Oxygen Vacancy-Mediated Activates Oxygen to Produce Reactive Oxygen Species (ROS) on Ce-Modified Activated Clay for Degradation of Organic Compounds without Hydrogen Peroxide in Strong Acid
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
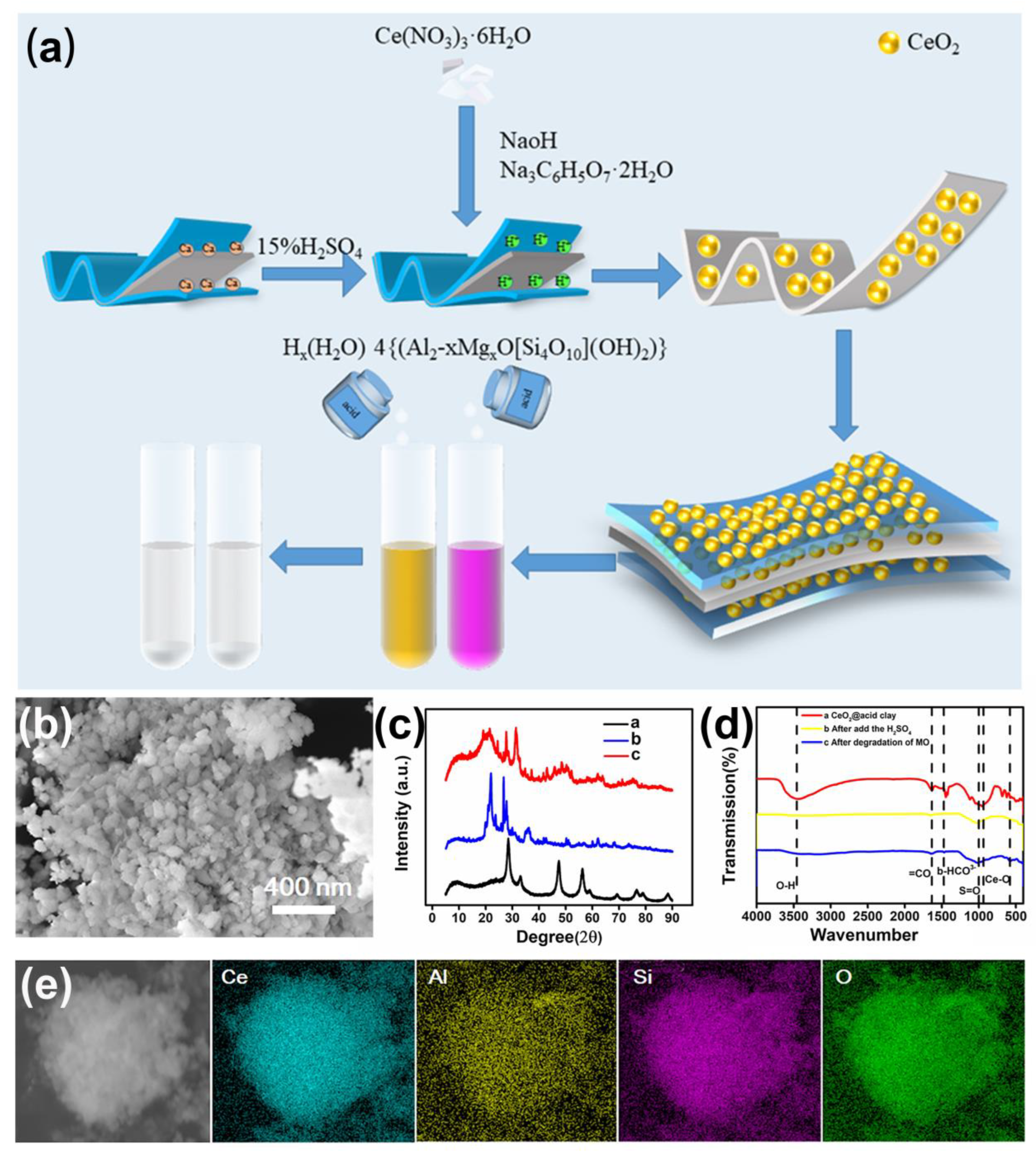
2.2. Synthesis of Activated Clay CeO2 and CeAY
2.2.1. Synthesis of Activated Clay
2.2.2. Synthesis of CeO2 and CeAY
2.3. Degradation Tests
2.4. Investigation of the Oxidase Mimetic Activity of CeAY
2.5. Characterization
3. Results and Discussion
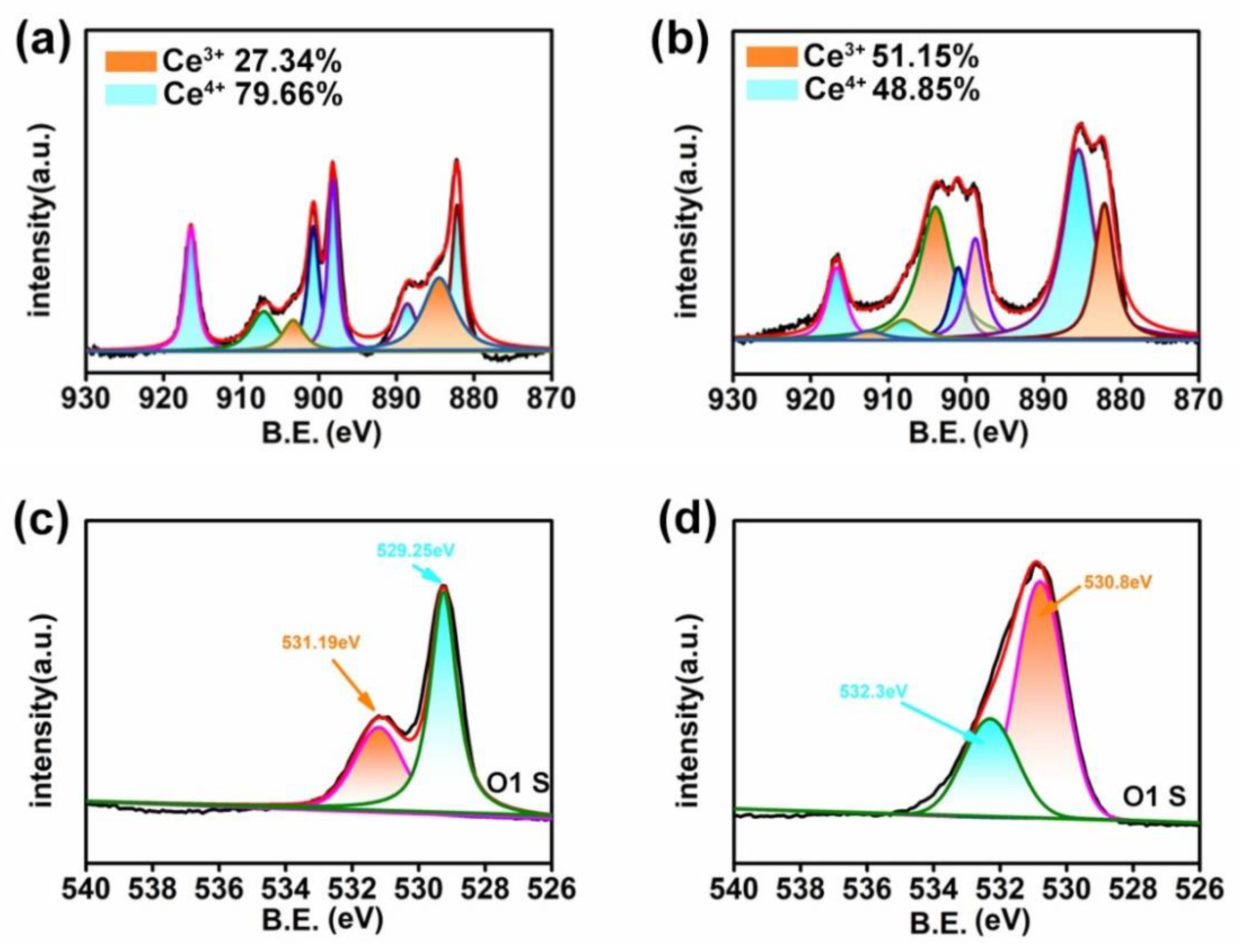
3.1. Characterization
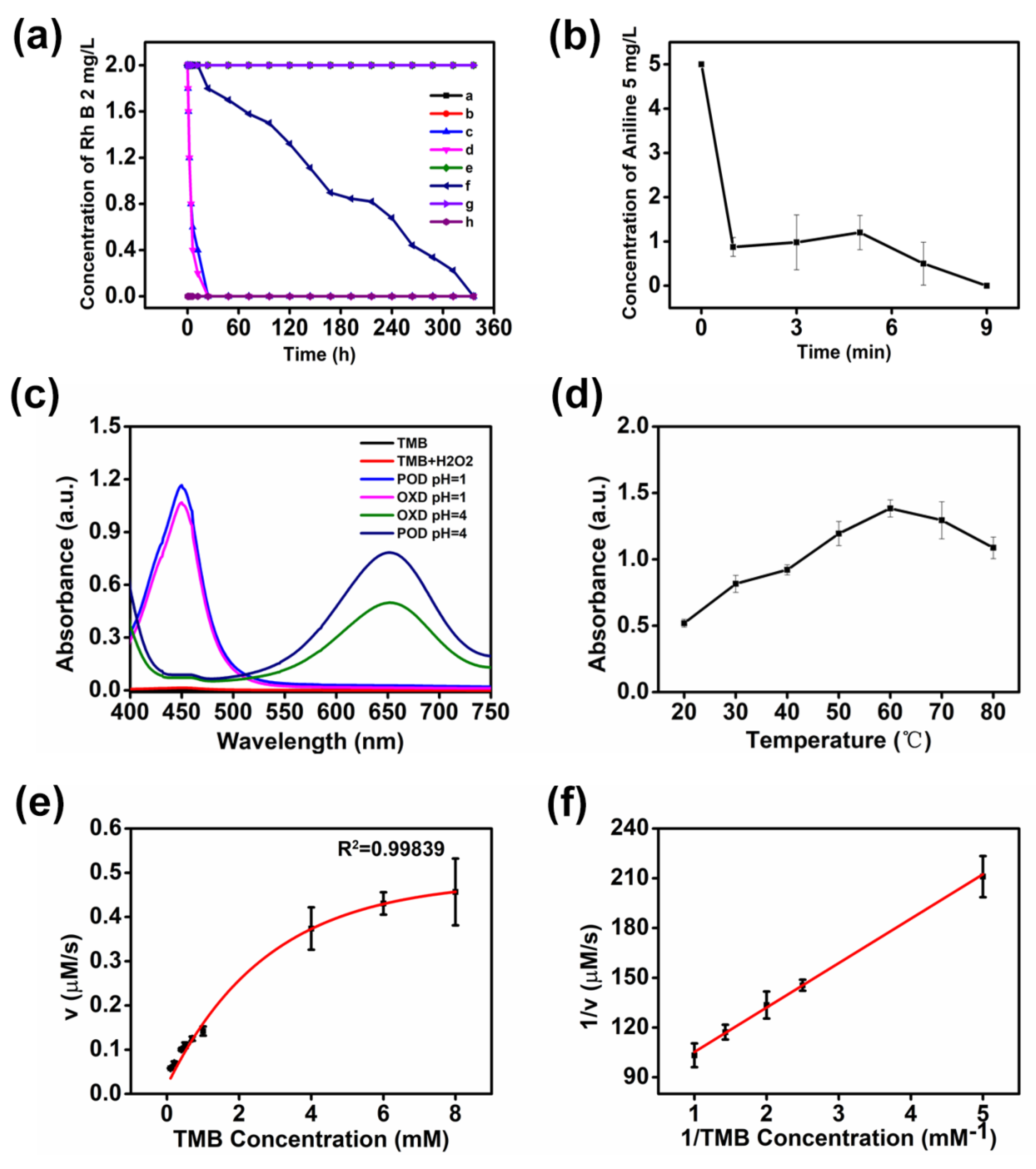
3.2. Catalytic Activity and Degradation Performance
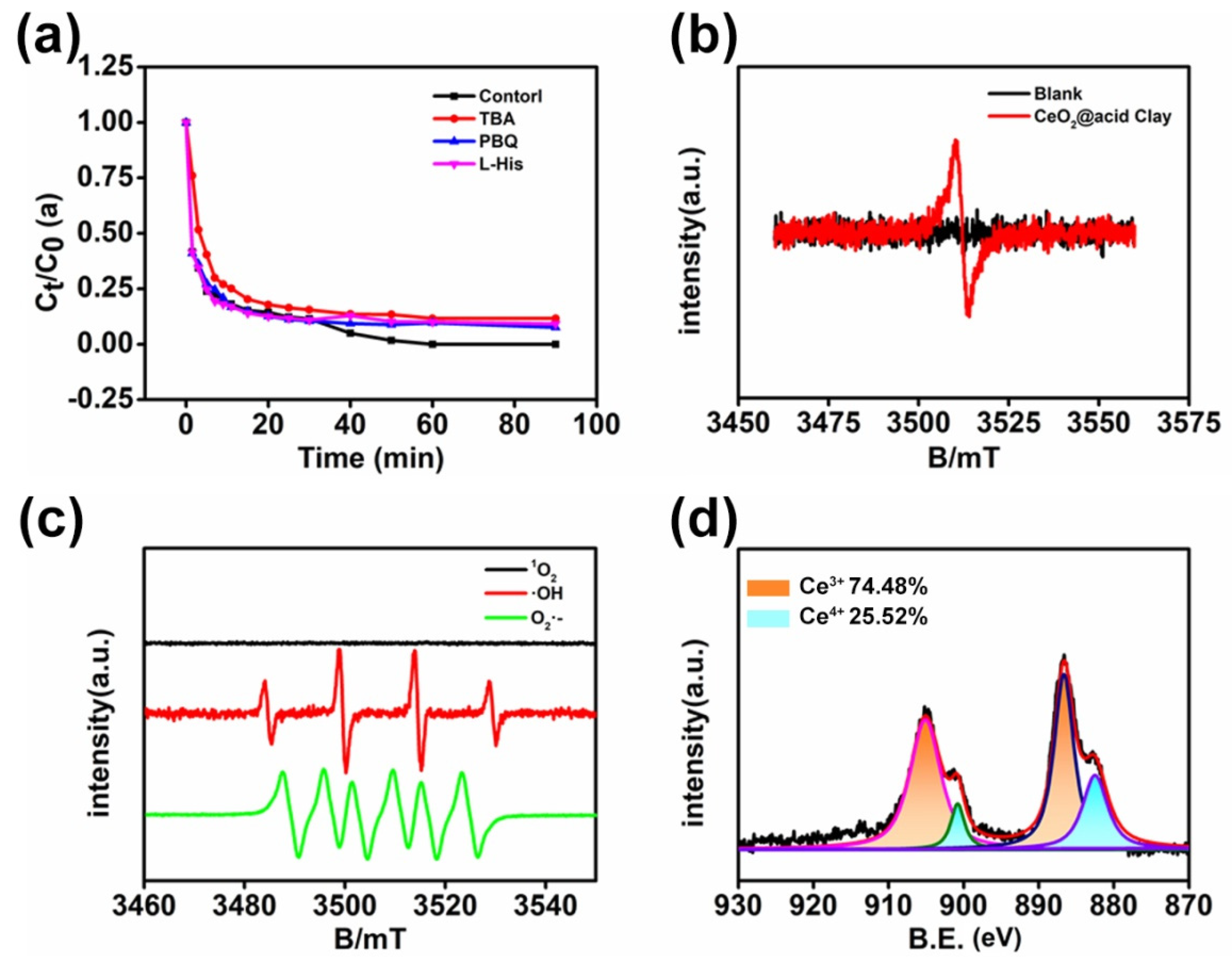
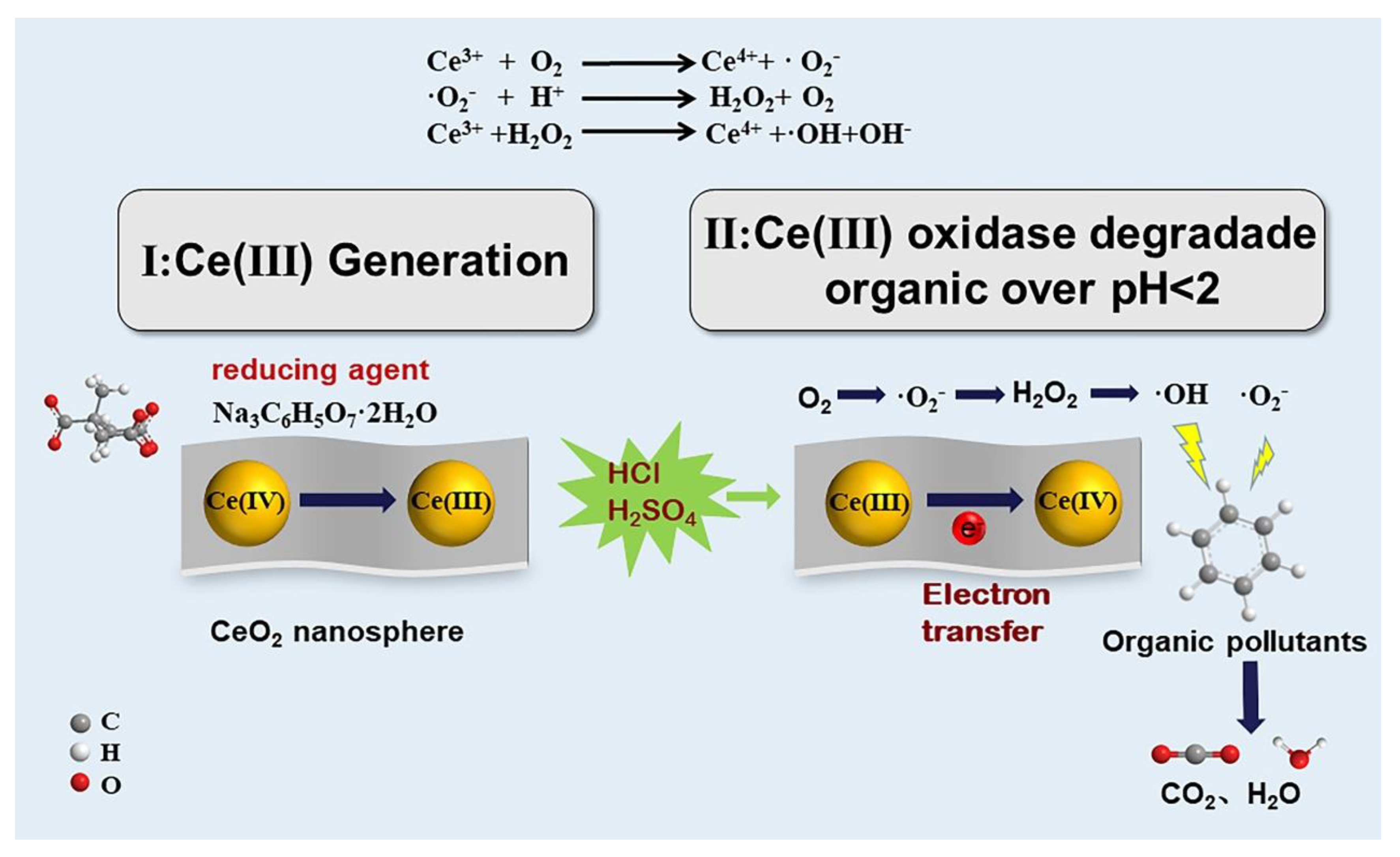
3.3. Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, D.; Zhang, C.; Tang, S.; Li, X.; Tang, J.; Rao, Y.; Wang, Z.; Zhang, Q. Enhancing CaO2 fenton-like process by Fe(II)-oxalic acid complexation for organic wastewater treatment. Water Res. 2019, 163, 114861. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiong, J.; Tian, C.; Gao, B.; Wang, L.; Jia, X. The degradation of methyl orange and membrane fouling behavior in anaerobic baffled membrane bioreactor. Chem. Eng. J. 2018, 338, 719–725. [Google Scholar] [CrossRef]
- Cai, F.; Lei, L.; Li, Y.; Chen, Y. A review of aerobic granular sludge (AGS) treating recalcitrant wastewater: Refractory organics removal mechanism, application and prospect. Sci. Total Environ. 2021, 782, 146852. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Orfao, J.J.M.; Pereira, M.F.R. Activated carbon and ceria catalysts applied to the catalytic ozonation of dyes and textile effluents. Appl. Catal. B Environ. 2009, 88, 341–350. [Google Scholar] [CrossRef]
- Jing, X.; Yuan, J.; Cai, D.; Li, B.; Hu, D.; Li, J. Concentrating and recycling of high-concentration printing and dyeing wastewater by a disc tube reverse osmosis-Fenton oxidation/low temperature crystallization process. Separat. Purif. Technol. 2021, 266, 118583. [Google Scholar] [CrossRef]
