Reduced Graphene Oxide-Wrapped Novel CoIn2S4 Spinel Composite Anode Materials for Li-ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Pristine CoIn2S4 and CoIn2S4/rGO Composite
2.2. Characterizations
2.3. Electrochemical Measurements
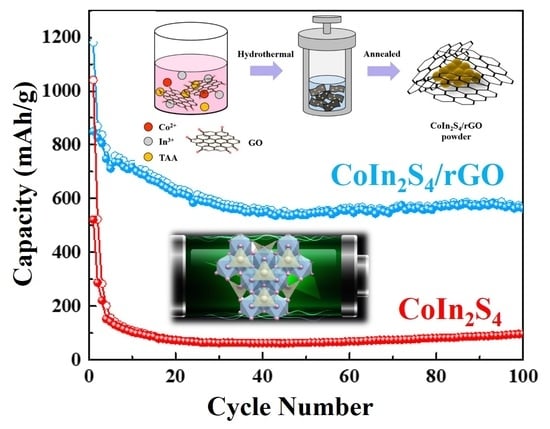
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Horowitz, Y.; Schmidt, C.; Yoon, D.H.; Riegger, L.M.; Katzenmeier, L.; Bosch, G.M.; Golodnitsky, D. Between liquid and all solid: A prospect on electrolyte future in Lithium-ion batteries for electric vehicles. Energy Technol. 2020, 8, 2000580. [Google Scholar] [CrossRef]
- Kong, L.; Li, C.; Jiang, J.; Pecht, M.G. Li-ion battery fire hazards and safety strategies. Energies 2018, 11, 2191. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, A. The underestimated potential of battery electric vehicles to reduce emissions. Joule 2019, 3, 1412–1414. [Google Scholar] [CrossRef] [Green Version]
- Vissers, D.R.; Chen, Z.; Shao, Y.; Engelhard, M.; Das, U.; Redfern, P.; Amine, K. Role of manganese deposition on graphite in the capacity fading of lithium ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 14244–14251. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A. Effect of electrode density on cycle performance and irreversible capacity loss for natural graphite anode in lithium-ion batteries. J. Power Sources 2003, 119, 934–937. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yan, D.; Xu, H.; Feng, J.; Jiang, X.; Yue, J.; Qian, Y. Hollow nanospheres of mesoporous Co9S8 as a high-capacity and long-life anode for advanced lithium ion batteries. Nano Energy 2015, 12, 528–537. [Google Scholar] [CrossRef]
- Qu, G.; Geng, H.; Ge, D.; Tang, M.; Zheng, J.; Gu, H. Porous carbon-wrapped mesoporous Co9S8 fibers as stable anode for Li-ion batteries. Electrochim. Acta 2016, 211, 305–312. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Q.; Peng, J.; Jiang, Y.; Ding, Y.; Wei, Q. Co9S8 nanoparticles embedded into amorphous carbon as anode materials for lithium-ion batteries. Nanotechnology 2020, 31, 235713. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Jiang, J.; Liu, F.; Hou, Y. Multifunctional Co3S4/graphene composites for lithium ion batteries and oxygen reduction reaction. Chem.–Eur. J. 2013, 19, 5183–5190. [Google Scholar] [CrossRef]
- Luo, F.; Ma, D.; Li, Y.; Mi, H.; Zhang, P.; Luo, S. Hollow Co3S4/C anchored on nitrogen-doped carbon nanofibers as a free-standing anode for high-performance Li-ion batteries. Electrochimica Acta 2019, 299, 173–181. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Y.; Wang, Y. Graphene-wrapped CoS nanoparticles for high-capacity lithium-ion storage. ACS Appl. Mater. Interfaces 2013, 5, 801–806. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Liu, S.; Nie, L.; Chai, Y.; Yang, X.; Yuan, R. CoS/CNTs hybrid structure for improved performance lithium ion battery. J. Alloys Compd. 2016, 676, 551–556. [Google Scholar] [CrossRef]
- Kong, S.; Jin, Z.; Liu, H.; Wang, Y. Morphological effect of graphene nanosheets on ultrathin CoS nanosheets and their applications for high-performance Li-ion batteries and photocatalysis. J. Phys. Chem. C 2014, 118, 25355–25364. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Gao, W.; Wang, Y.; Jiao, L.; Yuan, H.; Liu, S. Synthesis of novel CuS with hierarchical structures and its application in lithium-ion batteries. Powder Technol. 2011, 212, 64–68. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, P.; Liao, H.; Cheng, S. In situ preparation of CuS cathode with unique stability and high rate performance for lithium ion batteries. Electrochim. Acta 2012, 80, 264–268. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Saeed, A.; Zhou, K.; Shoaib, M.; Waqas, M. Electrochemical performance of 2D polyaniline anchored CuS/Graphene nano-active composite as anode material for lithium-ion battery. J. Colloid Interface Sci. 2017, 502, 16–23. [Google Scholar] [CrossRef]
- Dong, X.; Deng, Z.P.; Huo, L.H.; Zhang, X.F.; Gao, S. Large-scale synthesis of NiS@N and S co-doped carbon mesoporous tubule as high performance anode for lithium-ion battery. J. Alloys Compd. 2019, 788, 984–992. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Q.; Tao, L.; Su, X. Controlled-synthesis of NiS hierarchical hollow microspheres with different building blocks and their application in lithium batteries. J. Mater. Chem. 2011, 21, 9248–9254. [Google Scholar] [CrossRef]
