Polyacrylic Acid Functionalized Biomass-Derived Carbon Skeleton with Highly Porous Hierarchical Structures for Efficient Solid-Phase Microextraction of Volatile Halogenated Hydrocarbons
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents, Materials, and Instruments
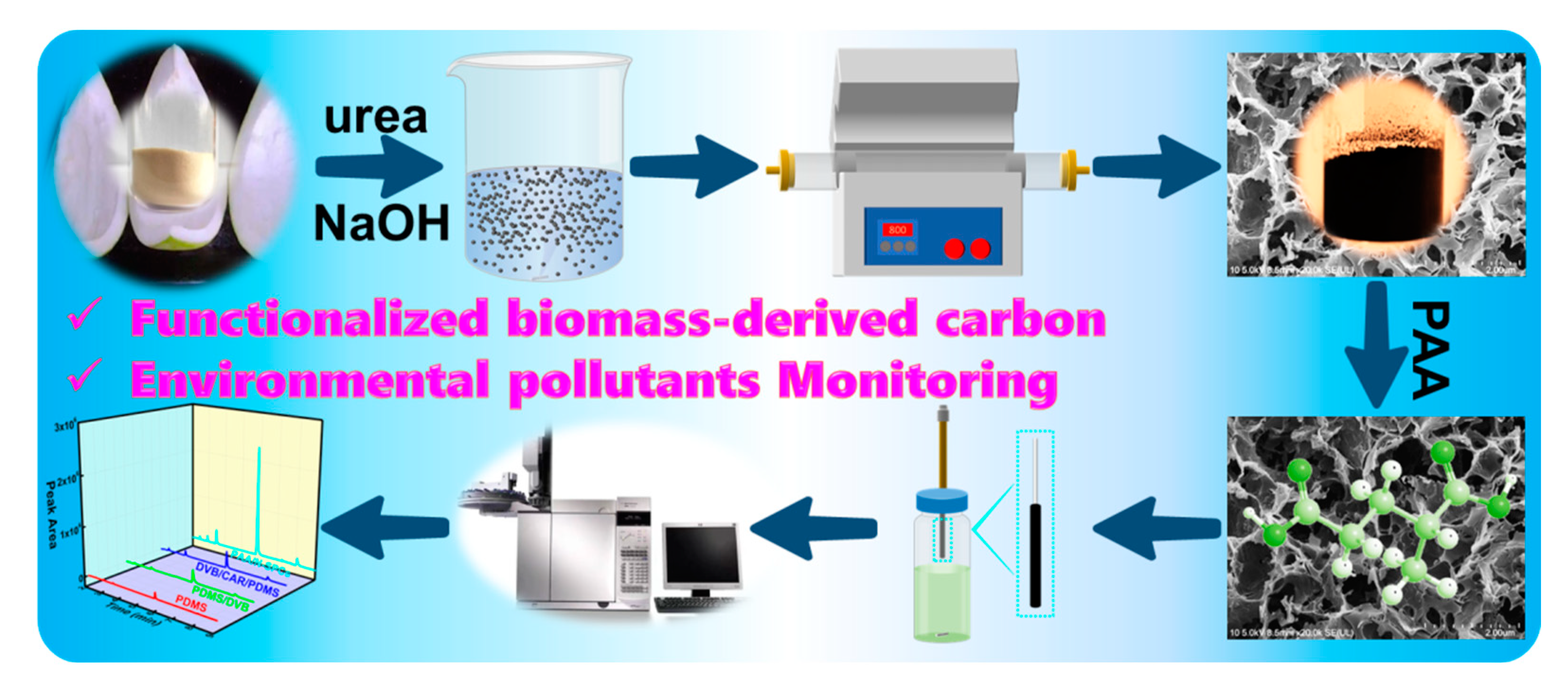
2.2. Preparation of PAA/N-SPCs
2.3. Fabrication of PAA/N-SPCs Coated SPME Fiber
2.4. SPME Procedures and GC–MS Analysis
2.5. Collection and Analysis of Real Water Samples
3. Results and Discussion
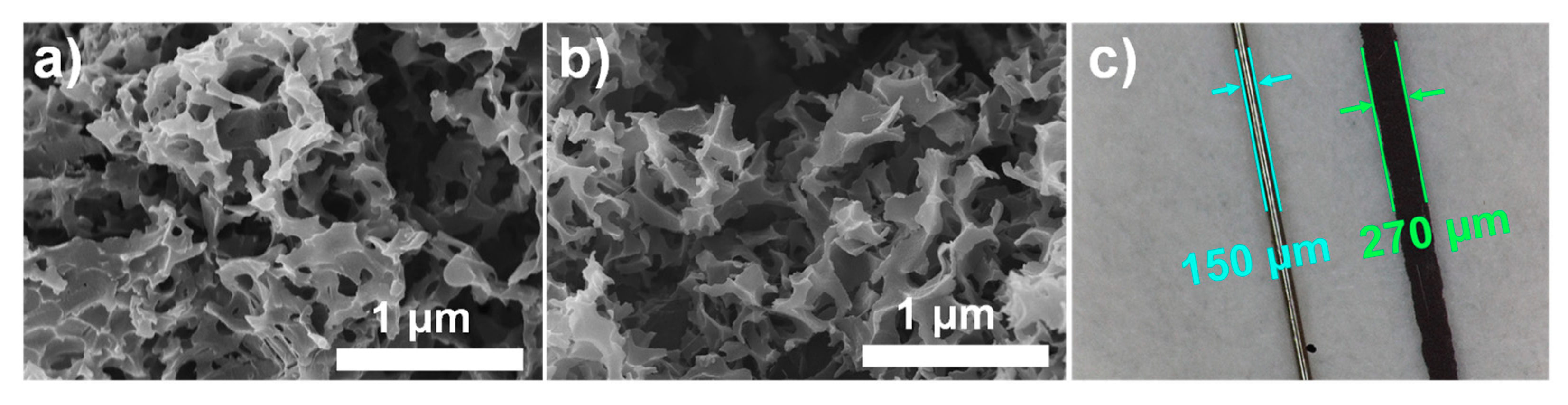
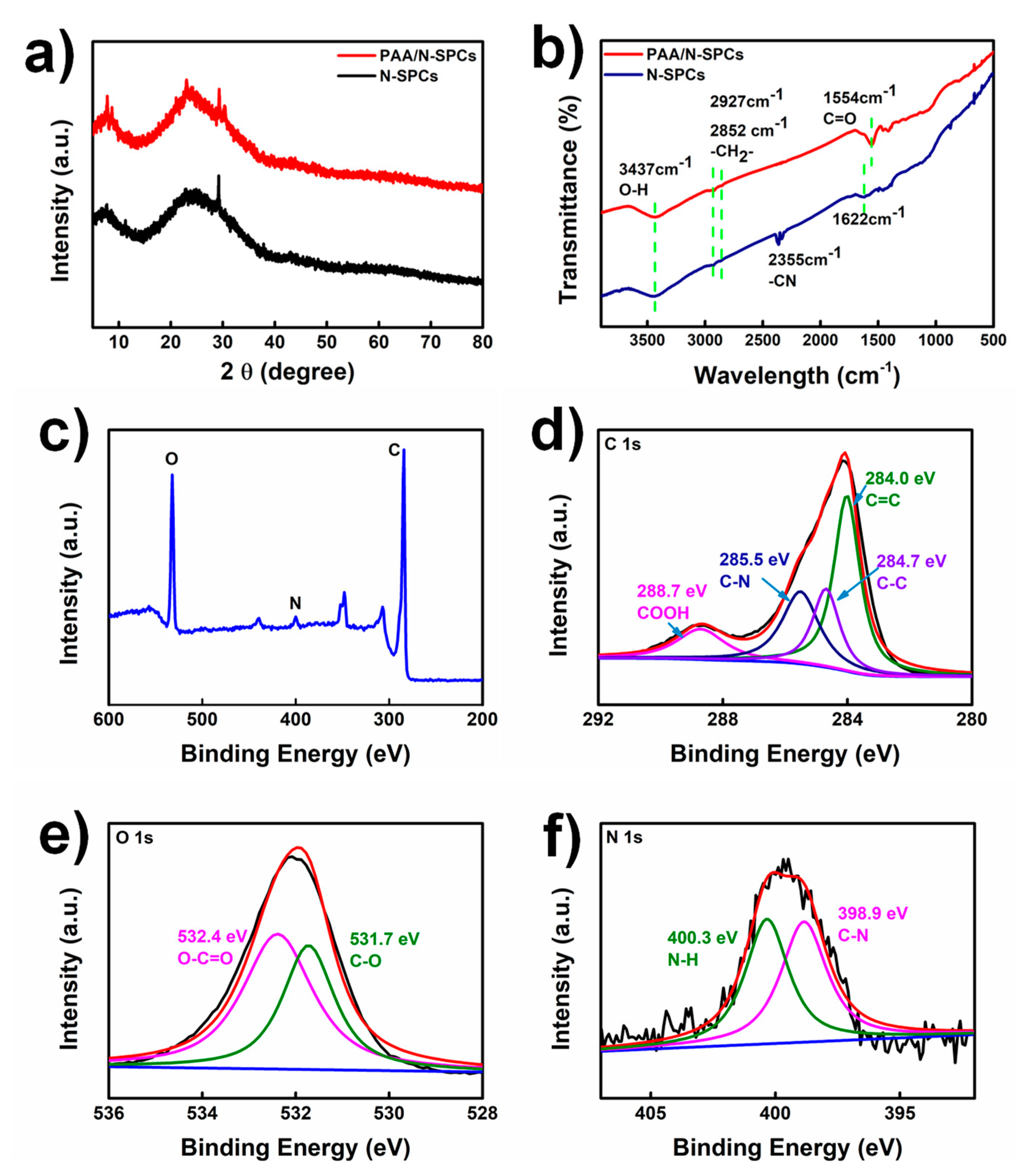
3.1. Characterizations of PAA/N-SPCs
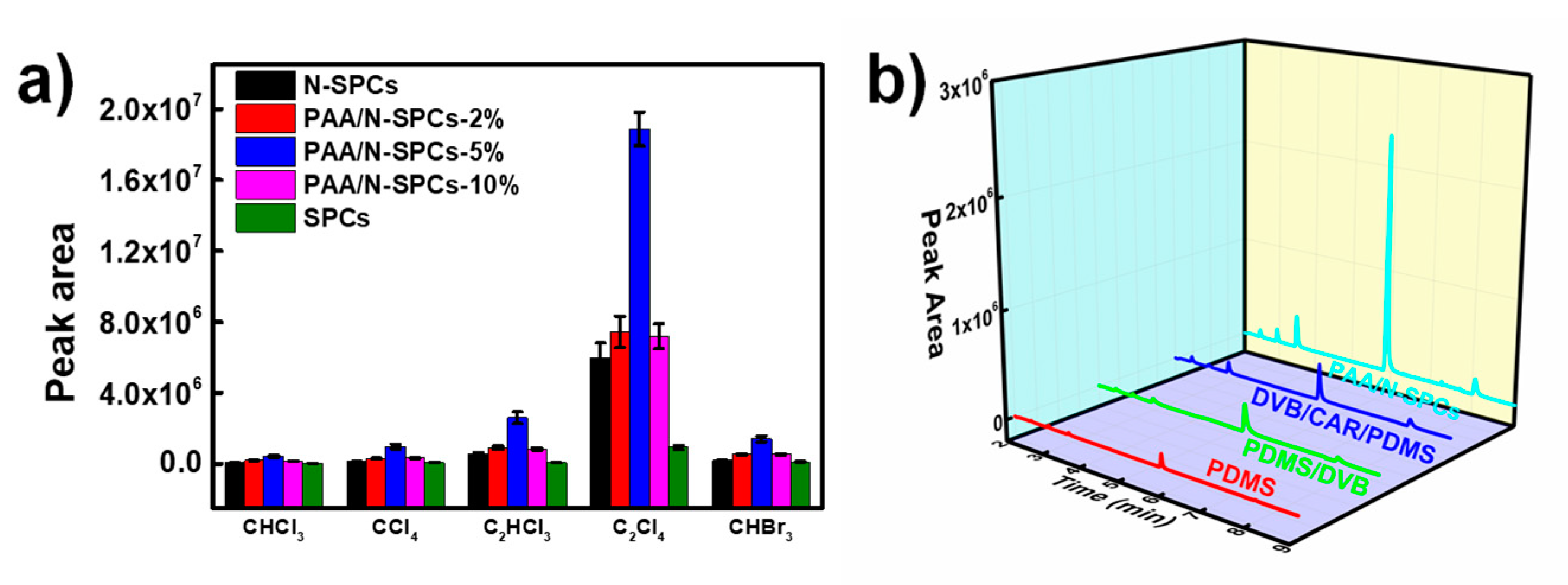
3.2. SPME Capacities of PAA/N-SPCs Coating for VHCs
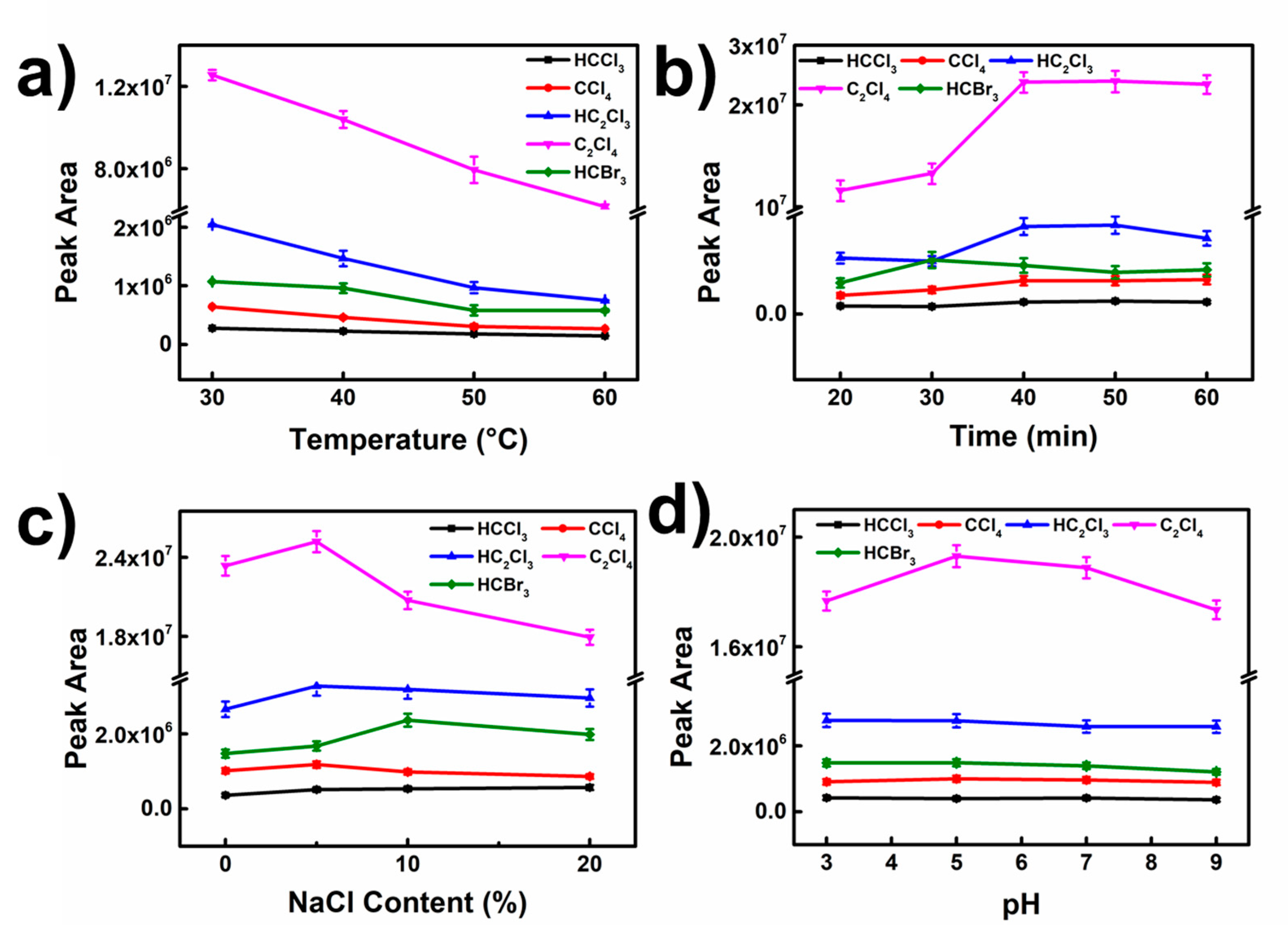
3.3. Optimization of SPME Conditions
3.4. Analytical Method Performance
3.5. Real Water Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R.; Wu, X.; Zhang, W.; Chen, Y.; Fu, J.; Ou, H. Volatile organic compounds generation pathways and mechanisms from microplastics in water: Ultraviolet, chlorine and ultraviolet/chlorine disinfection. J. Hazard. Mater. 2023, 441, 129813. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Yang, Q.; Gu, Y.; Wang, P.; Li, Z.; Li, L. Performance and mechanism of the in situ restoration effect on VHCs in the polluted river water based on the orthogonal experiment: Photosynthetic fluorescence characteristics and microbial community analysis. Environ. Sci. Pollut. R. 2022, 29, 43004–43018. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, C.; Zhou, Y.; Liu, S.; Liu, W. A new perspective on volatile halogenated hydrocarbons in Chinese agricultural soils. Sci. Total Environ. 2020, 703, 134646. [Google Scholar] [CrossRef]