- Stoica, A.; Sandberg, M.; Holby, O. Energy use and recovery strategies within wastewater treatment and sludge handling at pulp and paper mills. Bioresour. Technol. 2009, 100, 3497–3505. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, Q.-S.; Wei, W.; Sun, J.; Dai, X.; Ni, B.-J. Improving the treatment of waste activated sludge using calcium peroxide. Water Res. 2020, 187, 116440. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, L.; Sun, L.; Gao, Y.; Wang, T.; Lv, C. Effective and selective separation of perrhenate from acidic wastewater by super-stable, superhydrophobic, and recyclable biosorbent. Front. Environ. Sci. Eng. 2022, 16, 21. [Google Scholar] [CrossRef]
- Bhanot, P.; Celin, S.M.; Sreekrishnan, T.R.; Kalsi, A.; Sahai, S.K.; Sharma, P. Application of integrated treatment strategies for explosive industry wastewater—A critical review. J. Water Process Eng. 2020, 35, 101232. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Hameed, M.A.; Abrar, M.M.; Maitlo, A.A.; Noreen, S.; et al. Current and emerging adsorbent technologies for wastewater treatment: Trends, limitations, and environmental implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Won, S.W.; Vijayaraghavan, K.; Mao, J.; Kim, S.; Yun, Y.-S. Reinforcement of carboxyl groups in the surface of Corynebacterium glutamicum biomass for effective removal of basic dyes. Bioresour. Technol. 2009, 100, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, J.; Liu, J.-W.; Zhou, J.; Cheng, L.; Zhao, J.; Shao, Z.; Iris, C.; Pan, B.; Li, X.; et al. A review of China’s municipal solid waste (MSW) and comparison with international regions: Management and technologies in treatment and resource utilization. J. Clean. Prod. 2021, 293, 126144. [Google Scholar] [CrossRef]
- Gong, C.-T.; Xu, G.-D.; Chen, L.-J.; Jia, J.-H.; Peng, Y.-W. Catalytic advanced oxidation processes (AOPS) in water treatment by covalent organic frameworks-based materials: A review. Res. Chem. Intermd. 2021, 47, 3109–3130. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Ricardo, I.A.; Leitao, J.M.M.; da Silva, J.C.G.E. A review on advanced oxidation processes: From classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples. Trends Environ. Anal. Chem. 2019, 24, e00072. [Google Scholar] [CrossRef]
