Nanomaterials Based on Honey and Propolis for Wound Healing—A Mini-Review
Abstract
:1. Introduction
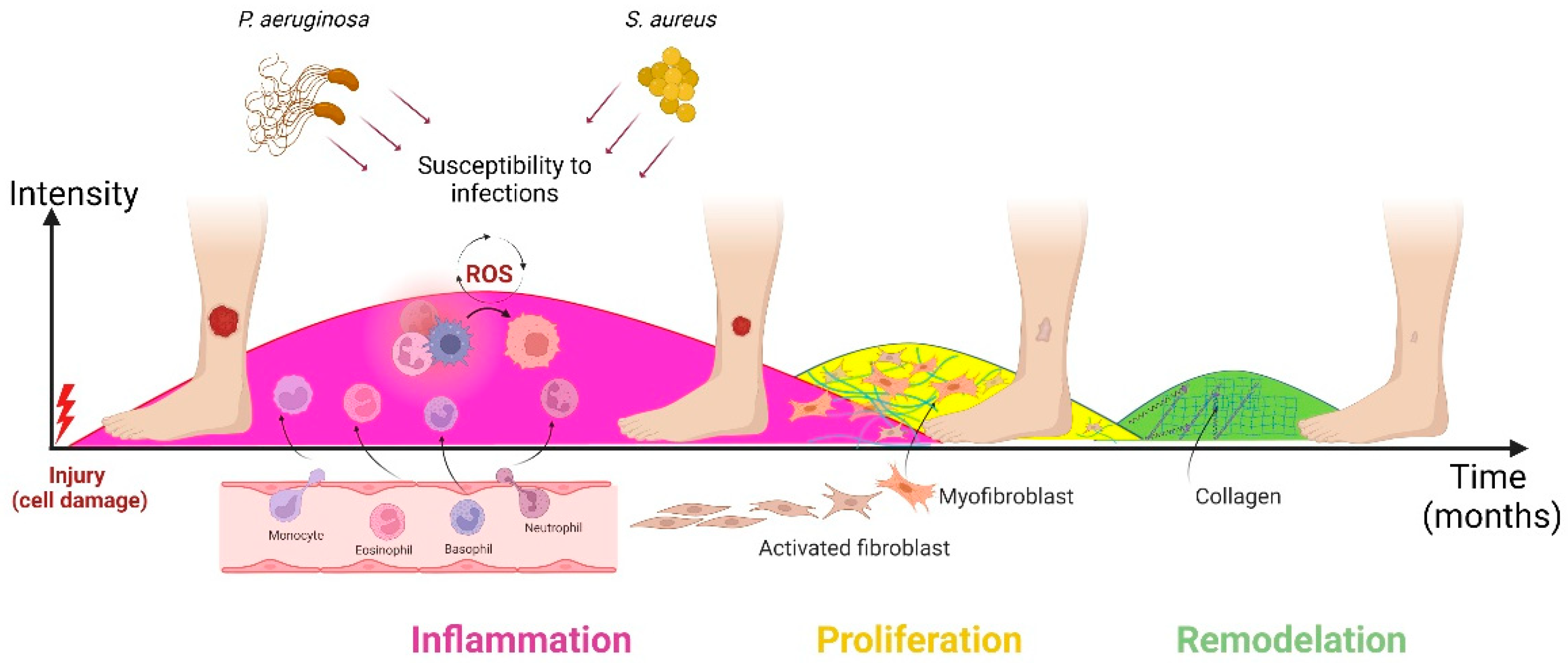
2. Wound Healing
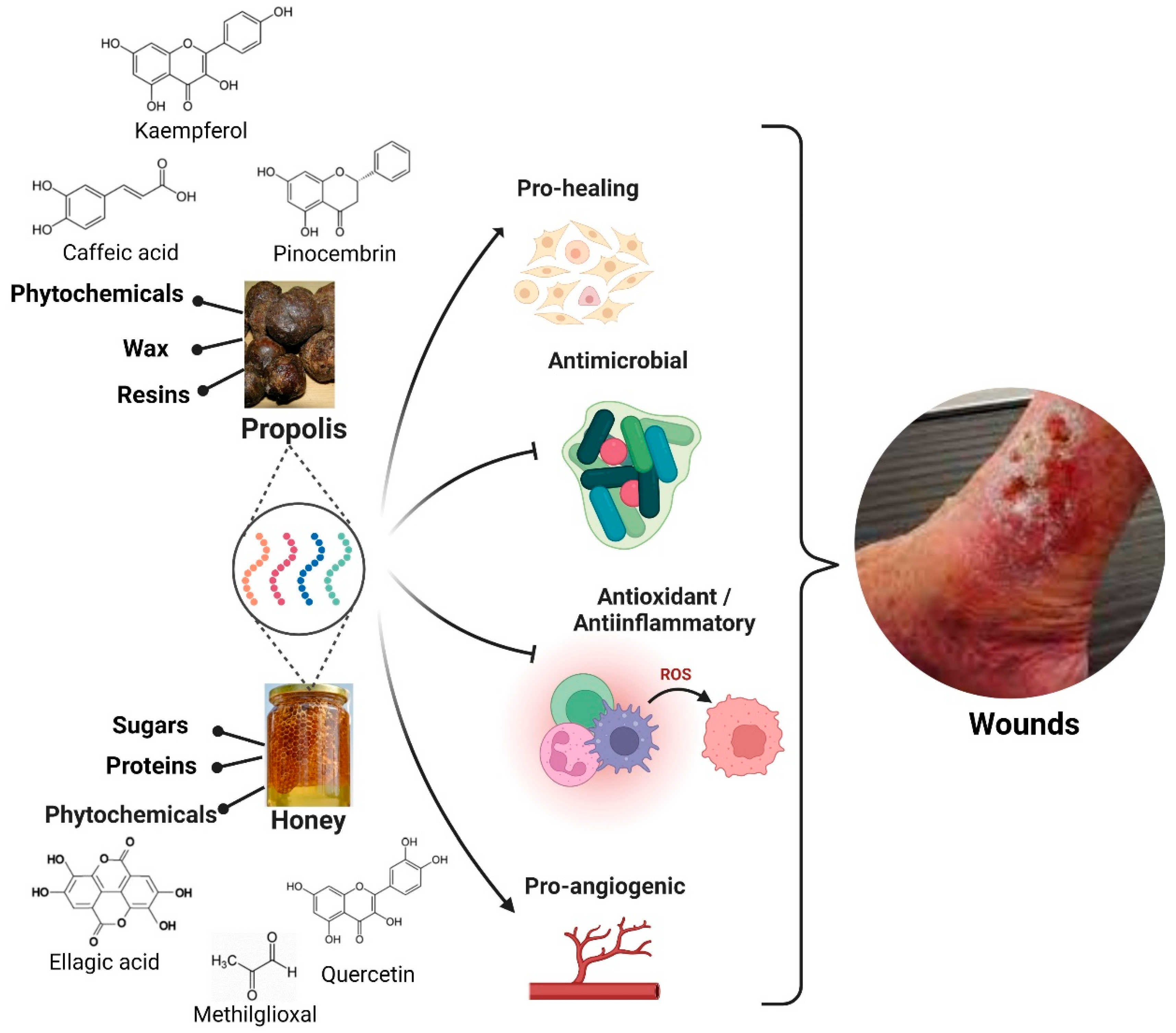
3. Honey and Propolis Properties
3.1. Honey
3.1.1. Antimicrobial Property
3.1.2. Anti-Inflammatory Property
3.1.3. Debriding Property
3.1.4. Tissue Regenerative Property
3.2. Propolis
3.2.1. Antimicrobial Properties
3.2.2. Antioxidant and Anti-Inflammatory Properties
3.2.3. Wound Healing Property
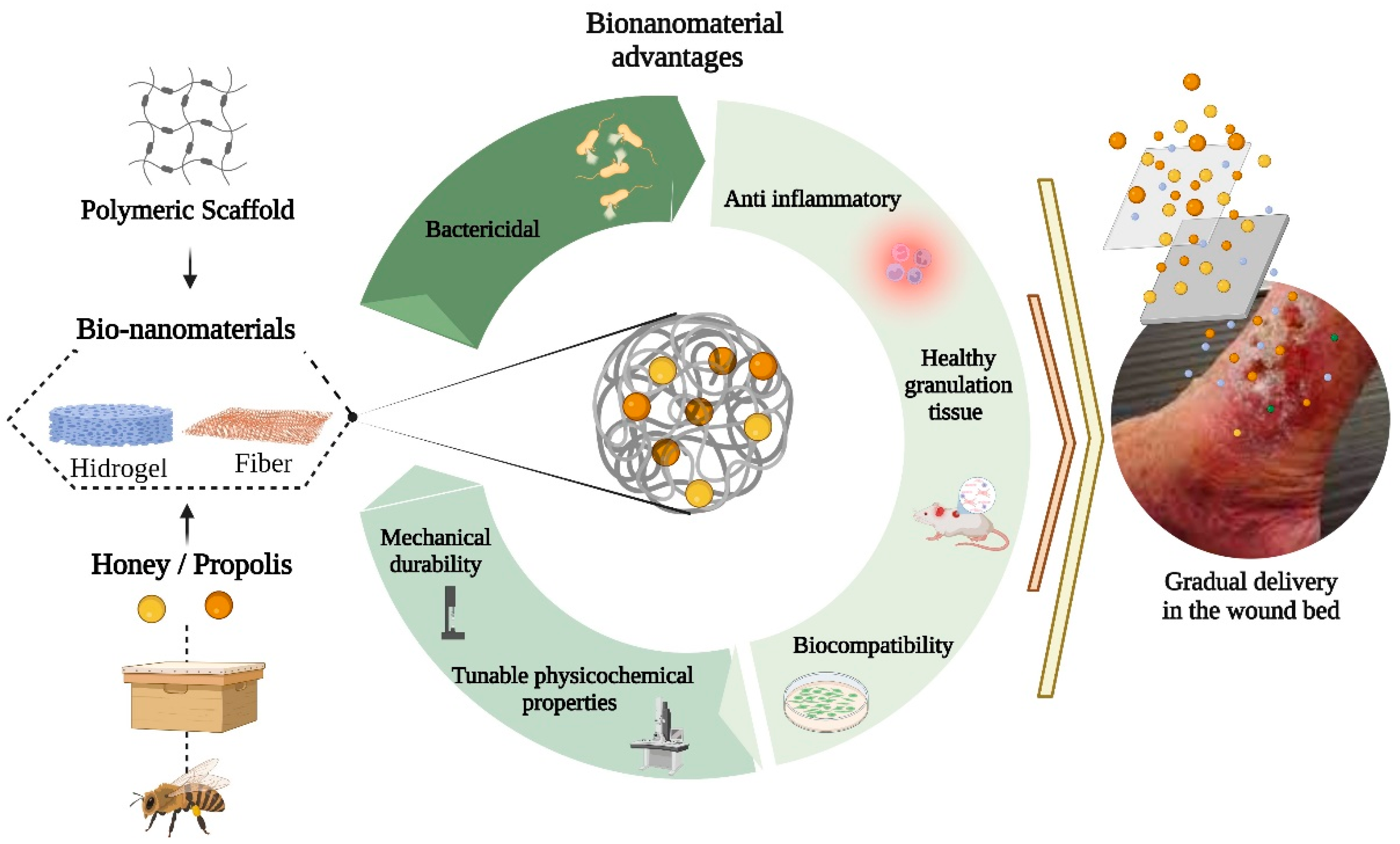
4. Bionanomaterials Based on Honey and Propolis
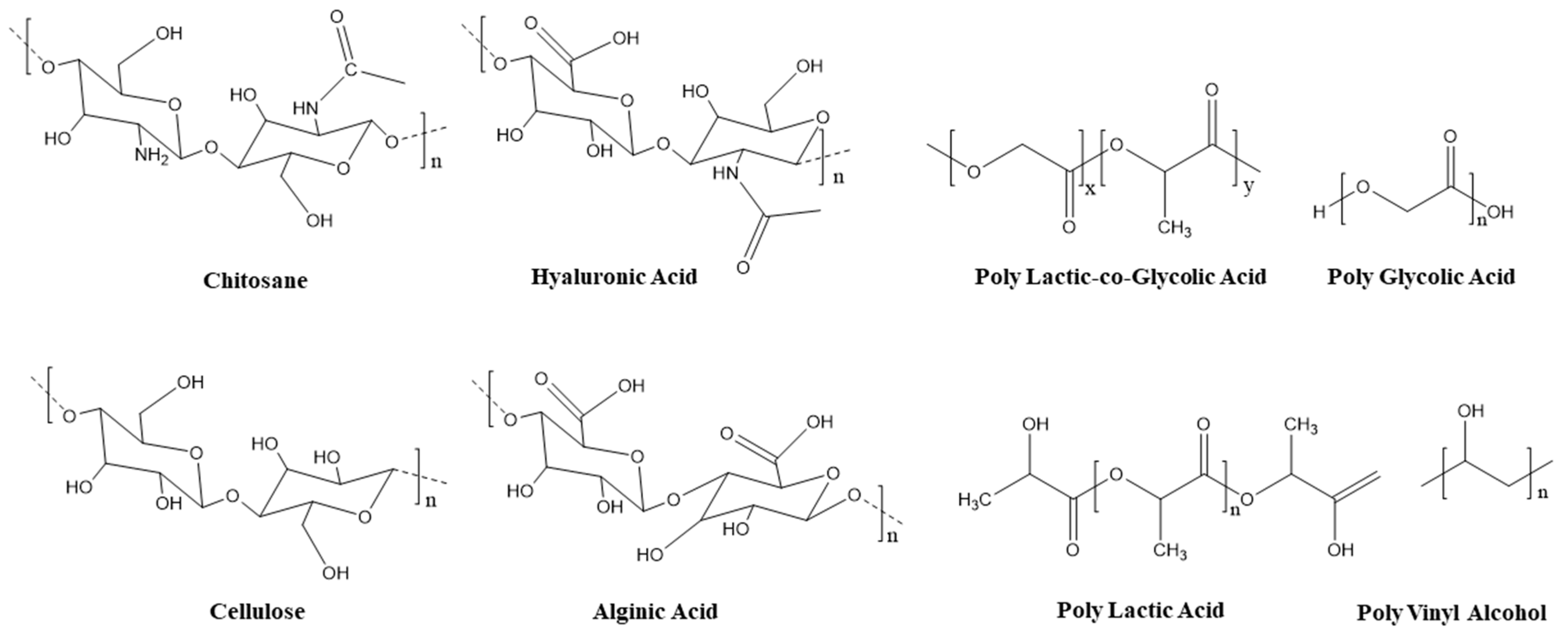
4.1. Polymeric Scaffolds
4.2. Applications of Bionanomaterial Based on Honey for Wound Healing
4.3. Bionanomaterial Based on Propolis for Wound Healing Applications
4.4. Stability of Bionanomaterial Based on Honey or Propolis for Wound Healing
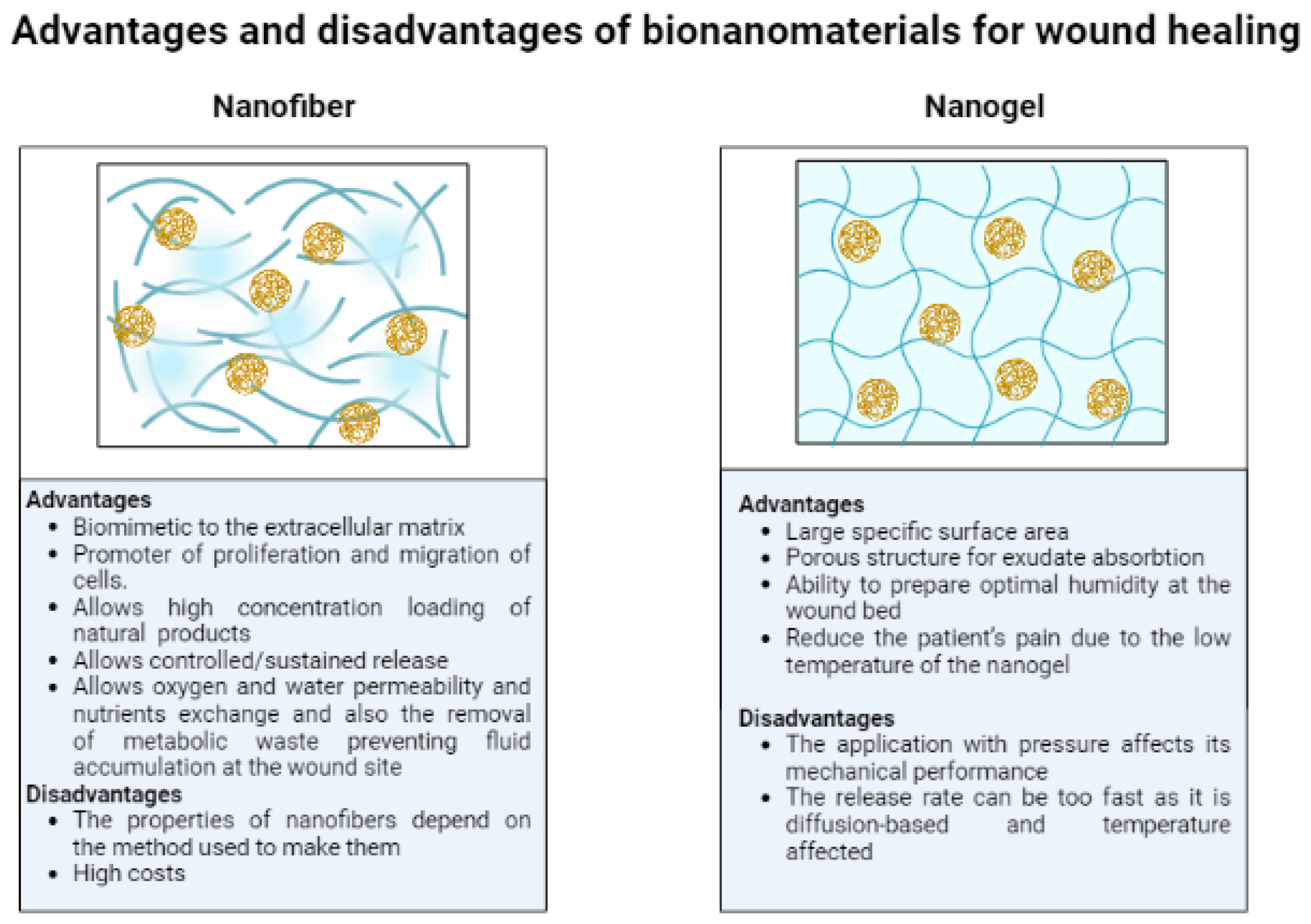
4.5. Advantages and Disadvantages of Different Types of Bionanomaterials (Nanofibers and Nanogels)
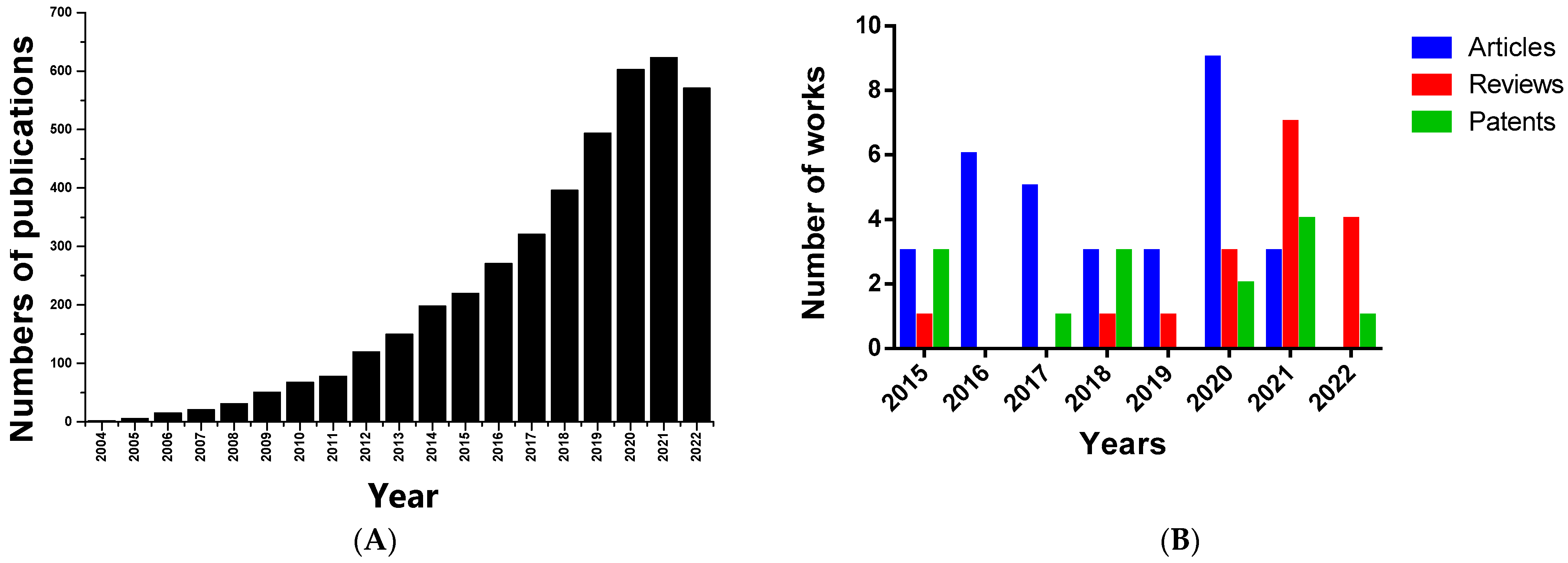
4.6. Patents Related to Bionanomaterials Based on Honey or Propolis for Wound Healing
5. Challenges, Future Directions, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A promising avenue in therapeutic solutions for wound care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Aygin, D. Honey Dressing in Wound Treatment: A Systematic Review. Complement. Ther. Med. 2020, 51, 102388. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, R.P. Introduction to bionanomaterials: An overview. In Bionanomaterials; IOP Publishing: Bristol, UK, 2021; p. 1. ISBN 978-0-7503-3767-0. [Google Scholar]
- Jeevanandam, J.; Ling, J.K.U.; Barhoum, A.; Chan, Y.S.; Danquah, M.K. Bionanomaterials: Definitions, sources, types, properties, toxicity, and regulations. In Fundamentals of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–29. ISBN 978-0-12-824147-9. [Google Scholar]
- Hoang, T.P.N.; Ghori, M.U.; Conway, B.R. Topical Antiseptic Formulations for Skin and Soft Tissue Infections. Pharmaceutics 2021, 13, 558. [Google Scholar] [CrossRef]
- Boyacı, D.; Kavur, P.B.; Gulec, Ş.; Yemenicioğlu, A. Physicochemical and Active Properties of Gelatine-Based Composite Gels Loaded with Lysozyme and Green Tea Polyphenols. Food Technol. Biotechnol. 2021, 59, 337–348. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.D.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Nour, S.; Imani, R.; Chaudhry, G.R.; Sharifi, A.M. Skin wound healing assisted by angiogenic targeted tissue engineering: A comprehensive review of bioengineered approaches. J. Biomed. Mater. Res. Part A 2021, 109, 453–478. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Münstedt, K.; Bogdanov, S. Bee products and their potential use in modern medicine. J. ApiProduct ApiMedical Sci. 2009, 1, 57–63. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent advances in honey-based hydrogels for wound healing applications: Towards natural therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Kumaran, P.; Gupta, A.; Sharma, S. Synthesis of wound-healing keratin hydrogels using chiken feathers proteins and its properties. Int. J. Pharm. Pharm. Sci. 2017, 9, 171. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Ullah, S.; Qamar, M.; Hashmi, M.; Duy, P.; Kato, Y. International Journal of Biological Macromolecules Manuka honey incorporated cellulose acetate nano fi brous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibiotics: Structural, physico-mechanical and in-vitro biomedical studies. Mater. Sci. Eng. C 2017, 77, 318–325. [Google Scholar] [CrossRef]
- Aslan, E. Electrospun PCL-Surgihoney meshes for skin wound healing applications. MATEC Web Conf. 2020, 318, 01029. [Google Scholar] [CrossRef]
- Kosimaningrum, W.E.; Barleany, D.R.; Sako, V.N.; Ristiyanti, R. Preparation of Gelatin-Chitosan-Honey-Based Hydrogel for Potential Active Material of Wound Care Dressing Application. Mater. Sci. Forum 2020, 988, 162–168. [Google Scholar] [CrossRef]
- Chao, C.Y.; Mani, M.P.; Jaganathan, S.K. Engineering electrospun multicomponent polyurethane scaffolding platform comprising grapeseed oil and honey/propolis for bone tissue regeneration. PLoS ONE 2018, 13, e0205699. [Google Scholar] [CrossRef]
- Bahari, N.; Hashim, N.; Md Akim, A.; Maringgal, B. Recent Advances in Honey-Based Nanoparticles for Wound Dressing: A Review. Nanomaterials 2022, 12, 2560. [Google Scholar] [CrossRef]