- Dowty, B.; Carlisle, D.; Laseter, J.L.; Storer, J. Halogenated hydrocarbons in New Orleans drinking water and blood plasma. Science 1975, 187, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Ueta, I. Gas chromatographic determination of volatile compounds. Anal. Sci. 2022, 38, 737–738. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trend. Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Xia, F.; Zhu, T.; Wu, D.; Chingin, K.; Chen, H. High throughput online sequential extraction of natural rare earth elements and determination by mass spectrometry. Sci. China Chem. 2021, 64, 642–649. [Google Scholar] [CrossRef]
- Guzowska, M.; Wasiak, W.; Wawrzyniak, R. Comparison of extraction techniques for the determination of volatile organic compounds in liverwort samples. Molecules 2022, 27, 2911. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Xiao, Y.; Fan, J.; Feng, S.; Xu, S. Application of cooling-assisted solid phase microextraction in analysis of complex matrix sample. Chin. J. Anal. Chem. 2022, 50, 1289–1298. [Google Scholar]
- Piri-Moghadam, H.; Ahmadi, F.; Pawliszyn, J. A critical review of solid phase microextraction for analysis of water samples. Trac-Trend. Anal. Chem. 2016, 85, 133–143. [Google Scholar] [CrossRef]
- Reyes-Garces, N.; Gionfriddo, E.; Gmez-Rios, G.A.; Alam, M.N.; Boyaci, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, H.; Wu, H.; Xiao, L.; Dong, P.; Feng, S.; Fan, J. A facile cooling-assisted solid-phase microextraction device for solvent-free sampling of polycyclic aromatic hydrocarbons from soil based on matrix solid-phase dispersion technique. Anal. Chim. Acta 2020, 1115, 7–15. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.; Chen, C.; Feng, S.; Fan, J. Ultrasound-assisted one-step reduction and self-assembly of carbon dots-reduced graphene oxide: Mechanism investigation and solid phase microextraction of ultra-trace organochlorine pesticides. Chem. Eng. J. 2023, 451, 138569. [Google Scholar] [CrossRef]