- Shen, B.; Dong, C.; Ji, J.; Xing, M.; Zhang, J. Efficient Fe(III)/Fe(II) cycling triggered by MoO2 in Fenton reaction for the degradation of dye molecules and the reduction of Cr(VI). Chin. Chem. Lett. 2019, 30, 2205–2210. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Xing, M. Cocatalytic fenton reaction for pollutant control. Cell Rep. Phys. Sci. 2020, 1, 100149. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Yan, L.; Jing, C. Oxygen vacancy modulated interface chemistry: Identifying iron (IV) in heterogeneous Fenton reaction. Environ. Sci. Nano 2021, 8, 978–985. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, C.; Tang, S.; Wang, Z.; Sun, Q.; Zhang, X.; Jiao, T.; Zhang, Q. Ferric ion-ascorbic acid complex catalyzed calcium peroxide for organic wastewater treatment: Optimized by response surface method. Chin. Chem. Lett. 2021, 32, 3387–3392. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S. Photo-Fenton applied to the removal of pharmaceutical and other pollutants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar]
- Ziembowicz, S.; Kida, M. Limitations and future directions of application of the Fenton-like process in micropollutants degradation in water and wastewater treatment: A critical review. Chemosphere 2022, 296, 134041. [Google Scholar]
- Chen, Y.; Miller, C.J.; Collins, R.N.; Waite, T.D. Key considerations when assessing novel Fenton catalysts: Iron oxychloride (FeOCl) as a case study. Environ. Sci. Technol. 2021, 55, 13317–13325. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, C.Z.S.; Gokcal, B.; Polat, M.; Hamaloglu, K.O.; Kip, C.; Tuncel, A. Porous, oxygen vacancy enhanced CeO2−x microspheres with efficient enzyme-mimetic and photothermal properties. ACS Sustain. Chem. Eng. 2022, 10, 9492–9505. [Google Scholar] [CrossRef]
- Chen, B.-H.; Inbaraj, B.S. Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles. Crit. Rev. Biotechnol. 2018, 38, 1003–1024. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.G.M.; Batalha, D.C.; Rodrigues, T.S.; Candido, E.G.; Luz, S.C.; de Freitas, I.C.; Fonseca, F.C.; de Oliveira, D.C.; Taylor, J.G.; de Torresi, S.I.C.; et al. Sub-15 nm CeO2 nanowires as an efficient non-noble metal catalyst in the room-temperature oxidation of aniline. Catal. Sci. Technol. 2018, 8, 1828–1839. [Google Scholar] [CrossRef]
