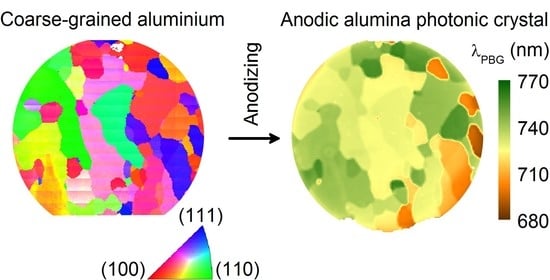
Mosaic of Anodic Alumina Inherited from Anodizing of Polycrystalline Substrate in Oxalic Acid
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wang, Z.; Mi, Y.; Xu, R.; Yu, S.-H.; Lei, Y. Designing Heterogeneous 1D Nanostructure Arrays Based on AAO Templates for Energy Applications. Small 2015, 11, 3408–3428. [Google Scholar] [CrossRef] [PubMed]
- Md Jani, A.M.; Losic, D.; Voelcker, N.H. Nanoporous Anodic Aluminium Oxide: Advances in Surface Engineering and Emerging Applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Sulka, G.D.; Brzózka, A.; Zaraska, L.; Wierzbicka, E.; Brudzisz, A. AAO Templates with Different Patterns and Channel Shapes. In Submicron Porous Materials; Springer: Cham, Switzerland, 2017; pp. 107–156. ISBN 978-3-319-53033-8. [Google Scholar]
- Roslyakov, I.V.; Koshkodaev, D.S.; Eliseev, A.A.; Hermida-Merino, D.; Ivanov, V.K.; Petukhov, A.V.; Napolskii, K.S. Growth of Porous Anodic Alumina on Low-Index Surfaces of Al Single Crystals. J. Phys. Chem. C 2017, 121, 27511–27520. [Google Scholar] [CrossRef]
- Beck, G.; Bretzler, R. Regularity of Nanopores in Anodic Alumina Formed on Orientated Aluminium Single-Crystals. Mater. Chem. Phys. 2011, 128, 383–387. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Ngan, A.H.W. Precise Control of Nanohoneycomb Ordering over Anodic Aluminum Oxide of Square Centimeter Areas. Chem. Mater. 2011, 23, 5264–5268. [Google Scholar] [CrossRef]
- Sacco, L.; Florea, I.; Châtelet, M.; Cojocaru, C.-S. Investigation of Porous Anodic Alumina Templates Formed by Anodization of Single-Crystal Aluminum Substrates. Thin Solid Films 2018, 660, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Roslyakov, I.V.; Eliseev, A.A.; Yakovenko, E.V.; Zabelin, A.V.; Napolskii, K.S. Longitudinal Pore Alignment in Anodic Alumina Films Grown on Polycrystalline Metal Substrates. J. Appl. Crystallogr. 2013, 46, 1705–1710. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Koshkodaev, D.S.; Eliseev, A.A.; Hermida-Merino, D.; Petukhov, A.V.; Napolskii, K.S. Crystallography-Induced Correlations in Pore Ordering of Anodic Alumina Films. J. Phys. Chem. C 2016, 120, 19698–19704. [Google Scholar] [CrossRef]
- Napolskii, K.S.; Roslyakov, I.V.; Romanchuk, A.Y.; Kapitanova, O.O.; Mankevich, A.S.; Lebedev, V.A.; Eliseev, A.A. Origin of Long-Range Orientational Pore Ordering in Anodic Films on Aluminium. J. Mater. Chem. 2012, 22, 11922–11926. [Google Scholar] [CrossRef]
- Evertsson, J.; Vinogradov, N.A.; Harlow, G.S.; Carlà, F.; McKibbin, S.R.; Rullik, L.; Linpé, W.; Felici, R.; Lundgren, E. Self-Organization of Porous Anodic Alumina Films Studied in Situ by Grazing-Incidence Transmission Small-Angle X-ray Scattering. RSC Adv. 2018, 8, 18980–18991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinogradov, N.A.; Harlow, G.S.; Carlà, F.; Evertsson, J.; Rullik, L.; Linpé, W.; Felici, R.; Lundgren, E. Observation of Pore Growth and Self-Organization in Anodic Alumina by Time-Resolved X-ray Scattering. ACS Appl. Nano Mater. 2018, 1, 1265–1271. [Google Scholar] [CrossRef]
- Gordeeva, E.O.; Roslyakov, I.V.; Napolskii, K.S. Aluminium Anodizing in Selenic Acid: Electrochemical Behaviour, Porous Structure, and Ordering Regimes. Electrochim. Acta 2019, 307, 13–19. [Google Scholar] [CrossRef]
- Sapoletova, N.A.; Kushnir, S.E.; Napolskii, K.S. Anodic Titanium Oxide Photonic Crystals Prepared by Novel Cyclic Anodizing with Voltage versus Charge Modulation. Electrochem. Commun. 2018, 91, 5–9. [Google Scholar] [CrossRef]
- Kushnir, S.E.; Sapoletova, N.A.; Roslyakov, I.V.; Napolskii, K.S. One-Dimensional Photonic Crystals with Nonbranched Pores Prepared via Phosphorous Acid Anodizing of Aluminium. Nanomaterials 2022, 12, 1548. [Google Scholar] [CrossRef] [PubMed]