- Xu, S.; Shuai, Q.; Pawliszyn, J. Determination of polycyclic aromatic hydrocarbons in sediment by pressure-balanced cold fiber solid phase microextraction. Anal. Chem. 2016, 88, 8936–8941. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Shen, M.; Li, X.; Tong, Y.; Guo, J.; Lin, W.; Ye, Y.; Xu, J.; Zhou, N.; Zhu, F.; et al. Rational design of ordered porous nanoparticles for selective extraction of nitrobenzene compounds. J. Hazard. Mater. 2023, 441, 129971. [Google Scholar] [CrossRef]
- Zang, X.; Chang, Q.; Li, H.; Zhao, X.; Zhang, S.; Wang, C.; Wang, Z. Construction of a ringent multi-shelled hollow MIL-88B as the solid-phase microextraction fiber coating for the extraction of organochlorine pesticides. Sep. Purif. Technol. 2023, 304, 122350. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Dong, P.; Wang, M.; Chen, C.-P.; Feng, S.; Fan, J. High-throughput profiling volatiles in edible oils by cooling assisted solid-phase microextraction technique for sensitive discrimination of edible oils adulteration. Anal. Chim. Acta 2022, 1221, 340159. [Google Scholar] [CrossRef]
- Tuzen, M.; Hazer, B.; Elik, A.; Altunay, N. Synthesized of poly(vinyl benzyl dithiocarbonate-dimethyl amino ethyl methacrylate) block copolymer as adsorbent for the vortex-assisted dispersive solid phase microextraction of patulin from apple products and dried fruits. Food Chem. 2022, 395, 133607. [Google Scholar] [CrossRef]
- Gao, Y.; Sheng, K.; Bao, T.; Wang, S. Recent applications of organic molecule-based framework porous materials in solid-phase microextraction for pharmaceutical analysis. J. Pharmaceut. Biomed. 2022, 221, 115040. [Google Scholar] [CrossRef]
- Filipiak, W.; Bojko, B. SPME in clinical, pharmaceutical, and biotechnological research—How far are we from daily practice? TrAC Trend. Anal. Chem. 2019, 115, 203–213. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, Y.; Hu, Q.; Yang, Y.; Ye, Y.; Zou, S.; Xu, J.; Ouyang, G. A polymeric solid-phase microextraction fiber for the detection of pharmaceuticals in water samples. J. Chromatogr. A 2020, 1623, 461171. [Google Scholar] [CrossRef]