- Li, X.; You, S.; Du, J.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived Co3O4@carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions. J. Mater. Chem. A 2019, 7, 25853–25864. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, C.; Xie, Y.; Pan, Z.; Xue, X.; Zhang, R. A study on the catalytic oxidation of soot by Sn-Ce composite oxides: Adsorbed oxygen and defect sites synergistically enhance catalytic activity. New J. Chem. 2019, 43, 17423–17432. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, M.; Liu, S.; Bao, C.; Liang, G.; Lai, C.; Shi, W.; Ma, S. Deactivation by HCl of CeO2-MoO3/TiO2 catalyst for selective catalytic reduction of NO with NH3. RSC Adv. 2018, 8, 17677–17684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, A.K.; Sinha, I. Synergistic effect of Ni doping and oxygen vacancies on the visible light photocatalytic properties of Ag2O nanoparticles. J. Phys. Chem. Solids 2022, 167, 110733. [Google Scholar] [CrossRef]
- Lyu, P.; Zhu, J.; Han, C.; Qiang, L.; Zhang, L.; Mei, B.; He, J.; Liu, X.; Bian, Z.; Li, H. Self-driven reactive oxygen species generation via interfacial oxygen vacancies on carbon-coated TiO2−x with versatile applications. ACS Appl. Mater. Interfaces 2021, 13, 2033–2043. [Google Scholar] [CrossRef]
- Mavuso, M.A.; Makgwane, P.R.; Ray, S.S. Morphology modulated photocatalytic activity of CeO2 nanostructures for selective oxidation of biobased alpha-pinene to oxygenates. Chemistryselect 2020, 5, 12940–12951. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, J.; Li, Y.; Tu, R.; Goto, T. Dispersion of CeO2 nanoparticles on hexagonal boron nitride by a simple CVD method. Transact. Indian Ceram. Soc. 2018, 77, 127–131. [Google Scholar] [CrossRef]
- Geng, L.; Li, G.; Zhang, X.; Wang, X.; Li, C.; Liu, Z.; Zhang, D.-S.; Zhang, Y.-Z.; Wang, G.; Han, H. Rational design of CuO/SiO2 nanocatalyst with anchor structure and hydrophilic surface for efficient hydrogenation of nitrophenol. J. Solid State Chem. 2021, 296, 121960. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Yu, G.; Huang, J.; Lim, V.C. As(V) and As(III) removal from water by a Ce-Ti oxide adsorbent: Behavior and mechanism. Chem. Eng. J. 2010, 161, 106–113. [Google Scholar] [CrossRef]
- Guo, J.; Wang, K.; Wang, X. Photocatalytic reduction of CO2 with H2O vapor under visible light over Ce doped ZnFe2O4. Catal. Sci. Technol. 2017, 7, 6013–6025. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H.; Mu, G.; Zhao, N. Synergism between rare earth cerium(IV) ion and vanillin on the corrosion of steel in H2SO4 solution: Weight loss, electrochemical, UV-vis, FTIR, XPS, and AFM approaches. Appl. Surf. Sci. 2008, 254, 5574–5586. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Hashmi, A.; Susan, M.A.B.H.; Lellouche, J.-P. Surfactant-assisted cerium oxide and its catalytic activity towards Fenton process for non-degradable dye. Adv. Compos. Hybrid Mater. 2020, 3, 430–441. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J. Nanostructured TiO2-CeO2 mixed oxides by an aqueous sol-gel process effect of Ce Ti molar ratio on physical and sensing properties. Sens. Actuators B Chem. 2010, 150, 631–640. [Google Scholar] [CrossRef]
- Anandan, C.; Bera, P. XPS studies on the interaction of CeO2 with silicon in magnetron sputtered CeO2 thin films on Si and Si3N4 substrates. Appl. Surf. Sci. 2013, 283, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhou, J.; Ma, H.; Qian, W.; Zhang, H.; Ying, W. Antisintering and high-activity Ni catalyst supported on Mesoporous silica incorporated by Ce/Zr for CO methanation. Ind. Eng. Chem. Res. 2018, 57, 14406–14416. [Google Scholar] [CrossRef]
- Dominguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodil, S.E.; Brachetti-Sibaja, S.B.; de la Cruz, W.; Flores-Vela, A. XPS and EIS studies of sputtered Al-Ce films formed on AA6061 aluminum alloy in 3.5% NaCl solution. J. Appl. Electrochem. 2010, 40, 639–651. [Google Scholar] [CrossRef]
- Li, C.; Zhao, W.; Liu, B.; Xu, G.; Liu, L.; Lv, H.; Shang, D.; Yang, D.; Damirin, A.; Zhang, J. Cytotoxicity of ultrafine monodispersed nanoceria on human gastric cancer cells. J. Biomed. Nanotechnol. 2014, 10, 1231–1241. [Google Scholar] [CrossRef]
- Yasyerli, S.; Dogu, G.; Dogu, T. Selective oxidation of H2S to elemental sulfur over Ce-V mixed oxide and CeO2 catalysts prepared by the complexation technique. Catal. Today 2006, 117, 271–278. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.-X.; Xing, Y.; Zhang, P.; Chen, J.-J.; Yin, J.; Bie, L.-J. Morphology-controlled synthesis of CeO2 nanocrystals and their facet-dependent gas sensing properties. Sens. Actuators B Chem. 2021, 330, 129374. [Google Scholar] [CrossRef]
- Deng, Q.-F.; Ren, T.-Z.; Agula, B.; Liu, Y.; Yuan, Z.-Y. Mesoporous CexZr1−xO2 solid solutions supported CuO nanocatalysts for toluene total oxidation. J. Ind. Eng. Chem. 2014, 20, 3303–3312. [Google Scholar] [CrossRef]