- Hixon, K.R.; Klein, R.C.; Eberlin, C.T.; Linder, H.R.; Ona, W.J.; Gonzalez, H.; Sell, S.A. A Critical Review and Perspective of Honey in Tissue Engineering and Clinical Wound Healing. Adv. Wound Care 2019, 8, 403–415. [Google Scholar] [CrossRef]
- Bonsignore, G.; Patrone, M.; Martinotti, S.; Ranzato, E. “Green” Biomaterials: The Promising Role of Honey. J. Funct. Biomater. 2021, 12, 72. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules 2021, 26, 5701. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Polysaccharides/propolis composite as promising materials with biomedical and packaging applications: A review. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Gómez, L.E.; Martel-Estrada, S.A.; Vargas-Requena, C.L.; Rodriguez-González, C.A.; Olivas-Armendariz, I. Apósitos de polímeros naturales para regeneración de piel. Rev. Mex. Ing. Bioméd. 2016, 37, 235–249. [Google Scholar]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Kim, K. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Teng, Y.; Wu, J.; Liu, S.; Tang, X.; Jia, Y.; Chen, Z.-H.; Zhang, K.-W.; Sun, Z.-L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing. Front. Cell Dev. Biol. 2021, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Walker, N.; Deshpande, S. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2013, 2013, CD005083. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on Manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasooriya, E.R.; Jayasinghe, C.D.; Jayawardena, U.A.; Ruwanthika, R.W.D.; Mendis de Silva, R.; Udagama, P.V. Honey Mediated Green Synthesis of Nanoparticles: New Era of Safe Nanotechnology. J. Nanomater. 2017, 2017, 5919836. [Google Scholar] [CrossRef]
- Maruhashi, E. Honey in Wound Healing. In Therapeutic Dressings and Wound Healing Applications; Wiley Online Library: New York, NY, USA, 2020; pp. 235–254. [Google Scholar]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef] [Green Version]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Mayba, J.N.; Gooderham, M.J. A Guide to Topical Vehicle Formulations. J. Cutan. Med. Surg. 2018, 22, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Karthik, T.; Rathinamoorthy, R. Sustainable biopolymers in textiles: An overview. Handb. Ecomater. 2019, 3, 1435–1460. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Iacopetti, I.; Perazzi, A.; Martinello, T.; Gemignani, F.; Patruno, M. Hyaluronic acid, Manuka honey and Acemannan gel: Wound-specific applications for skin lesions. Res. Vet. Sci. 2020, 129, 82–89. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef]
- Cazander, G.; Ottelander, B.K.; Kamga, S.; Doomen, M.C.H.A.; Damen, T.H.C.; Well, A.M.E. Importance of Debriding and Wound Cleansing Agents in Wound Healing. In Therapeutic Dressings and Wound Healing Applications; Wiley: New York, NY, USA, 2020; pp. 59–89. [Google Scholar]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Feresín, G.E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial activity, antioxidant effect and chemical composition of propolis from the Región del Maule, central Chile. Molecules 2015, 20, 18144–18167. [Google Scholar] [CrossRef] [Green Version]
- Velazquez, C.; Navarro, M.; Acosta, A.; Angulo, A.; Dominguez, Z.; Robles, R.; Robles-Zepeda, R.; Lugo, E.; Goycoolea, F.M.; Velazquez, E.F.; et al. Antibacterial and free-radical scavenging activities of Sonoran propolis. J. Appl. Microbiol. 2007, 103, 1747–1756. [Google Scholar] [CrossRef]
- Ibrahim, M.E.E.-D.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (Bbee glue) extracts. Int. J. Food Microbiol. 2022, 361, 109463. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Longo, R.; Russo, A.; Borrelli, F.; Sautebin, L. The role of the phenethyl ester of caffeic acid (CAPE) in the inhibition of rat lung cyclooxygenase activity by propolis. Fitoterapia 2002, 73, S30–S37. [Google Scholar] [CrossRef] [Green Version]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based. Complement. Alternat. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis modulates fibronectin expression in the matrix of thermal injury. Biomed Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef]