- Song, L.; Chingin, K.; Wang, M.; Zhong, D.; Chen, H.; Xu, J. Polarity-specific profiling of metabolites in single cells by probe electrophoresis mass spectrometry. Anal. Chem. 2022, 94, 4175–4182. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Q.; Wang, C.; Xiao, L.; Feng, S.; Li, N.; Chen, C.-P. Three-dimensional pompon-like Au/ZnO porous microspheres as solid phase microextraction coating for determination of volatile fatty acids from foot odor. Talanta 2020, 209, 120519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Zhang, X.; Han, L.; Qin, P.; Tian, S.; Zhou, Q.; Zhang, X.; Lu, M. Preparation of Al-doped mesoporous crystalline material-41 as fiber coating material for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons from human urine. J. Chromatogr. A 2020, 1626, 461354. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhu, W.; Qin, P.; Lu, M.; Zhang, X.; Miao, Y.; Cai, Z. Mesoporous graphitic carbon nitride@NiCo2O4 nanocomposite as a solid phase microextraction coating for sensitive determination of environmental pollutants in human serum samples. Chem. Commun. 2019, 55, 10019–10022. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, X.; Huang, Y.; Huang, Y.; Zheng, J.; Zhu, F.; Xu, J.; Ouyang, G. Novel solid-phase microextraction fiber coatings: A review. J. Sep. Sci. 2022, 45, 282–304. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.; Long, A.; Li, H.; Chen, C.; Feng, S.; Fan, J. Carbon dot-decorated graphite carbon nitride composites for enhanced solid-phase microextraction of chlorobenzenes from water. Nanomaterials 2022, 12, 335. [Google Scholar] [CrossRef]
- Xu, S.; Dong, P.; Liu, H.; Li, H.; Chen, C.; Feng, S.; Fan, J. Lotus-like Ni@NiO nanoparticles embedded porous carbon derived from MOF-74/cellulose nanocrystal hybrids as solid phase microextraction coating for ultrasensitive determination of chlorobenzenes from water. J. Hazard. Mater. 2022, 429, 128384. [Google Scholar] [CrossRef]