- Shen, Z.; He, L.; Xu, Z.; Mu, R.; Huang, G. Morphological evolution and failure of LZC/YSZ DCL TBCs by electron beam-physical vapor deposition. Materialia 2018, 4, 340–347. [Google Scholar] [CrossRef]
- Cervantes-Lopez, J.L.; Rangel, R.; Espino, J.; Martinez, E.; Garcia-Gutierrez, R.; Bartolo-Perez, P.; Alvarado-Gil, J.J.; Contreras, O.E. Photoluminescence on cerium-doped ZnO nanorods produced under sequential atomic layer deposition-hydrothermal processes. Appl. Phys. A 2017, 123, 86. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, X.; Mei, Z.; Cai, S.; An, Q.; Fu, Y.; Wang, H.; Liu, T.; Guo, H. Understanding rich oxygen vacant hollow CeO2@MoSe2 heterojunction for accelerating photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 611, 644–653. [Google Scholar] [CrossRef]
- Bera, P.; Anandan, C. XRD and XPS studies of room temperature spontaneous interfacial reaction of CeO2 thin films on Si and Si3N4 substrates. RSC Adv. 2014, 4, 62935–62939. [Google Scholar] [CrossRef]
- Qi, J.; Liu, G.; Dong, Y. Probing the hydrophobic mechanism of N-(3-hydroxyamino)-propoxy-N-octyl dithiocarbamate toward bastnaesite flotation by in situ AFM, FTIR and XPS. J. Colloid Interface Sci. 2020, 572, 179–189. [Google Scholar] [CrossRef]
- Bai, Y.; Bian, X.; Wu, W. Catalytic properties of CuO/CeO2-Al2O3 catalysts for low concentration NO reduction with CO. Appl. Surf. Sci. 2019, 463, 435–444. [Google Scholar] [CrossRef]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the properties and applications of nanoceria: Is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390–405. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Kang, Y.; Li, D.; Liu, Y.C. Catalytic oxidation of sulfur dioxide over alpha-Fe2O3/SiO2 catalyst promoted with Co and Ce oxides. Korean J. Chem. Eng. 2020, 37, 623–632. [Google Scholar] [CrossRef]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically structured ternary heterojunctions based on Ce3+/Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Bi, X.; Wang, X.; Liu, X.; Meng, X. Activation of O2 over three-dimensional manganese oxide nanoprisms under ambient conditions towards oxidative removal of aqueous organics. Environ. Sci. Nano 2022, 9, 1541–1552. [Google Scholar] [CrossRef]
