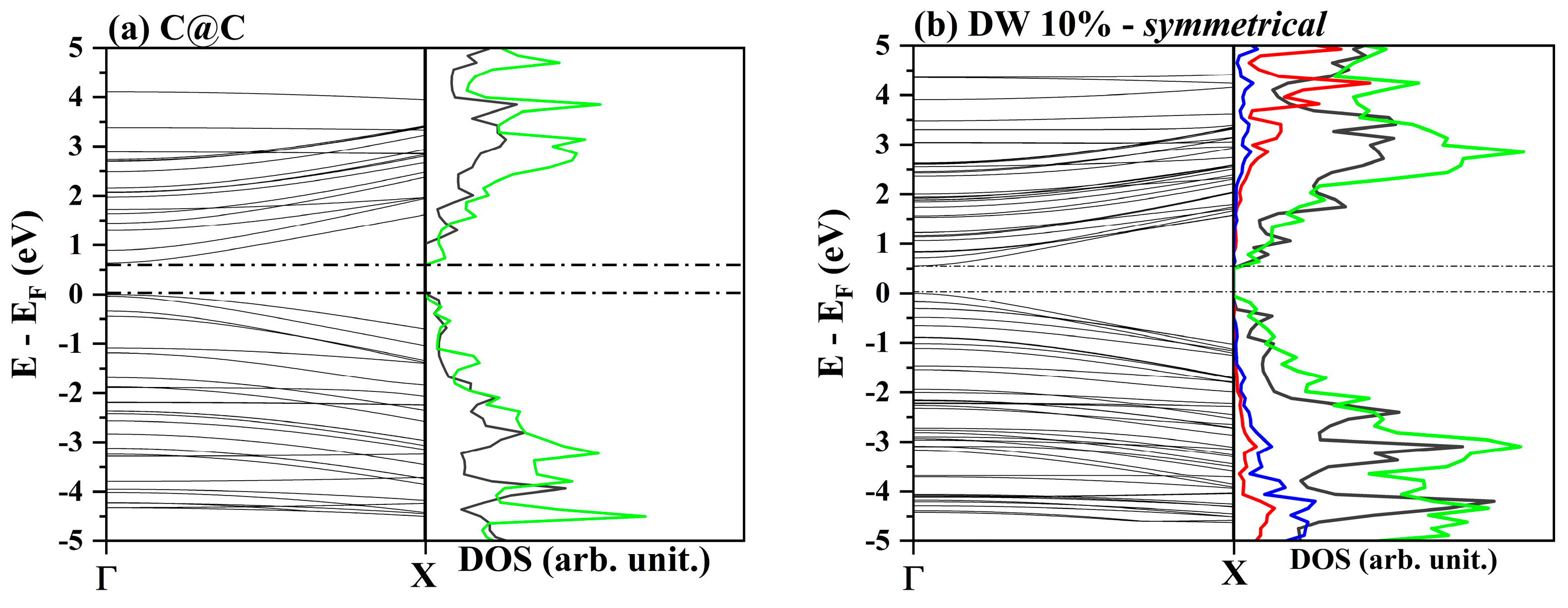
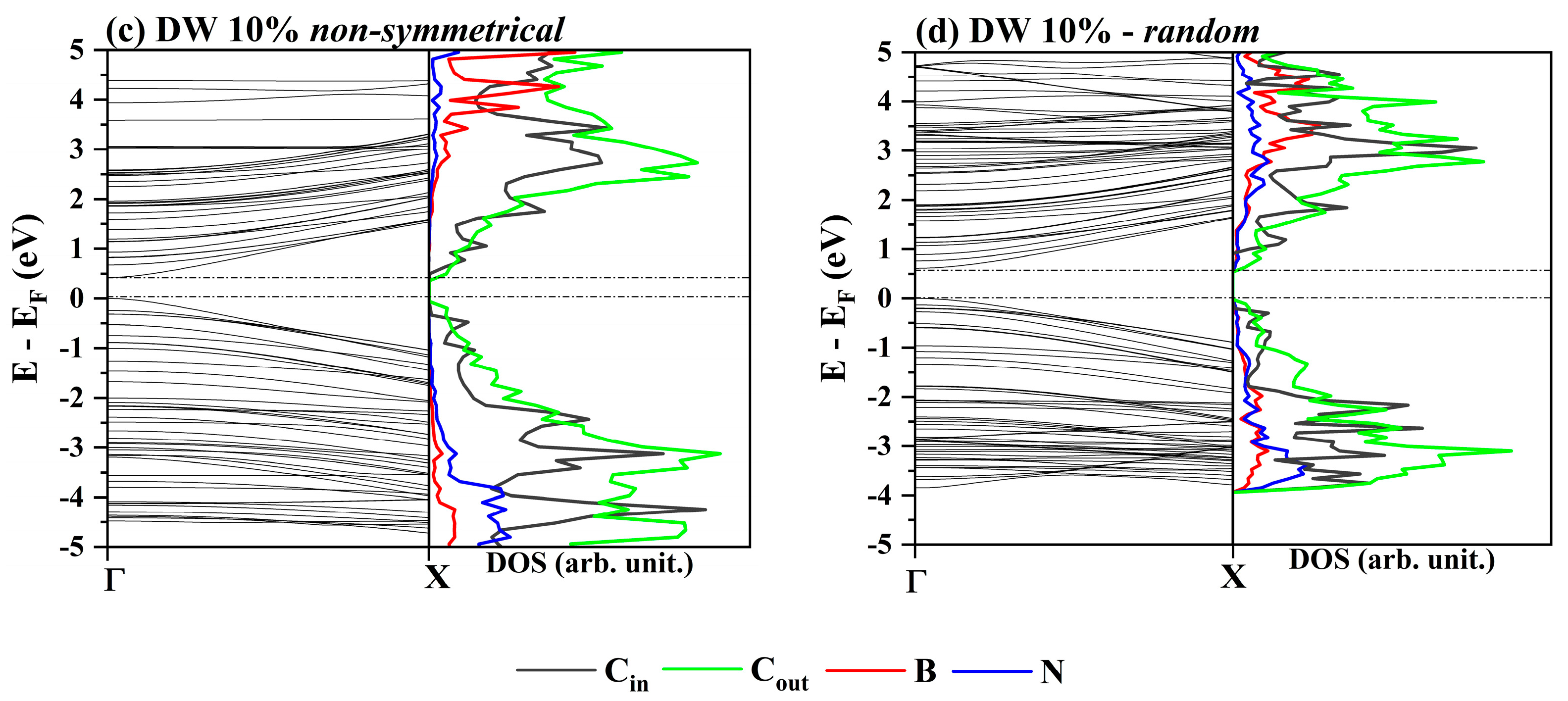
3.2. One-Electron Properties
3.3. Thermoelectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Chen, Z. Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (<C60) and Single-Walled Carbon Nanotubes. Chem. Rev. 2005, 105, 3643–3696. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Tanabe, S.; Mizuno, S.; Kageshima, H.; Hibino, H.; Tanabe, S.; Mizuno, S.; Kageshima, H. Growth and Electronic Transport Properties of Epitaxial Graphene on SiC. J. Phys. D Appl. Phys. 2012, 45, 154008. [Google Scholar] [CrossRef]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential Applications of Carbon Nanotubes. In Carbon Nanotubes; Springer: Berlin/Heidelberg, Germany, 2007; pp. 13–62. [Google Scholar]
- Sharma, P.; Kumar Mehra, N.; Jain, K.; Jain, N.K. Biomedical Applications of Carbon Nanotubes: A Critical Review. Curr. Drug Deliv. 2016, 13, 796–817. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.D.; Zhou, B.H.; Lee, J.; Lee, E.S.; Miller, E.M.; Ihly, R.; Wesenberg, D.; Mistry, K.S.; Guillot, S.L.; Zink, B.L.; et al. Tailored Semiconducting Carbon Nanotube Networks with Enhanced Thermoelectric Properties. Nat. Energy 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Hung, N.T.; Nugraha, A.R.T.; Hasdeo, E.H.; Dresselhaus, M.S.; Saito, R. Diameter Dependence of Thermoelectric Power of Semiconducting Carbon Nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 165426. [Google Scholar] [CrossRef]
- Blase, X.; Rubio, A.; Louie, S.G.; Cohen, M.L. Stability and Band Gap Constancy of Boron Nitride Nanotubes. EPL 1994, 28, 335. [Google Scholar] [CrossRef]
- Vaccarini, L.; Goze, C.; Henrard, L.; Hernández, E.; Bernier, P.; Rubio, A. Mechanical and Electronic Properties of Carbon and Boron–Nitride Nanotubes. Carbon N. Y. 2000, 38, 1681–1690. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhuang, F.D.; Wang, R.B.; Wang, X.C.; Cao, X.Y.; Wang, J.Y.; Pei, J. A Straightforward Strategy toward Large BN-Embedded π-Systems: Synthesis, Structure, and Optoelectronic Properties of Extended BN Heterosuperbenzenes. J. Am. Chem. Soc. 2014, 136, 3764–3767. [Google Scholar] [CrossRef]