- Vazquez-Garrido, I.; Flores-Aguilar, J.F.; Miranda, J.M.; Santos, E.M.; Jardinez, C.; Ibarra, I.S. Carbonaceous materials in sample treatment techniques in the determination of pesticides in food and environmental analysis. A review. Int. J. Environ. Anal. Chem. 2022, 1–35. [Google Scholar] [CrossRef]
- Kang, J.Y.; Shi, Y.P. Recent advances and application of carbon nitride framework materials in sample preparation. TrAC Trend. Anal. Chem. 2022, 153, 116661. [Google Scholar] [CrossRef]
- Shahhoseini, F.; Azizi, A.; Bottaro, C.S. A critical evaluation of molecularly imprinted polymer (MIP) coatings in solid phase microextraction devices. TrAC Trend. Anal. Chem. 2022, 156, 116695. [Google Scholar] [CrossRef]
- Torabi, E.; Mirzaei, M.; Bazargan, M.; Amiri, A. A critical review of covalent organic frameworks-based sorbents in extraction methods. Anal. Chim. Acta 2022, 1224, 340207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Ihara, H.; Guan, M.; Qiu, H.D. Preparation of porous carbon nanomaterials and their application in sample preparation: A review. TrAC Trend. Anal. Chem. 2021, 143, 116421. [Google Scholar] [CrossRef]
- Kusmierek, K.; Borucka, M.; Swiatkowski, A.; Dabek, L. Evaluation of different carbon materials in adsorption and solid-phase microextraction of 2,4,6-trichlorophenol from water. Desalin. Water Treat. 2019, 157, 129–137. [Google Scholar] [CrossRef]
- Xie, X.; Yang, H.; Han, J.; Tong, Y.; Hu, Y.; Ouyang, S.; Cui, S.; Zheng, J.; Ouyang, G. Nitrogen, oxygen-codoped hierarchically porous biochar for simultaneous enrichment and ultrasensitive determination of o-xylene and its hydroxyl metabolites in human urine by solid phase microextraction-gas chromatography-mass spectrometry. Microchem. J. 2022, 178, 107384. [Google Scholar] [CrossRef]