- Parkinson, C.R.; Walker, M.; McConville, C.F. Reaction of atomic oxygen with a Pt(111) surface: Chemical and structural determination using XPS, CAICISS and LEED. Surf. Sci. 2003, 545, 19–33. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sandan, M.Z.; Nayan, N.; Embong, Z.; Hak, C.R.C.; Adriyanto, F. Neutron beam interaction with rutile TiO2 single crystal (111): Raman and XPS study on Ti3+-oxygen vacancy formation. Mater. Lett. 2020, 263, 127143. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zhang, F.; Chan, K.-Y. Preparation and characterization of Pt-TiO2-SiO2 mesoporous materials and visible-light photocatalytic performance. Mater. Lett. 2007, 61, 2231–2234. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Z.; Liu, J. Boosting the oxidase mimicking activity of nanoceria by fluoride capping: Rivaling protein enzymes and ultrasensitive F(-) detection. Nanoscale 2016, 8, 13562–13567. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. 2009, 48, 2308–2312. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, S.; Li, W.; Jalil Shah, S.; Zhao, Z.; Pan, L.; Zhao, Z. Confined construction of COF@Cu-nanozyme with high activity and stability as laccase biomimetic catalyst for the efficient degradation of phenolic pollutants. Chem. Eng. J. 2022, 448, 137701. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; Binks, B.P.; He, Z. Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl. Catal. B Environ. 2019, 254, 452–462. [Google Scholar] [CrossRef]
- Chen, S.; Ma, L.; Du, Y.; Zhan, W.; Zhang, T.C.; Du, D. Highly efficient degradation of rhodamine B by carbon nanotubes-activated persulfate. Sep. Purif. Technol. 2021, 256, 117788. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Li, W.; Zhang, L.; Chen, T.; Ding, L. Degradation of methyl orange through hydroxyl radical generated by optically excited biochar: Performance and mechanism. Colloids Surf. A Physicochem. Eng. Aspects 2020, 601, 125034. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, S.; Wang, D.; Wu, J.; Jia, T.; Liu, Q.; Qi, Y.; Qi, X.; He, P.; Zhou, M. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Appl. Surf. Sci. 2020, 530, 147116. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, H.; Gao, G.; Liu, L.; Chen, W. Facet-dependent catalytic activity of nanosheet-assembled bismuth oxyiodide microspheres in degradation of bisphenol A. Environ. Sci. Technol. 