- Hellgren, N.; Berlind, T.; Gueorguiev, G.K.; Johansson, M.P.; Stafström, S.; Hultman, L. Fullerene-like BCN Thin Films: A Computational and Experimental Study. Mater. Sci. Eng. B 2004, 113, 242–247. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wang, H.; Cang, D.; Ji, X.; Liu, B.; Yang, W.; Li, D.; Liu, J. Defective Carbon-Doped Boron Nitride Nanosheets for Highly Efficient Electrocatalytic Conversion of N2 to NH3. ACS Sustain. Chem. Eng. 2020, 8, 5278–5286. [Google Scholar] [CrossRef]
- Azevedo, S.; De Paiva, R. Structural Stability and Electronic Properties of Carbon-Boron Nitride Compounds. EPL (Europhys. Lett.) 2006, 75, 126. [Google Scholar] [CrossRef]
- Li, Y.; Gao, D.; Zhao, S.; Xiao, Y.; Guo, Z.; Fang, Y.; Lin, J.; Liu, Z.; Huang, Y.; Guo, K.; et al. Carbon Doped Hexagonal Boron Nitride Nanoribbon as Efficient Metal-Free Electrochemical Nitrogen Reduction Catalyst. Chem. Eng. J. 2021, 410, 128419. [Google Scholar] [CrossRef]
- Kah, C.B.; Smith, L.; Jayanthi, C.S.; Yu, M. The Electronic Structure Studies of Hybrid H-BNC Sheets Based on a Semi-Empirical Hamiltonian. Mater. Today Commun. 2021, 26, 102142. [Google Scholar] [CrossRef]
- Young, S.K.; Park, J.; Hyun, C.C.; Jae, P.A.; Jin, Q.H.; Hong, S.K. X-ray Photoelectron Spectroscopy and First Principles Calculation of BCN Nanotubes. J. Am. Chem. Soc. 2007, 129, 1705–1716. [Google Scholar] [CrossRef]
- Chiang, W.H.; Hsieh, C.Y.; Lo, S.C.; Chang, Y.C.; Kawai, T.; Nonoguchi, Y. C/BCN Core/Shell Nanotube Films with Improved Thermoelectric Properties. Carbon N. Y. 2016, 109, 49–56. [Google Scholar] [CrossRef]
- Medrano Sandonas, L.; Cuba-Supanta, G.; Gutierrez, R.; Landauro, C.V.; Rojas-Tapia, J.; Cuniberti, G. Doping Engineering of Thermoelectric Transport in BNC Heteronanotubes. Phys. Chem. Chem. Phys. 2019, 21, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Erba, A.; Desmarais, J.; Casassa, S.; Civalleri, B.; Donà, L.; Bush, I.; Searle, B.; Maschio, L.; Daga, L.E.; Cossard, A.; et al. CRYSTAL23: A Program for Computational Solid State Physics and Chemistry. J. Chem. Theory Comput. 2022; submited. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef]
- Dovesi, R.; Causa, M.; Orlando, R.; Roetti, C.; Saunders, V.R. Ab Initio Approach to Molecular Crystals: A Periodic Hartree–Fock Study of Crystalline Urea. J. Chem. Phys. 1990, 92, 7402–7411. [Google Scholar] [CrossRef]
- Orlando, R.; Dovesi, R.; Roetti, C.; Saunders, V.R. Ab Initio Hartree-Fock Calculations for Periodic Compounds: Application to Semiconductors. J. Phys. Condens. Matter 1990, 2, 7769. [Google Scholar] [CrossRef]