- Okur, M.E.; Bülbül, E.Ö.; Mutlu, G.; Eleftherıadou, K.; Karantas, I.D.; Okur, N.Ü.; Siafaka, P.I. An Updated Review for the Diabetic Wound Healing Systems. Curr. Drug Targets 2022, 23, 393–419. [Google Scholar] [CrossRef]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bianchera, A.; Catanzano, O.; Boateng, J.; Elviri, L. The Place of Biomaterials in Wound Healing. In Therapeutic Dressings and Wound Healing Applications; Wiley: New York, NY, USA, 2020; pp. 337–366. [Google Scholar]
- Preem, L.; Kogermann, K. Electrospun Antimicrobial Wound Dressings: Novel Strategies to Fight Against Wound Infections. In Chronic Wounds, Wound Dressings and Wound Healing; Springer: Berlin/Heidelberg, Germany, 2018; pp. 213–253. [Google Scholar]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, X.; Chen, J.; Li, C.; Liu, L.; Liu, X.; Wang, F.; Chen, G.; Wang, L. Constructing Nanoscale Topology on the Surface of Microfibers Inhibits Fibroblast Fibrosis. Adv. Fiber Mater. 2022, 4, 1219–1232. [Google Scholar] [CrossRef]
- Sutjarittangtham, K.; Tragoolpua, Y.; Tunkasiri, T.; Chantawannakul, P.; Intatha, U.; Eitssayeam, S. The Preparation of Electrospun Fiber Mats Containing Propolis Extract/CL-CMS for Wound Dressing and Cytotoxicity, Antimicrobial, Anti-Herpes Simplex Virus. J. Comput. Theor. Nanosci. 2015, 12, 804–808. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, G.; Tang, J.; Cheng, R.; Shen, X.; Gu, Y.; Wu, L.; Xi, K.; Zhao, Y.; Cui, W.; et al. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci. Adv. 2020, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rafati, Z.; Sirousazar, M.; Muhammad, Z.; Farshad, H. Honey-Loaded Egg White/Poly (vinyl alcohol)/Clay Bionanocomposite Hydrogel Wound Dressings: In Vitro and In Vivo Evaluations. J. Polym. Environ. 2020, 28, 32–46. [Google Scholar] [CrossRef]
- Noori, S.; Kokabi, M.; Hassan, Z.M. Poly(vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 46311. [Google Scholar] [CrossRef]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. WIREs Nanomed. Nanobiotechnology 2009, 1, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Jahani-Javanmardi, A.; Sirousazar, M.; Shaabani, Y.; Kheiri, F. Egg white/poly (vinyl alcohol)/MMT nanocomposite hydrogels for wound dressing. J. Biomater. Sci. Polym. Ed. 2016, 27, 1262–1276. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Feketshane, Z.; Alven, S.; Aderibigbe, B.A. Gellan Gum in Wound Dressing Scaffolds. Polymers 2022, 14, 4098. [Google Scholar] [CrossRef]
- Holloway, S.; Harding, K.G. Wound dressings. Surgery 2022, 40, 25–32. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Rudnicka, K.; Gatkowska, J.; Sobczak-Kupiec, A.; Jampilek, J. In vitro biosafety of pro-ecological chitosan-based hydrogels modified with natural substances. J. Biomed. Mater. Res.-Part A 2019, 107, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M. High concentration honey chitosan electrospun nanofibers: Biocompatibility and antibacterial effects. Carbohydr. Polym. 2015, 122, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.; Balaji, A.; Ismail, A.F.; Rajasekar, R. Fabrication and hemocompatibility assessment of novel polyurethane-based bio-nanofibrous dressing loaded with honey and Carica papaya extract for the management of burn injuries. Int. J. Nanomed. 2016, 11, 4339–4355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamar, M.; Lee, H.; Khatri, Z.; Kharaghani, D.; Khatri, M.; Ishikawa, T.; Im, S.; Soo, I. Fabrication and characterization of nanofibers of honey/poly(1,4-cyclohexane dimethylene isosorbide trephthalate) by electrospinning. Mater. Sci. Eng. C 2017, 81, 247–251. [Google Scholar] [CrossRef]
- Behere, I.; Ingavle, G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J. Biomed. Mater. Res. Part A 2022, 110, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Saremi, Z.; Yari, R.; Khodadadi, I.; Tabatabaei, S.M. The Combined Effects of Nano-Zinc, Nano-Albumin and Honey in Healing Wounds Caused by Third-Degree Burn in Male Mice. J. Ski. Stem Cell 2016, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In vivo evaluation of the wound healing properties of bio-nanofiber chitosan/ polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Kanuganti, J.S.; Sharma, C.S. Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 2020, 35, 600–609. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nano fi brous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef]
- Hassan, W.A.S.; Azzazy, M. Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine 2017, 35, 600–609. [Google Scholar] [CrossRef]