2015, 49, 6240–6248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xing, L.; Ma, H.; Cheng, L.; Liu, J.; Yang, J.; Zhang, Q. Sulfated ce-doped TiO2 as visible light driven photocatalyst: Preparation, characterization and promotion effects of Ce doping and sulfation on catalyst performance. Environ. Progress Sustain. Energy 2017, 36, 494–504. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Akid, R.; Zorainy, M. Corrosion protection mechanism of Ce4+/organic inhibitor for AA2024 in 3.5% NaCl. RSC Adv. 2020, 10, 2227–2240. [Google Scholar] [CrossRef] [Green Version]
- Bellardita, M.; Fiorenza, R.; D’Urso, L.; Spitaleri, L.; Gulino, A.; Compagnini, G.; Scire, S.; Palmisano, L. Exploring the photothermo-catalytic performance of brookite TiO2-CeO2 composites. Catalysts 2020, 10, 765. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Leng, D.; Yin, F. The enhanced activity of Pt-Ce nanoalloy for oxygen electroreduction. Sci. Rep. 2020, 10, 14837. [Google Scholar] [CrossRef]
- Hernandez-Alonso, M.D.; Hungria, A.B.; Martinez-Arias, A.; Fernandez-Garcia, M.; Coronado, J.M.; Conesa, J.C.; Soria, J. EPR study of the photoassisted formation of radicals on CeO2 nanoparticles employed for toluene photooxidation. Appl. Catal. B Environ. 2004, 50, 167–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Cui, J.; Wang, C.; Zhang, G.; Li, L.; Qu, Y.; Niu, Y. Oxygen Vacancy-Mediated Activates Oxygen to Produce Reactive Oxygen Species (ROS) on Ce-Modified Activated Clay for Degradation of Organic Compounds without Hydrogen Peroxide in Strong Acid. Nanomaterials 2022, 12, 4410. https://doi.org/10.3390/nano12244410
Wu T, Cui J, Wang C, Zhang G, Li L, Qu Y, Niu Y. Oxygen Vacancy-Mediated Activates Oxygen to Produce Reactive Oxygen Species (ROS) on Ce-Modified Activated Clay for Degradation of Organic Compounds without Hydrogen Peroxide in Strong Acid. Nanomaterials. 2022; 12(24):4410. https://doi.org/10.3390/nano12244410
Chicago/Turabian StyleWu, Tianming, Jing Cui, Changjiang Wang, Gong Zhang, Limin Li, Yue Qu, and Yusheng Niu. 2022. "Oxygen Vacancy-Mediated Activates Oxygen to Produce Reactive Oxygen Species (ROS) on Ce-Modified Activated Clay for Degradation of Organic Compounds without Hydrogen Peroxide in Strong Acid" Nanomaterials 12, no. 24: 4410. https://doi.org/10.3390/nano12244410