- Behbahan, A.K.; Mahdavi, V.; Roustaei, Z.; Bagheri, H. Preparation and evaluation of various banana-based biochars together with ultra-high performance liquid chromatography-tandem mass spectrometry for determination of diverse pesticides in fruiting vegetables. Food Chem. 2021, 360, 130085. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.T.; Wu, Y.R.; Bian, Y.R.; Song, Y.; Sun, Q.; Jiang, X.; Zhang, L.J.; Han, J.G.; Cheng, H. Nitrogen-doped porous biochar derived from marine algae for efficient solid-phase microextraction of chlorobenzenes from aqueous solution. J. Hazard. Mater. 2021, 407, 124785. [Google Scholar] [CrossRef]
- Yin, L.; Hu, Q.; Mondal, S.; Xu, J.; Ouyang, G. Peanut shell-derived biochar materials for effective solid-phase microextraction of polycyclic aromatic hydrocarbons in environmental. Talanta 2019, 202, 90–95. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef]
- Gokmen, F.O.; Yaman, E.; Temel, S. Eco-friendly polyacrylic acid based porous hydrogel for heavy metal ions adsorption: Characterization, adsorption behavior, thermodynamic and reusability studies. Microchem. J. 2021, 168, 106357. [Google Scholar] [CrossRef]
- Bibi, A.; Bibi, S.; Abu-Dieyeh, M.; Al-Ghouti, M.A. New material of polyacrylic acid-modified graphene oxide composite for phenol remediation from synthetic and real wastewater. Environ. Technol. Inno. 2022, 27, 102795. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Liu, M.; Su, L.; Wang, Y.; Li, S.; Zhong, M.-e.; Zhou, Z.; Zhou, N. Simultaneous removal of organic inorganic composite contaminants by in situ double modified biochar: Performance and mechanisms. J. Taiwan Inst. Chem. E. 2022, 139, 104523. [Google Scholar] [CrossRef]
- Salami, M.; Talebpour, Z.; Alizadeh, R. Fabrication of a new SPME fiber based on Polyacrylic acid/ MIL-88(Fe)-NH2 composite as a self-healing coating for the analysis of breast cancer biomarkers in the urine sample. J. Pharmaceut. Biomed. 2022, 219, 114902. [Google Scholar] [CrossRef]
- Xu, S.; Dong, P.; Qin, M.; Liu, H.; Long, A.; Chen, C.; Feng, S.; Wu, H. Core-shell structured Fe2O3/CeO2@MnO2 microspheres with abundant surface oxygen for sensitive solid-phase microextraction of polycyclic aromatic hydrocarbons from water. Microchim. Acta 2021, 188, 337. [Google Scholar] [CrossRef]