- Shahid, M.A.; Ali, A.; Uddin, M.N.; Miah, S.; Islam, S.M.; Mohebbullah, M.; Jamal, M.S.I. Antibacterial wound dressing electrospun nanofibrous material from polyvinyl alcohol, honey and Curcumin longa extract. J. Ind. Text. 2021, 51, 455–469. [Google Scholar] [CrossRef]
- Sarkar, R.; Ghosh, A.; Barui, A.; Datta, P. Repositing honey incorporated electrospun nanofiber membranes to provide anti-oxidant, anti-bacterial and anti-inflammatory microenvironment for wound regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 31. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Neuhalfen, R.M.; Janowiak, B.E.; Sell, S.A. Preliminary Investigation and Characterization of Electrospun Polycaprolactone and Manuka Honey Scaffolds for Dermal Repair. J. Eng. Fiber. Fabr. 2015, 10, 15. [Google Scholar] [CrossRef]
- Fard, G.C.; Maleknia, L.; Giahi, M.; Almasian, A.; Shabani, M.; Dehdast, S.A. Synthesis and characterization of novel antibacterial PdDA/ honey nanofiber against Gram-positive and Gram-negative bacteria. Nanomedicine Res. J. 2020, 5, 75–89. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Gupta, B. Scar free healing mediated by the release of aloe vera and manuka honey from dextran bionanocomposite wound dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. The effect of increasing honey concentration on the properties of the honey/polyvinyl alcohol/chitosan nanofibers. Mater. Sci. Eng. C 2016, 67, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, S.; Kathiresan, G.; Kathalingam, A.; Kim, H.-S.; Doss, M.N.R. Organic nanocomposite Band-Aid for chronic wound healing: A novel honey-based nanofibrous scaffold. Appl. Nanosci. 2020, 10, 1639–1652. [Google Scholar] [CrossRef]
- Ghalei, S.; Li, J.; Douglass, M.; Garren, M.; Handa, H. Synergistic Approach to Develop Antibacterial Electrospun Scaffolds Using Honey and S-Nitroso-N-acetyl Penicillamine. ACS Biomater. Sci. Eng. 2021, 7, 517–526. [Google Scholar] [CrossRef]
- Fatma Nur, P.; Pınar, T.; Uğur, P.; Ayşenur, Y.; Murat, E.; Kenan, Y. Fabrication of polyamide 6/honey/boric acid mats by electrohydrodynamic processes for wound healing applications. Mater. Today Commun. 2021, 29, 102921. [Google Scholar] [CrossRef]
- Patil, S.; Desai, N.; Mahadik, K.; Paradkar, A. Can green synthesized propolis loaded silver nanoparticulate gel enhance wound healing caused by burns? Eur. J. Integr. Med. 2015, 7, 243–250. [Google Scholar] [CrossRef]
- Cavalu, S.; Pasca, P.M.; Brocks, M. Natural Polymeric Film Encapsulating Propolis Nano-Formulation for Cutaneous Wound Healing. Mater. Plast. 2019, 56, 479–483. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [Green Version]
- Adomavičiūtė, E.; Stanys, S.; Žilius, M.; Juškaitė, V.; Pavilonis, A.; Briedis, V. Formation and Biopharmaceutical Characterization of Electrospun PVP Mats with Propolis and Silver Nanoparticles for Fast Releasing Wound Dressing. Biomed Res. Int. 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, S.; El-Naggar, M.E. Eco-friendly technology for preparation, characterization and promotion of honey bee propolis extract loaded cellulose acetate nanofibers in medical domains. Cellulose 2018, 25, 5195–5204. [Google Scholar] [CrossRef]
- do Nascimento, T.G.; da Silva, P.F.; Azevedo, L.F.; da Rocha, L.G.; de Moraes Porto, I.C.C.; Lima e Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; da Silva Fonseca, E.J.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Alberti, T.B.; Coelho, D.S.; de Prá, M.; Maraschin, M.; Veleirinho, B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J. Mater. Sci. 2020, 55, 9712–9727. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A propolis enriched polyurethane-hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Zeighampour, F.; Alihosseini, F.; Morshed, M.; Rahimi, A.A. Comparison of prolonged antibacterial activity and release profile of propolis-incorporated PVA nanofibrous mat, microfibrous mat, and film. J. Appl. Polym. Sci. 2018, 135, 45794. [Google Scholar] [CrossRef]
- Baygar, T. Characterization of silk sutures coated with propolis and biogenic silver nanoparticles (AgNPs); an eco-friendly solution with wound healing potential against surgical site infections (SSIs). Turkish J. Med. 2019, 50, 258–266. [Google Scholar] [CrossRef]