- Ermolaev, G.A.; Kushnir, S.E.; Sapoletova, N.A.; Napolskii, K.S. Titania Photonic Crystals with Precise Photonic Band Gap Position via Anodizing with Voltage versus Optical Path Length Modulation. Nanomaterials 2019, 9, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushnir, S.E.; Napolskii, K.S. Thickness-Dependent Iridescence of One-Dimensional Photonic Crystals Based on Anodic Alumina. Mater. Des. 2018, 144, 140–150. [Google Scholar] [CrossRef]
- Sapoletova, N.A.; Kushnir, S.E.; Cherepanova, Y.M.; Napolskii, K.S. Effect of Heat Treatment on the Structure and Optical Properties of Porous Anodic Titanium Oxide Films. Inorg. Mater. 2022, 58, 40–47. [Google Scholar] [CrossRef]
- Sapoletova, N.A.; Kushnir, S.E.; Napolskii, K.S. Polarization-Enhanced Cell Walls Etching of Anodic Titanium Oxide. Nanotechnology 2022, 33, 065602. [Google Scholar] [CrossRef]
- Ozin, G.A.; Arsenault, A. Nanochemistry: A Chemical Approach to Nanomaterials; Royal Society of Chemistry: London, UK, 2015; ISBN 978-1-78262-626-8. [Google Scholar]
- Choy, T.C. Effective Medium Theory: Principles and Applications, 2nd ed.; Oxford University Press: Oxford, UK, 2016; ISBN 978-0-19-870509-3. [Google Scholar]
- Kushnir, S.E.; Pchelyakova, T.Y.; Napolskii, K.S. Anodizing with Voltage versus Optical Path Length Modulation: A New Tool for the Preparation of Photonic Structures. J. Mater. Chem. C 2018, 6, 12192–12199. [Google Scholar] [CrossRef]
- Acosta, L.K.; Bertó-Roselló, F.; Xifre-Perez, E.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F. Stacked Nanoporous Anodic Alumina Gradient-Index Filters with Tunable Multi-Spectral Photonic Stopbands as Sensing Platforms. ACS Appl. Mater. Interfaces 2019, 11, 3360–3371. [Google Scholar] [CrossRef] [PubMed]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Voelcker, N.H.; Santos, A. Nanoporous Anodic Alumina Photonic Crystals for Optical Chemo- and Biosensing: Fundamentals, Advances, and Perspectives. Nanomaterials 2018, 8, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Structural and Optical Nanoengineering of Nanoporous Anodic Alumina Rugate Filters for Real-Time and Label-Free Biosensing Applications. Anal. Chem. 2014, 86, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Rahman, M.M.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F.; Losic, D. Nanoporous Anodic Alumina Rugate Filters for Sensing of Ionic Mercury: Toward Environmental Point-of-Analysis Systems. ACS Appl. Mater. Interfaces 2014, 6, 12971–12978. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Law, C.S.; Markovic, M.; Marsal, L.F.; Voelcker, N.H.; Abell, A.D.; Santos, A. Rational Management of Photons for Enhanced Photocatalysis in Structurally-Colored Nanoporous Anodic Alumina Photonic Crystals. ACS Appl. Energy Mater. 2019, 2, 1169–1184. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushnir, S.E.; Kuznetsov, M.E.; Roslyakov, I.V.; Lyskov, N.V.; Napolskii, K.S. Mosaic of Anodic Alumina Inherited from Anodizing of Polycrystalline Substrate in Oxalic Acid. Nanomaterials 2022, 12, 4406. https://doi.org/10.3390/nano12244406
Kushnir SE, Kuznetsov ME, Roslyakov IV, Lyskov NV, Napolskii KS. Mosaic of Anodic Alumina Inherited from Anodizing of Polycrystalline Substrate in Oxalic Acid. Nanomaterials. 2022; 12(24):4406. https://doi.org/10.3390/nano12244406
Chicago/Turabian StyleKushnir, Sergey E., Mikhail E. Kuznetsov, Ilya V. Roslyakov, Nikolay V. Lyskov, and Kirill S. Napolskii. 2022. "Mosaic of Anodic Alumina Inherited from Anodizing of Polycrystalline Substrate in Oxalic Acid" Nanomaterials 12, no. 24: 4406. https://doi.org/10.3390/nano12244406
APA StyleKushnir, S. E., Kuznetsov, M. E., Roslyakov, I. V., Lyskov, N. V., & Napolskii, K. S. (2022). Mosaic of Anodic Alumina Inherited from Anodizing of Polycrystalline Substrate in Oxalic Acid. Nanomaterials, 12(24), 4406. https://doi.org/10.3390/nano12244406