- Wu, S.; Qiao, Y.; Jiang, K.; He, Y.; Guo, S.; Zhou, H. Tailoring sodium anodes for stable sodium–oxygen batteries. Adv. Funct. Mater. 2018, 28, 1706374. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Fu, Y.; Wang, J.; Lvov, Y.; Liu, J.; Lu, Y.; Zhang, L. Enhanced electrical and mechanical properties of rubber/graphene film through layer-by-layer electrostatic assembly. Compos. Part B: Eng. 2016, 90, 457–464. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Lou, Z.; Shan, W.; Xiong, Y. Polyacrylic acid-functionalized graphene oxide for high-performance adsorption of gallium from aqueous solution. J. Colloid. Interf. Sci. 2019, 556, 102–110. [Google Scholar] [CrossRef]
- Xu, G.; Yan, Q.; Kushima, A.; Zhang, X.; Pan, J.; Li, J. Conductive graphene oxide-polyacrylic acid (GOPAA) binder for lithium-sulfur battery. Nano Energy 2017, 31, 568–574. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.; Li, J.; Song, X.; Dai, H.; Liang, Z. Construction of carbon quantum dots/proton-functionalized graphitic carbon nitride nanocomposite via electrostatic self-assembly strategy and its application. Appl. Surf. Sci. 2016, 370, 514–521. [Google Scholar] [CrossRef]
- Singh, G.; Aher, S.C.; Varma, P.V.S.; Sathe, D.B.; Bhatt, R.B. Analysis of BET specific surface area in several recycled oxide powders. J. Radioanal. Nucl. Chem. 2021, 327, 555–564. [Google Scholar] [CrossRef]

| Analytes | Linear Ranges (ng mL−1) | R2 | LODs (ng mL−1) | LOQs (ng mL−1) | RSDs (%) (n = 5) |
|---|---|---|---|---|---|
| CHCl3 | 0.5–50 | 0.9973 | 0.0054 | 0.0181 | 7.1 |
| CCl4 | 0.5–50 | 0.9879 | 0.0086 | 0.0285 | 5.8 |
| C2HCl3 | 0.5–50 | 0.9918 | 0.0031 | 0.0102 | 6.8 |
| C2Cl4 | 0.5–50 | 0.9922 | 0.0005 | 0.0015 | 7.2 |
| CHBr3 | 0.5–250 | 0.9938 | 0.0060 | 0.0199 | 6.1 |