- Md Abu, T.; Zahan, K.A.; Rajaie, M.A.; Leong, C.R.; Ab Rashid, S.; Mohd Nor Hamin, N.S.; Tan, W.N.; Tong, W.Y. Nanocellulose as drug delivery system for honey as antimicrobial wound dressing. Mater. Today Proc. 2020, 31, 14–17. [Google Scholar] [CrossRef]
- Andleeb, A.; Dikici, S.; Waris, T.S.; Bashir, M.M.; Akhter, S.; Chaudhry, A.A.; MacNeil, S.; Yar, M. Developing affordable and accessible pro-angiogenic wound dressings; incorporation of 2 deoxy D-ribose (2dDR) into cotton fibres and wax-coated cotton fibres. J. Tissue Eng. Regener. Med. 2020, 14, 973–988. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology Approaches in Chronic Wound Healing. Adv. Wound Care 2021, 10, 234–256. [Google Scholar] [CrossRef]
| Type of Bionanomaterial | Composition | Size (nm) | Evaluation of Biological Parameters | Parameters Tested | Potential Applications | Ref. | |
|---|---|---|---|---|---|---|---|
| Biological | Physicochemical (PCP) | ||||||
| Mechanical (MP) | |||||||
| Nanogel | Honey (10% w/v), PVA (60, 63.3, 66.7% w/v) dried egg-white (30, 31, 33.3% w/v), MMT (0.5, 10% w/v) | <100 nm | Female BALB/c mice | Wound closure for 10 days | PCP: Size, shape, swelling, water vapor permeability, thermal degradation, transparency, and honey release | Wounds with medium exudate and low microbial load | [66,69] |
| Histological observations of healed wounds in BALB/c mice | Inflammation, cell proliferation, re-epithelization, angiogenesis, collagenization and tensile strength in healed skin | MP: Tensile strength and elongation at maximum stress | |||||
| Human peripheral blood mononuclear cells | In vitro cytotoxicity assay | ||||||
| Nanogel | Honey (15% w/w), PVA, (10% w/w), chitosan (2% w/w), MMT (0–3% w/w) | Undeclared | Total plate count method | Antibacterial (S. aureus) | PCP: Shape, chemical interaction, water vapor permeability, swelling and honey release | Wounds with medium exudate and medium microbial load | [67] |
| Female Syrian mice | Wound closure for 12 days | MP: Tensile strength | |||||
| Human peripheral blood mononuclear cells | Cytotoxicity | ||||||
| Nanogel | Honey (5% w/w), PVA (94% w/w), borax (1% w/w) | <100 nm | Viable cell count method | Antibacterial (S. aureus, E. coli) | PCP: Size, shape, swelling, antibiotic release and bio-adhesion | Wounds with medium exudate and medium microbial load | [19] |
| Human fibroblast cells | Proliferation | MP: Tensile strength an elongation at maximum stress | |||||
| Cytotoxicity | |||||||
| Nanogel | Honey (40% v/v), Nano-Zinc (20% v/v), nano-albumin (40% v/v) | <100 nm | Male albino mice | Wound closure for 10 days | NT | Third degree Burns | [78] |
| Histological observations of the healed wounds in albino mice | Cell proliferation, angiogenesis and collagen synthesis | ||||||
| Nanogel | Honey (~1.5% w/w), PVA (10% w/w), Chitosan (3% w/w), Nepeta dschyparensis (~1.5% w/w) | 95–150 nm | Male Wistar rats | Wound closure for 21 days. | FCP: Size, shape, chemical interactions and thermal degradation | Second-degree Burns | [79] |
| Histological observations of the healed wounds in Wistar rats | Cell proliferation, angiogenesis and collagen synthesis | MP: NT | |||||
| Nanogel | Honey (6% w/v), PVA (6% w/v), cellulose acetate (16% w/v), Curcuma longa extract (1% w/v) | 262–695 nm | Disc diffusion assay | Antibacterial (E. coli) | PCP: Size, shape, chemical interactions, water vapor permeability and wettability | Wounds with medium exudate and medium microbial load | [80] |
| Nanofiber | Honey (0, 5, 10, 15, 20% v/v), PVA (7.2% w/v), Sodium alginate (0.8% w/v) | 95–528 nm | Disc diffusion assay and dynamic contact assay | Antibacterial (S. aureus and E. coli) | PCP: Size, shape, chemical interactions, swelling, viscosity and conductivity. | Wounds with medium exudate and medium microbial load | [81] |
| DPPH assay | Antioxidant capacity | ||||||
| NIH/3T3 cells | Cytotoxicity | ||||||
| DPPH assay | Antioxidant capacity | ||||||
| Nanofiber | Manuka honey (10, 20, 25% w/v) and Lyophilized multiflora honey powder (10, 20, 25% w/v), Bee venom (0.01% w/v), PVA (9.7, 10.5, 12% w/v), extract of Punica grantum (1, 2, 2.5% p/v) | 511–879 nm | Viable cell count method | Antibacterial (S. aureus, E. coli) | PCP: Size, shape and swelling | Wounds with medium exudate and medium microbial load | [44] |
| Mouse fibroblast cells (L929) | Cytotoxicity | ||||||
| Female Sprague–Dawley rats | Wound closure for 14 days. | MP: NT | |||||