- Pinhal, G.B.; Marana, N.L.; Fabris, G.S.L.; Sambrano, J.R. Structural, Electronic and Mechanical Properties of Single-Walled AlN and GaN Nanotubes via DFT/B3LYP. Theor. Chem. Acc. 2019, 138, 1–11. [Google Scholar] [CrossRef]
- Marana, N.L.; Albuquerque, A.R.; La Porta, F.A.; Longo, E.; Sambrano, J.R. Periodic Density Functional Theory Study of Structural and Electronic Properties of Single-Walled Zinc Oxide and Carbon Nanotubes. J. Solid State Chem. 2016, 237, 36–47. [Google Scholar] [CrossRef]
- Fletcher, R. A New Approach to Variable Metric Algorithms. Comput. J. 1970, 13, 317–322. [Google Scholar] [CrossRef]
- Goldfarb, D. A Family of Variable-Metric Methods Derived by Variational Means. Math. Comput. 1970, 24, 23–26. [Google Scholar] [CrossRef]
- Shanno, D.F. Conditioning of Quasi-Newton Methods for Function Minimization. Math. Comput. 1970, 24, 647–656. [Google Scholar] [CrossRef]
- Marana, N.L.; Noel, Y.; Sambrano, J.R.; Ribaldone, C.; Casassa, S. Ab Initio Modeling of MultiWall: A General Algorithm First Applied to Carbon Nanotubes. J. Phys. Chem. A 2021, 125, 4003–4012. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, C.; Casassa, S. Condensed-Phase Hybrid Molecular Dynamics Made Feasible: Theory and Implementation. J. Chem. Theory Comput. 2022; submited. [Google Scholar]
- Marana, N.L.; Ribaldone, C.; Casassa, S.M. Condensed-Phase Hybrid Molecular Dynamics Made Feasible: First Applications. 2022; in preparation. [Google Scholar]
- Lundstrom, M. Fundamentals of Carrier Transport. Fundam; Cambridge University Press: Cambridg, UK, 2000. [Google Scholar] [CrossRef]
- Sansone, G.; Ferretti, A.; Maschio, L. Ab Initio Electronic Transport and Thermoelectric Properties of Solids from Full and Range-Separated Hybrid Functionals. J. Chem. Phys. 2017, 147, 114101. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Han, W.Q.; Zettl, A. Thermal Conductivity of B–C–N and BN Nanotubes. Appl. Phys. Lett. 2005, 86, 173102. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2005, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wang, J.; Kayatsha, V.K.; Huang, J.Y.; Yap, Y.K. Effective Growth of Boron Nitride Nanotubes by Thermal Chemical Vapor Deposition. Nanotechnology 2008, 19, 455605. [Google Scholar] [CrossRef]