- Deng, C.; Yang, L.; Yang, C.; Shen, P.; Zhao, L.; Wang, Z.; Qian, D. Spinel FeCo2S4 nanoflower arrays grown on Ni foam as novel binder-free electrodes for long-cycle-life supercapacitors. Appl. Surf. Sci. 2018, 428, 148–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Konya, M.; Kutsuma, A.; Lim, S.; Mandai, T.; Munakata, H.; Kanamura, K. Magnesium storage performance and mechanism of 2D-ultrathin nanosheet-assembled spinel MgIn2S4 cathode for high-temperature Mg batteries. Small 2019, 15, 1902236. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Sinhamahapatra, A.; Ko, J.J.; Yu, J.S. High capacity and exceptional cycling stability of ternary metal sulfide nanorods as Li ion battery anodes. Chem. Commun. 2015, 51, 13350–13353. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, C.; Zhong, Y.; Chen, M.; Cai, L.; Wang, H.; Li, W. Highly dispersed Co-Mo sulfide nanoparticles on reduced graphene oxide for lithium and sodium ion storage. Nano Res. 2020, 13, 188–195. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, A.; Sun, Z.; Ning, X.; Guo, J.; Lv, Y.; Jia, D. Boosting the performance of half/full lithium-ion batteries by designing smart architecture anode of SnS2 nanosheet coating on NiCo2S4 hollow spheres. J. Alloys Compd. 2020, 847, 156505. [Google Scholar] [CrossRef]
- Verma, R.; Kothandaraman, R.; Varadaraju, U.V. In-situ carbon coated CuCo2S4 anode material for Li-ion battery applications. Appl. Surf. Sci. 2017, 418, 30–39. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Feng, X.; Zhao, Y.; Li, H.; Feng, C.; Bai, X. One-pot synthesis of CuCo2S4 sub-microspheres for high-performance Lithium-/sodium-ion batteries. ChemElectroChem 2019, 6, 1558–1566. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, Z.; Liu, L.; Yang, J.; Zhang, C.; Pan, X.; Chi, F. Spray-drying assisted hydrothermal synthesis of ZnIn2S4@GO as anode material for improved lithium ion batteries. Int. J. Electrochem. Sci 2020, 15, 8797–8807. [Google Scholar] [CrossRef]
- Hsu, T.H.; Muruganantham, R.; Liu, W.-R. High-energy ball-milling for fabrication of CuIn2S4/C composite as an anode material for lithium-ion batteries. Ceram. Int. 2022, 48, 11561–11572. [Google Scholar] [CrossRef]
- Kumar, S.; Augustine, S.; Yadav, S.; Yadav, B.K.; Chauhan, R.P.; Malhotra, B.D. Effect of Brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosens. Bioelectron. 2018, 102, 247–255. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Zuo, X.; Song, Y.; Zhen, M. Carbon-coated NiCo2S4 multi-shelled hollow microspheres with porous structures for high rate lithium ion battery applications. Appl. Surf. Sci. 2020, 500, 144000. [Google Scholar] [CrossRef]
- Park, G.D.; Choi, S.H.; Lee, J.-K.; Kang, Y.C. One-pot method for synthesizing spherical-like metal sulfide–reduced graphene oxide composite powders with superior electrochemical properties for Lithium-ion batteries. Chem. Eur. J 2014, 20, 12183–12189. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Kang, Y.C. Sodium-ion storage properties of nickel sulfide hollow nanosphere/reduced graphene oxide composite powders prepared by a spray drying process and the nanoscale Kirkendall effect. Nanoscale 2015, 7, 16781. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-Y.; Liu, W.-R. Reduced Graphene Oxide-Wrapped Novel CoIn2S4 Spinel Composite Anode Materials for Li-ion Batteries. Nanomaterials 2022, 12, 4367. https://doi.org/10.3390/nano12244367
Lee T-Y, Liu W-R. Reduced Graphene Oxide-Wrapped Novel CoIn2S4 Spinel Composite Anode Materials for Li-ion Batteries. Nanomaterials. 2022; 12(24):4367. https://doi.org/10.3390/nano12244367
Chicago/Turabian StyleLee, Ting-Yu, and Wei-Ren Liu. 2022. "Reduced Graphene Oxide-Wrapped Novel CoIn2S4 Spinel Composite Anode Materials for Li-ion Batteries" Nanomaterials 12, no. 24: 4367. https://doi.org/10.3390/nano12244367
APA StyleLee, T.-Y., & Liu, W.-R. (2022). Reduced Graphene Oxide-Wrapped Novel CoIn2S4 Spinel Composite Anode Materials for Li-ion Batteries. Nanomaterials, 12(24), 4367. https://doi.org/10.3390/nano12244367