| Analytes | Campus Tap Water (1#) | Lake Water (2#) | Lake Water (3#) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Found (ng mL−1) | RSDs (%, n = 3) | Recoveries (%, Spiked with 0.5 ng mL−1) | Found (ng mL−1) | RSDs (%, n = 3) | Recoveries (%, Spiked with 5 ng mL−1) | Found (ng mL−1) | RSDs (%, n = 3) | Recoveries (%, Spiked with 5 ng mL−1) | |
| CHCl3 | ND | 5.9 | 96 | 2.23 | 8.0 | 75 | 1.31 | 7.5 | 76 |
| CCl4 | ND | 8.2 | 102 | ND | – | 103 | ND | – | 106 |
| C2HCl3 | ND | 7.5 | 110 | ND | – | 103 | ND | – | 109 |
| C2Cl4 | ND | 8.1 | 103 | ND | – | 101 | 0.027 | 12.0 | 111 |
| CHBr3 | ND | 15.0 | 106 | 0.43 | 8.5 | 116 | ND | – | 112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, A.; Liu, H.; Xu, S.; Feng, S.; Shuai, Q.; Hu, S. Polyacrylic Acid Functionalized Biomass-Derived Carbon Skeleton with Highly Porous Hierarchical Structures for Efficient Solid-Phase Microextraction of Volatile Halogenated Hydrocarbons. Nanomaterials 2022, 12, 4376. https://doi.org/10.3390/nano12244376
Long A, Liu H, Xu S, Feng S, Shuai Q, Hu S. Polyacrylic Acid Functionalized Biomass-Derived Carbon Skeleton with Highly Porous Hierarchical Structures for Efficient Solid-Phase Microextraction of Volatile Halogenated Hydrocarbons. Nanomaterials. 2022; 12(24):4376. https://doi.org/10.3390/nano12244376
Chicago/Turabian StyleLong, Anying, Hailin Liu, Shengrui Xu, Suling Feng, Qin Shuai, and Shenghong Hu. 2022. "Polyacrylic Acid Functionalized Biomass-Derived Carbon Skeleton with Highly Porous Hierarchical Structures for Efficient Solid-Phase Microextraction of Volatile Halogenated Hydrocarbons" Nanomaterials 12, no. 24: 4376. https://doi.org/10.3390/nano12244376
APA StyleLong, A., Liu, H., Xu, S., Feng, S., Shuai, Q., & Hu, S. (2022). Polyacrylic Acid Functionalized Biomass-Derived Carbon Skeleton with Highly Porous Hierarchical Structures for Efficient Solid-Phase Microextraction of Volatile Halogenated Hydrocarbons. Nanomaterials, 12(24), 4376. https://doi.org/10.3390/nano12244376