- Guedes, J.P.; de Brito Mota, F.; Azevedo, S.; de Castilho, C.M.C. Structure, Energetic Stability and Tunable Electronic Properties of BxCyNz Armchair Nanotubes: A Theoretical Study on the Influence of Diameter and Local Carbon Concentration. Eur. Phys. J. B 2015, 88, 1–10. [Google Scholar] [CrossRef]
- Högberg, H.; Lai, C.C.; Broitman, E.; Ivanov, I.G.; Goyenola, C.; Näslund, L.Å.; Schmidt, S.; Hultman, L.; Rosen, J.; Gueorguiev, G.K. Reactive Sputtering of CSx Thin Solid Films Using CS2 as Precursor. Vacuum 2020, 182, 109775. [Google Scholar] [CrossRef]
- Gillis, P.P. Calculating the Elastic Constants of Graphite. Carbon N. Y. 1984, 22, 387–391. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Q.; Gong, Z.; Zhang, H. The Effects of Different Defects on the Elastic Constants of Single-Walled Carbon Nanotubes. In Proceedings of the 2010 IEEE 5th International Conference on Nano/Micro Engineered and Molecular Systems, Xiamen, China, 20–23 January 2010; pp. 777–780. [Google Scholar] [CrossRef]
- Lu, J.P. Elastic Properties of Carbon Nanotubes and Nanoropes. Phys. Rev. Lett. 1997, 79, 1297. [Google Scholar] [CrossRef]
- Popov, V.N.; Van Doren, V.E.; Balkanski, M. Elastic Properties of Crystals of Single-Walled Carbon Nanotubes. Solid State Commun. 2000, 114, 395–399. [Google Scholar] [CrossRef]
- Rubio, A.; Corkill, J.L.; Cohen, M.L. Theory of Graphitic Boron Nitride Nanotubes. Phys. Rev. B 1994, 49, 5081. [Google Scholar] [CrossRef] [PubMed]
- Stephan, O.; Ajayan, P.M.; Colliex, C.; Redlich, P.; Lambert, J.M.; Bernier, P.; Lefin, P. Doping Graphitic and Carbon Nanotube Structures with Boron and Nitrogen. Science 1994, 266, 1683–1685. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Arenal, R.; Loiseau, A.; Rubio, A.; Pichler, T. The Physical and Chemical Properties of Heteronanotubes. Rev. Mod. Phys. 2010, 82, 1843–1885. [Google Scholar] [CrossRef]
- Chang, C.W.; Han, W.-Q.; Zettl, A. Thermal Conductivity of B–C–N and BN Nanotubes. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process. Meas. Phenom. 2005, 23, 1883. [Google Scholar] [CrossRef]



| C–C | B–N | diw | Dint | Dext | Eform | Eiw | Egap | c11 | e11 | |
|---|---|---|---|---|---|---|---|---|---|---|
| BN@BN | - | 1.45 | 3.45 | 9.07 | 15.98 | 0.018 | −0.009 | 5.84 | 210.11 | 67.94 |
| C@C | 1.43 | - | 3.50 | 8.71 | 15.71 | 0.067 | −0.002 | 0.64 | 261.75 | 0 |