| Nanofiber | Honey (30% w/v), Propolis (10% w/v), bee venom (0.01% w/v), PVA (7% w/v), chitosan (3.1% w/v), bacteriophage (10% v/v) | 319–997 nm | Viable cell count method | Antibacterial (MRSA, P. aeruginosa, E. coli), | PCP: Size, shape and chemical interactions | Infected chronic wounds with low to medium exudate | [82] |
| Male mice | Wound closure for 12 days. | ||||||
| Histological observations of the healed wounds in albino mice | Necrosis, inflammation, collagen synthesis, vascularization and epithelialization | ||||||
| Human fibroblast cells | Cytotoxicity | ||||||
| Nanofiber | Honey (33–50% v/v), PVA (5–6.7% w/v), Curcuma longa extract (0.03–0.06% w/v) | 340 nm | Disc diffusion method | Antibacterial (S. aureus) | PCP: Size, shape, chemical interactions and wetness | Wounds with medium exudate | [83] |
| Nanofiber | Honey (0.2, 0.5, 1% w/v), PVA (12% w/v) | 280–410 nm | Agar diffusion test, surface staining | Antibacterial, antibiofilm (E. coli) | PCP: Size, shape, chemical interactions, swelling, stability, conductivity and viscosity. | Control infections and inflammation and promote regeneration of the wound bed | [84] |
| DPPH assay | Antioxidant capacity | MP: Roughness | |||||
| Vero cells (kidney epithelial cells), quantification of bromodeoxyouridine (BrdU), scratch assay and expression of pre-inflammatory cytokines by Vero cells. | Cytotoxicity, proliferation, migration and inflammation | ||||||
| Nanofiber | Honey (10, 20, 30, 40% w/v), PVA (5, 7, 10% w/v), chitosan (1.5, 3.5, 4.5, 5.5% w/v) | ~500 nm | Viable cell count method | Antibacterial (S. aureus, E. coli) | PCP: Size, shape, chemical interactions, viscosity and swelling. | Wounds with medium exudate and medium microbial load | [74] |
| Primary skin fibroblast cells of neonatal mice | Cytotoxicity | ||||||
| Nanofiber | Honey (4% w/v), PU (4% w/v), Carica papaya extract (4% v/v) | 170–210 nm | Human blood samples of healthy adults | Hemocompatibility (hemolysis) | PCP: Size, shape, chemical interactions, thermal degradation, porosity and pore size distribution, wettability, swelling, thermal degradation and protein absorption | Burns | [75] |
| Coagulation (PT and APTT) | |||||||
| Nanofiber | Honey (10, 15, 20% w/v), PICT (10% w/v) | 190–482 nm | NT | NT | PCP: Size, shape, chemical interactions, honey release and wetness | Active wound dressing | [76] |
| MP: Tensile strength | |||||||
| Nanofiber | Manuka honey (1, 5, 10, 20% v/v), PCL (15% w/v) | 500–5000 nm | Fibroblasts (CRL-252) | Cell viability | PCP: Size, shape, swelling and thermal degradation | Promoting healing and clearing bacteria from wound environment | [85] |
| Proliferation, infiltration, and migration in vitro | MP: Elasticity | ||||||
| Agar diffusion test | Antibacterial (S. agalactiae, E. coli) | PCP: Size, shape, water vapor permeability and honey release | |||||
| Nanofiber | Honey (30–70% w/w), PDDA (30, 60, 70% w/v) | 40–180 nm | Viable cell count method | Antibacterial (S. aureus, E. coli, P. aeruginosa) | PCP: Size, shape, chemical interactions and solubility | Antibiotic wound dressing | [86] |
| Nanofiber | Manuka honey (10, 20, 30, 40% v/v), Chitosan (7–35% w/v) loaded on a nanocomposite membrane: glycerol (30% v/v), dextran (48% v/v), nanosoy protein (22% v/v) | Undeclared | Zone of inhibition test and colony count method | Antibacterial (S. aureus and E. coli), | PCP: Shape, water vapor permeability, wettability and honey release | Multipurpose wound care membranes | [87] |
| BALB/c mice | Wound closure for 21 days | ||||||
| Histological observations of the healed wounds in albino mice | Inflammation, migration, proliferation, angiogenesis, collagen synthesis, re-epithelialization | MP: TS | |||||
| Nanofiber | Manuka honey (10, 30, 50, 70% w/v), silk fibroin (20% w/v), PEO (2% w/v) | 484–2229 nm | BALB/c mice | Wound closure for 21 days | PCP: Size, shape and chemical interactions | Control infections and promote the regeneration of the wound bed | [88] |
| Measuring the bacterial growth-inhibition halos and bactericidal kinetics | Antibacterial (S. aureus, MRSA, P. aeruginosa, E. coli) | ||||||
| Mouse fibroblast cell line (L929) | Cell viability | ||||||
| Nanofiber | Honey (10, 20 or 30%)/polyvinyl alcohol (7%)/chitosan (3.5%) (HPCS) | 84 ± 97, 371 ± 110 or 464 ± 185 nm | Broth dilution method | Antibacterial (S. aureus, E. coli) | PCP: Size, shape, porosity, crystallinity, thermal degradation, swelling and degradation rate. | Wound healing and tissue engineering | [89] |
| Nanofiber | Honey (1–4 mL to 50%)/PVA (8%) | Undeclared | Mouse fibroblast cell line (L929) | Cytotoxicity | PCP: shape, composition, chemical interaction, swelling, crystallinity, conductivity, in vitro releasing kinetics analysis | Fabricated Band-Aids | [90] |
| Nanofiber | PLA (12%)/honey (5,10,15%) and PLA (12%)/honey/SNAP (10%) | 624.92 ± 137.69 nm | In vitro bacterial adhesion assay Mouse fibroblast cell line (3T3) | Antibacterial (S. aureus, E. coli) Cytotoxicity, cell adhesion, cell proliferation | PCP: Size, shape, chemical interaction, wettability, swelling, water vapor transmission rate, NO release measurements, in vitro honey release, exudate absorption | Wound healing and tissue engineering | [91] |
| MP: Tensile strength | |||||||
| Nanofiber | polyamide 6 (16%)/honey (20%) nanofiber mats with boric acid (0, 5, 10, 15 and 20%) | 253–304 nm | Disk diffusion method | Antibacterial (A. baumannii, E. coli, P. aeruginosa and S. aureus) | PCP: Size, shape, chemical interaction, thermal analysis, wettability, in vitro honey release, exudate absorption. | Wound healing applications | [92] |
| Type of Bionanomaterial | Composition | Size (nm) | Evaluation of Biological Parameters | Parameters Tested | Potential Applications | Ref. | |
|---|---|---|---|---|---|---|---|
| Biological | Physicochemical (PCP) | ||||||
| Mechanical (MP) | |||||||
| Nanogel | Propolis (0.15% w/v), Carbapol 934 (0.5% w/v), nanosilver (0.05% w/v), Gelucire (0.1% w/v) | 10.6–52.7 nm | Cup plate and broth dilution method | Antibacterial (S. aureus) | PCP: Size, shape and chemical interaction | Second-degree skin burns | [93] |
| Wistar rats | Wound closure for 18 days | MP: NT | |||||
| Nanogel | Propolis (0.01, 0.02% w/v), collagen (2% w/v), chitosan (0.01, 0.02% w/v) | 120 nm | Agar diffusion method | Antibacterial (S. aureus, E. coli) | PCP: Size, shape and chemical interaction. | Cutaneous wound healing applications | [94] |
| MP: Elongation at maximum stress | |||||||
| Nanofiber | Propolis (0.5, 1, 2 w/v %), PU (10 w/v %), HA (10 w/v %), DTA (7 w/v %) | 294, 325, 718 nm | Female Wistar rats | Wound closure for 21 days | PCP: Size, shape, chemical interaction, thermal degradation, wettability and propolis release | Wounds with medium exudate and low microbial load | [95] |
| Disc-diffusion method | Antibacterial (S. aureus, E. coli) | MP: Tensile strength and elongation at maximum stress | |||||
| Histological observations of the healed wounds in Wistar rats | Inflammation and collagen synthesis | ||||||
| L929 mouse fibroblast cells (ATCC) | In vitro cytotoxicity assay | ||||||
| Nanofiber | Propolis (40% v/v), PVP (6, 8% w/v), glycerol (40% v/v), nanosilver (10, 20% w/v) | ~450 nm | Agar diffusion method | Antibacterial (S. aureus, S. epidermidis, E. faecalis, E. coli, P. aeruginosa, Proteus vulgaris, Bacillus subtilis, Bacillus cereus), Antifungal (C. albicans) | PCP: Size, shape, chemical interaction, AgNP release and propolis release | Wound healing stimulation with low microbial load | [96] |
| MP: NT | |||||||
| Nanofiber | Propolis (10, 20, 30, 40% v/v), Cellulose acetate (12% w/v) | 150–200 nm | Inhibition zone method | Antibacterial (S. aureus and E. coli) | PCP: Size, shape, chemical interactions, propolis release and thermal degradation | Wound healing and antibacterial action | [97] |
| MP: NT | |||||||
| Nanofiber | Brazilian red propolis (10–60% w/v), PCL (27–60% w/v), Poloxamer (13–46% w/v) | 200–400 nm | Culture in biphasic medium of Leishmania chagasi | Antimicrobial (Leishmania braziliensis) | PCP: Size, shape, chemical interactions and thermal degradation | Chronic wounds | [98] |
| DPPH assay | Antioxidant capacity | MP: NT | |||||
| Nanofiber | Propolis (1.25% w/v), PVA (10, 15, 20, 30% w/v), PEG (1, 2% w/v) | 282–984 nm | Male Swiss mice induced to diabetes with a single dose (150 mg/kg) of streptozotocin | Wound closure for 7 days. | PCP: Size and shape | Chronic wounds | [99] |
| Murine NIH/3T3 fibroblast cells | Cytotoxicity | MP: NT | |||||
| Nanofiber | Propolis (undeclared), cellulose acetate (8, 10, 12, 14% w/w), PCL (14% w/v) | 50–400 nm | Minimum inhibitory concentration assay | Antibacterial (S. aureus, S. epidermidis, P. aeruginosa, E. coli) | PCP: Size, shape, chemical interactions, and wettability | Wound healing system | [100] |