| C | 1.42 | - | - | - | 15.72 | 0.043 | - | 0.67 | 174.04 | 0 |
| BN | - | 1.45 | - | - | 16.16 | 0.010 | - | 6.74 | 136.30 | 44.08 |
| C–C | B–N | C–B | C–N | diw | Dint | Dext | Emix | Eiw | Egap | c11 | |e11| | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symmetrical | ||||||||||||
| 10%SW | 1.42 | 1.46 | 1.51 | 1.40 | - | - | 16.70 | 0.045 | - | 0.57 | 165.03 | 88.77 |
| 30%SW | 1.43 | 1.45 | 1.50 | 1.42 | - | - | 15.84 | 0.087 | - | 1.44 | 157.57 | 96.61 |
| 10%DW | 1.43 | 1.45 | 1.51 | 1.39 | 3.35 | 8.94 | 15.63 | 0.028 | −0.081 | 0.55 | 255.74 | 74.58 |
| 30%DW | 1.43 | 1.46 | 1.51 | 1.40 | 3.53 | 8.72 | 15.77 | 0.057 | −0.074 | 1.07 | 251.62 | 95.27 |
| Non-symmetrical | ||||||||||||
| 10%SW | 1.42 | 1.45 | 1.52 | 1.40 | - | - | 16.39 | 0.021 | - | 0.45 | 161.80 | 89.83 |
| 30%SW | 1.43 | 1.45 | 1.52 | 1.40 | - | - | 16.33 | 0.016 | - | 0.55 | 156.69 | 104.61 |
| 10%DW | 1.43 | 1.45 | 1.52 | 1.40 | 3.44 | 8.70 | 15.58 | 0.013 | −0.087 | 0.43 | 256.21 | 92.23 |
| 30%DW | 1.43 | 1.47 | 1.52 | 1.39 | 3.36 | 8.93 | 15.65 | 0.011 | −0.085 | 0.52 | 250.82 | 100.94 |
| Random | ||||||||||||
| 10%SW | 1.43 | 1.45 | 1.49 | 1.40 | - | - | 15.78 | 0.049 | - | 0.81 | 164.43 | 76.48 |
| 30%SW | 1.43 | 1.45 | 1.49 | 1.40 | - | - | 15.74 | 0.139 | - | 1.45 | 156.87 | 102.62 |
| 10%DW | 1.43 | 1.45 | 1.49 | 1.39 | 3.47 | 8.71 | 15.65 | 0.029 | −0.086 | 0.67 | 247.35 | 81.55 |
| 30%DW | 1.43 | 1.45 | 1.49 | 1.39 | 3.52 | 8.71 | 15.75 | 0.088 | −0.091 | 0.99 | 244.41 | 100.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marana, N.L.; Sambrano, J.R.; Casassa, S. Modeling of BN-Doped Carbon Nanotube as High-Performance Thermoelectric Materials. Nanomaterials 2022, 12, 4343. https://doi.org/10.3390/nano12234343
Marana NL, Sambrano JR, Casassa S. Modeling of BN-Doped Carbon Nanotube as High-Performance Thermoelectric Materials. Nanomaterials. 2022; 12(23):4343. https://doi.org/10.3390/nano12234343
Chicago/Turabian StyleMarana, Naiara L., Julio R. Sambrano, and Silvia Casassa. 2022. "Modeling of BN-Doped Carbon Nanotube as High-Performance Thermoelectric Materials" Nanomaterials 12, no. 23: 4343. https://doi.org/10.3390/nano12234343
APA StyleMarana, N. L., Sambrano, J. R., & Casassa, S. (2022). Modeling of BN-Doped Carbon Nanotube as High-Performance Thermoelectric Materials. Nanomaterials, 12(23), 4343. https://doi.org/10.3390/nano12234343