| DPPH assay | Antioxidant capacity | ||||||
| Nanofiber | Propolis (0.5, 1, 2% w/v), PU (10% w/w), HA (10% w/v) | ~718 nm | Female Wistar rats | Wound closure for 21 days | PCP: Size, shape, chemical interactions, swelling, wettability, thermal decomposition and propolis release | Effective wound dressing for biomedical applications | [101] |
| Histological observations of the healed wounds in Wistar rats | Cell proliferation, angiogenesis and collagen synthesis | ||||||
| Disc-diffusion method | Antibacterial (S. aureus, E. coli) | ||||||
| DPPH assay | Antioxidant capacity | MP: Tensile strength | |||||
| L929 fibroblast cells | Cytotoxicity, proliferation, migration and inflammation | ||||||
| Nanofiber | Propolis (5, 10, 20, 40, 60% w/w), PVA (8% w/v) | 85–329 nm | Broth microdilution method | Antibacterial (S. aureus, E. coli) | PCP: Size, shape, chemical interactions, swelling, viscosity, and propolis release. | High exudate wounds and infection | [102] |
| Primary skin fibroblast cells of neonatal mice | Cytotoxicity | ||||||
| Nanofiber | Propolis (undeclared %), nonabsorbable 4.0 silk sutures, nanosilver (undeclared %) | Undeclared | Murine NIH/3T3 fibroblast cells | Cytotoxicity | PCP: Shape and thermal degradation | Antibacterial biomaterial for wound healing | [103] |
| Migration in vitro | MP: NT | ||||||
| Agar diffusion test | Antibacterial (S. aureus, E. coli) | ||||||
| Patent N° | Publication Date | Applicant | Name |
|---|---|---|---|
| IN202241032202 | 10.06.2022 | DR. K KULATHURAAN. | Antibacterial nanomembrane for wound dressing and healing |
| CN211485249 | 15.09.2020 | TAIZHOU ROOSIN MEDICAL PRODUCT Co., Ltd. | Novel honey dressing |
| IN202014043490 | 21.05.2021 | SMART PRODUCTS & SERVICES INC. (DBA ALOEVIVE) | A combination of a novel topical gel and oral supplements for healing diabetic foot and other wounds |
| US20150030688 | 29.01.2015 | SAINT LOUIS UNIVERSITY | Honey and growth factor eluting scaffold for wound healing and tissue engineering |
| CN1082837271 | 17.07.2018 | GUANGDONG UNIVERSITY OF TECHNOLOGY | Nano-fiber dressing and preparation method thereof |
| CN112442278 | 05.03.2021 | YANCHENG POLYTECHNIC COLLEGE | Preparation method of biomedical multifunctional nanofiber membrane |
| CN108359140 | 03.08.2018 | SHAANXI YANGLING SHAANXI SPECIALTY AGRICULTURAL DEVELOPMENT CO., LTD | Honey-containing nanosilver antibacterial film and preparation method thereof |
| CN104342775 | 11.02.2015 | NATIONAL DONG HWA UNIVERSITY | Method for preparing composite nanofiber membrane with honey and natural materials on basis of environmentally friendly electrospinning technology |
| CN108283727 | 17.07.2018 | GUANGDONG UNIVERSITY OF TECHNOLOGY | Nanofiber dressing and preparation method thereof |
| CN111333918 | 26.06.2020 | TIANJIN UNIVERSITY OF SCIENCE & TECHNOLOGY | Preparation method of dialdehyde nanocellulose/manuka honey antibacterial composite film |
| CN106620652 | 10.05.2017 | PAN WEIFANG | Nano-ion antibacterial tissue regeneration promoting care solution and preparation method thereof |
| WO/2015/183228 | 03.12.2015 | DUYMUŞ, Ethem | Nanofiber cover for wounds with an additive containing natural antiseptic |
| CN112442278 | 05.03.2021 | YANCHENG POLYTECHNIC COLLEGE | Preparation method of biomedical multifunctional nanofiber membrane |
| CN110664725 | 10.01.2020 | BEIJING ZHONGMI TECHNOLOGY DEVELOPMENT CO., LTD. | Preparation method of emulsion containing nanopropolis extract and prepared emulsion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaldin-Crespo, L.; Silva, N.; Martínez, J. Nanomaterials Based on Honey and Propolis for Wound Healing—A Mini-Review. Nanomaterials 2022, 12, 4409. https://doi.org/10.3390/nano12244409
Jaldin-Crespo L, Silva N, Martínez J. Nanomaterials Based on Honey and Propolis for Wound Healing—A Mini-Review. Nanomaterials. 2022; 12(24):4409. https://doi.org/10.3390/nano12244409
Chicago/Turabian StyleJaldin-Crespo, Limberg, Nataly Silva, and Jessica Martínez. 2022. "Nanomaterials Based on Honey and Propolis for Wound Healing—A Mini-Review" Nanomaterials 12, no. 24: 4409. https://doi.org/10.3390/nano12244409
APA StyleJaldin-Crespo, L., Silva, N., & Martínez, J. (2022). Nanomaterials Based on Honey and Propolis for Wound Healing—A Mini-Review. Nanomaterials, 12(24), 4409. https://doi.org/10.3390/nano12244409








