Enhanced Electrocatalytic Water Oxidation of Ultrathin Porous Co3O4 Nanosheets by Physically Mixing with Au Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Co3O4 Bulk
2.2. Synthesis of Co3O4/Au Bulk Composites
2.3. Synthesis of Au Nanoparticles
2.4. Synthesis of Co3O4 Ultrathin Porous Nanosheets
2.5. Synthesis of Co3O4/Au Nanocomposites
2.6. Characterization
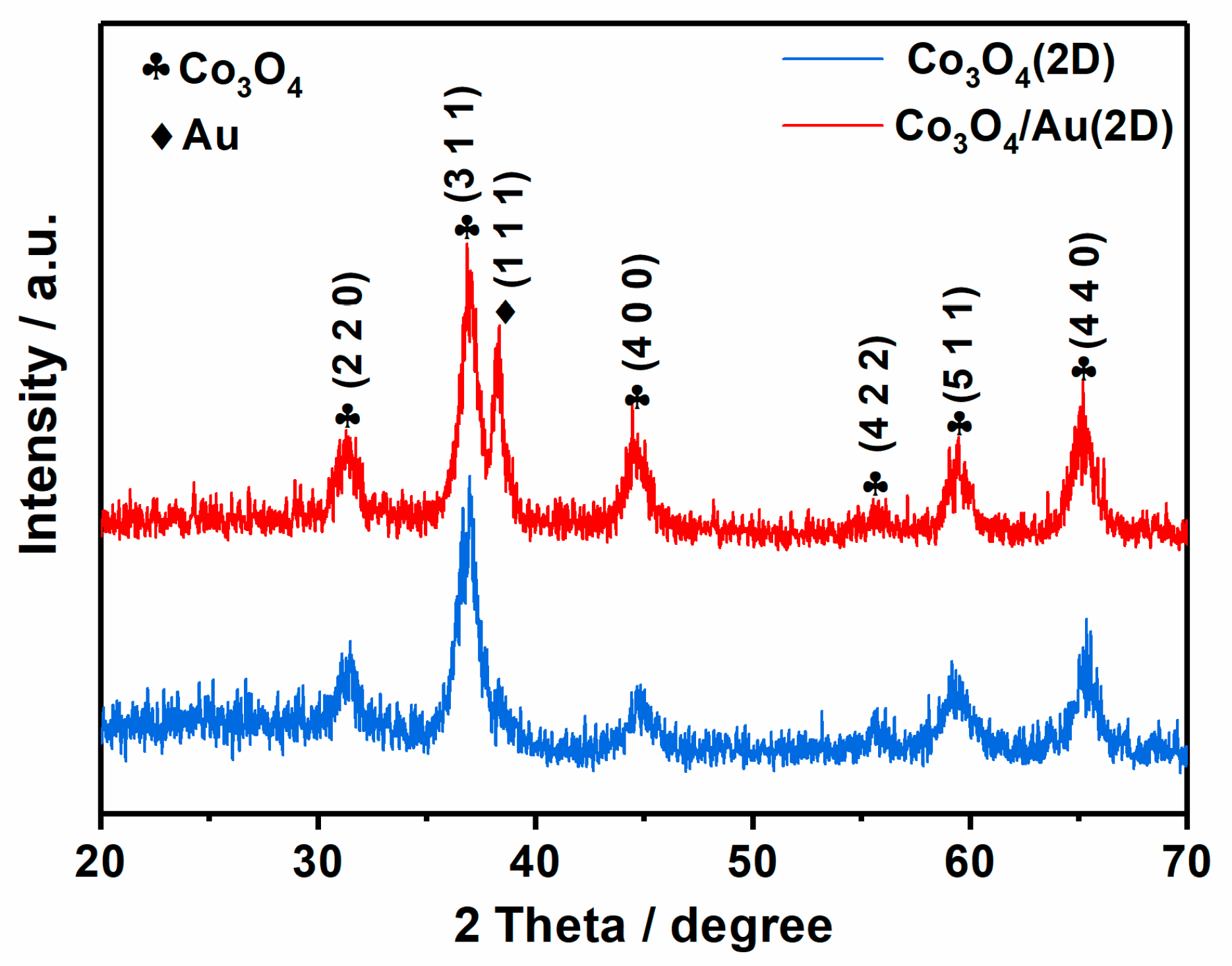
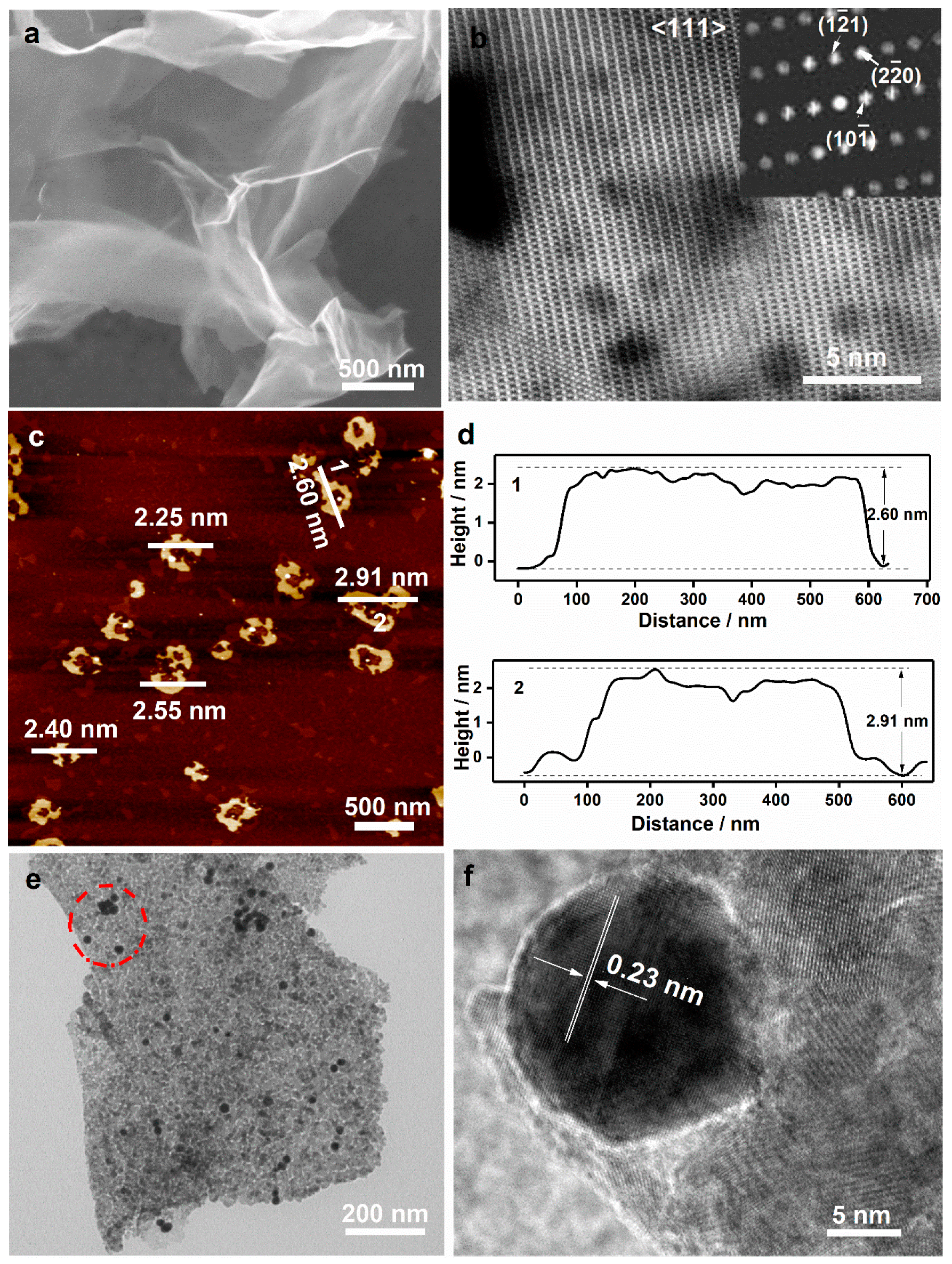
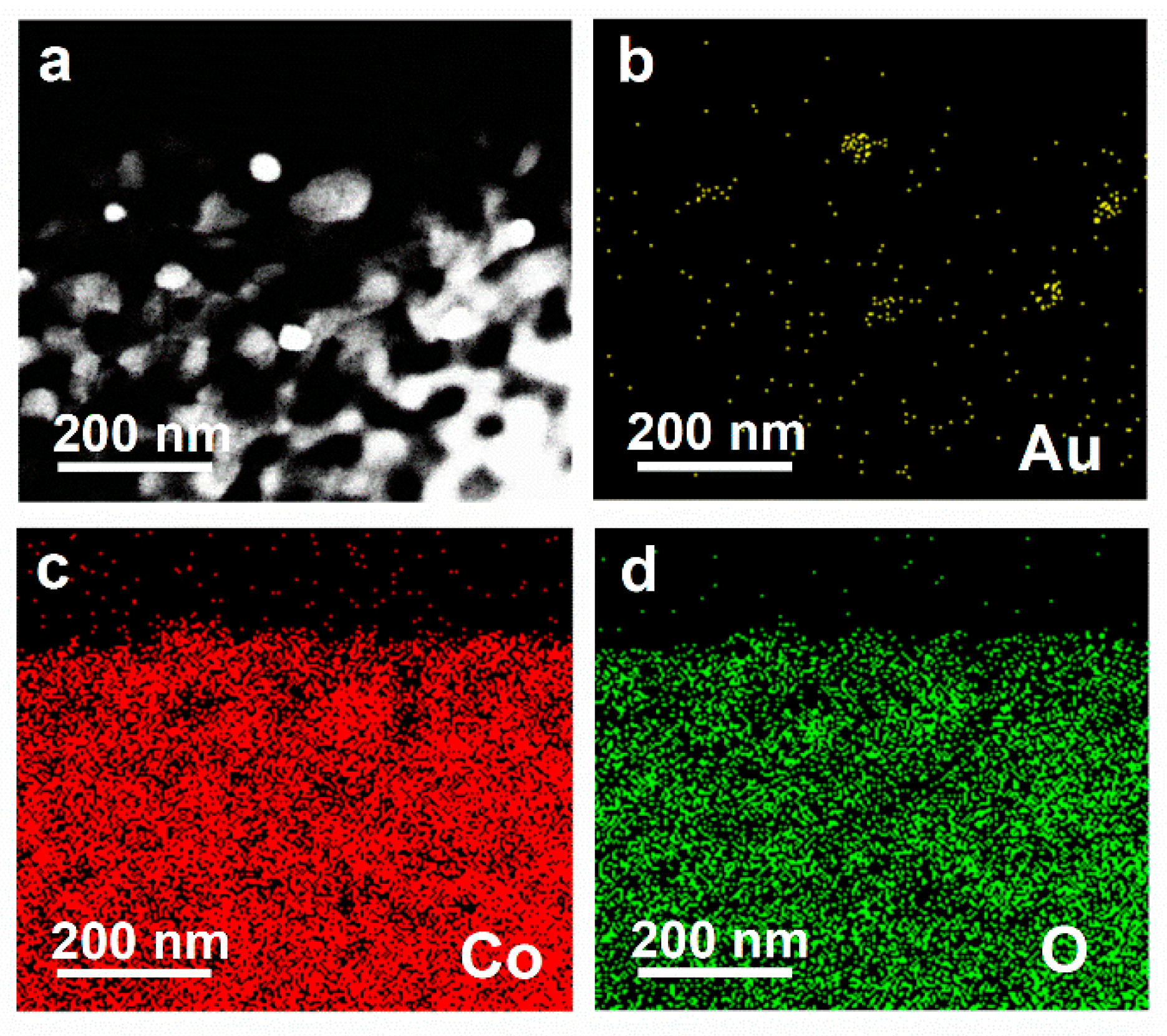
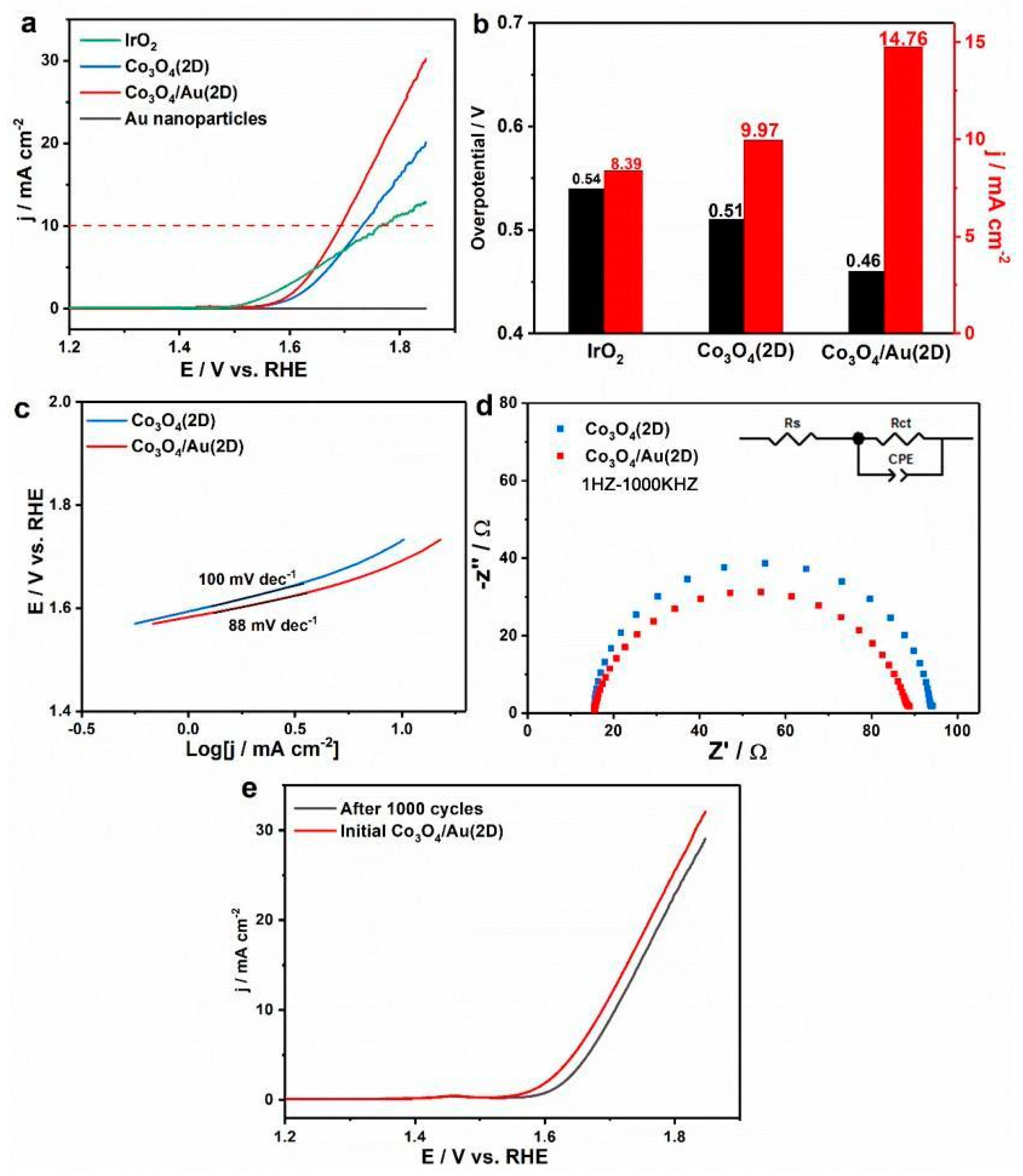
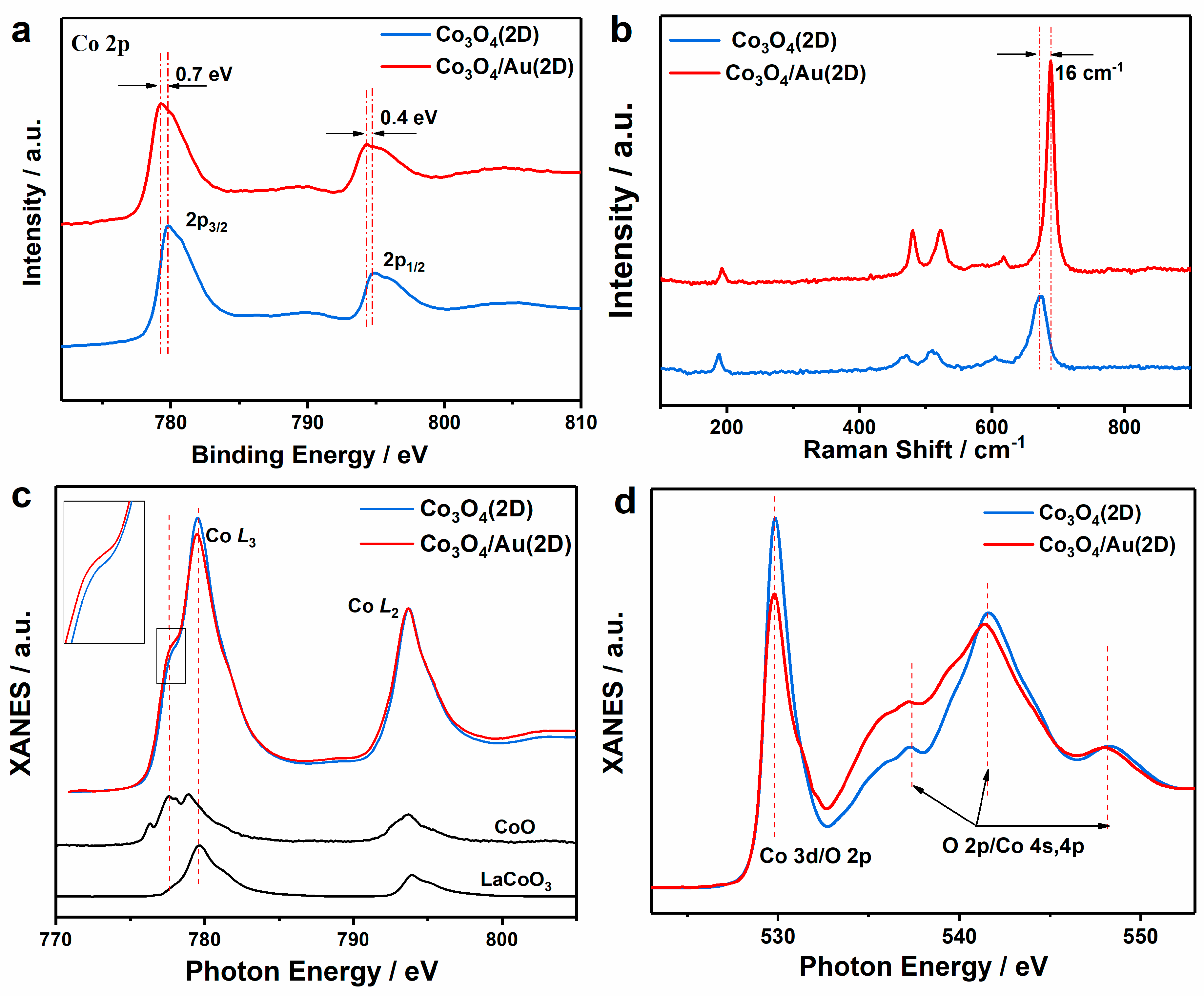
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2013, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Jensen, B.W.; Jensen, O.W.; Forsyth, M.; Macfarlane, D.R. High rates of oxygen reduction over a vapor phase-polymerized PEDOT electrode. Science 2008, 321, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Carlton, C.; Risch, M.; Surendranath, Y.; Chen, S.; Furutsuki, S.; Yamada, A.; Nocera, D.G.; Shao-Horn, Y. The nature of lithium battery materials under oxygen evolution reaction conditions. J. Am. Chem. Soc. 2012, 134, 16959–16962. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Gerken, J.B.; McAlpin, J.G.; Chen, J.Y.; Rigsby, M.L.; Casey, W.H.; Britt, R.D.; Stahl, S.S. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0-14: The thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 2011, 133, 14431–14442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, S.; Lei, F.; Liu, J.; Liang, L.; Xie, Y. Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem. Sci. 2014, 5, 3976–3982. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Zhang, J.H.; Wang, J.; Li, Q.Y.; Xu, C.W.; Lu, X. Three-dimensional ordered mesoporous Co3O4 enhanced by Pd for oxygen evolution reaction. Sci. Rep. 2017, 7, 41542. [Google Scholar] [CrossRef]
- Wu, J.; Xue, Y.; Yan, X.; Yan, W.; Cheng, Q.; Xie, Y. Co3O4 nanocrystals on single-walled carbon nanotubes as a highly efficient oxygen-evolving catalyst. Nano Res. 2012, 5, 521–530. [Google Scholar]
- Zhang, J.J.; Wang, H.H.; Zhao, T.J.; Zhang, K.X.; Wei, X.; Jiang, Z.D.; Hirano, S.I.; Li, X.H.; Chen, J.S. Oxygen Vacancy Engineering of Co3O4 Nanocrystals through Coupling with Metal Support for Water Oxidation. ChemSusChem 2017, 10, 2875–2879. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chem. Int. Ed. Engl. 2015, 54, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Liao, T.; Ma, Z.; Tian, D.; Liu, Q.; Xiao, F.; Sun, Z.; Ho Kim, J.; Xue Dou, S. Graphene-like holey Co3O4 nanosheets as a highly efficient catalyst for oxygen evolution reaction. Nano Energy 2016, 30, 267–275. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.W.D.; García-Melchor, M.; Bajdich, M.; Chakthranont, P.; Kirk, C.; Vojvodic, A.; Jaramillo, T.F. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nature Energy 2016, 1, 16053–16060. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Jin, R.; Abroshan, H.; Zeng, C.; Zhang, H.; House, S.D.; Gottlieb, E.; Kim, H.J.; Yang, J.C.; Jin, R. Gold Nanoclusters Promote Electrocatalytic Water Oxidation at the Nanocluster/CoSe2 Interface. J. Am. Chem. Soc. 2017, 139, 1077–1080. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Dou, Y.; Hwang, S.M.; Park, M.S.; Jiang, L.; Kim, J.H.; Dou, S.X. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813–3821. [Google Scholar] [CrossRef] [Green Version]
- Meher, S.K.; Rao, G.R. Effect of Microwave on the Nanowire Morphology, Optical, Magnetic, and Pseudocapacitance Behavior of Co3O4. J. Phys. Chem. C 2011, 115, 25543–25556. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, H.L.; Yan, J.M.; Ping, Y.; Song, O., II; Li, S.J.; Jiang, Q. DNA-directed growth of ultrafine CoAuPd nanoparticles on graphene as efficient catalysts for formic acid dehydrogenation. Chem. Commun. 2014, 50, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jin, R.; Song, Y.; Zhang, H.; House, S.D.; Yang, J.C.; Jin, R. Atomically Precise Gold Nanoclusters Accelerate Hydrogen Evolution over MoS2 Nanosheets: The Dual Interfacial Effect. Small 2017, 13, 1701519–1701525. [Google Scholar] [CrossRef]
- Zheng, Y.R.; Gao, M.R.; Gao, Q.; Li, H.H.; Xu, J.; Wu, Z.Y.; Yu, S.H. An efficient CeO2/CoSe2 Nanobelt composite for electrochemical water oxidation. Small 2015, 11, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Hadjiev, V.G.; Ilievl, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, 199–201. [Google Scholar] [CrossRef]
- Cui, Z.; Xu, H.; Yun, Y.; Guo, J.; Chuang, Y.-D.; Huang, H.; Meng, D.; Wang, J.; Fu, Z.; Peng, R.; et al. Soft X-ray absorption spectroscopy investigations of Bi6FeCoTi3O18 and LaBi5FeCoTi3O18 epitaxial thin films. J. Appl. Phys. 2016, 120, 084101–084105. [Google Scholar] [CrossRef]
- Zhu, J.; Bai, L.; Sun, Y.; Zhang, X.; Li, Q.; Cao, B.; Yan, W.; Xie, Y. Topochemical transformation route to atomically thick Co3O4 nanosheets realizing enhanced lithium storage performance. Nanoscale 2013, 5, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Diao, H.; Luo, W.; Song, Y.F. Facile Preparation of Ultrathin Co3O4/Nanocarbon Composites with Greatly Improved Surface Activity as a Highly Efficient Oxygen Evolution Reaction Catalyst. Chemistry 2017, 23, 4010–4016. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hung, S.F.; Chen, H.Y.; Chan, T.S.; Chen, H.M.; Liu, B. In Operando Identification of Geometrical-Site-Dependent Water Oxidation Activity of Spinel Co3O4. J. Am. Chem. Soc. 2016, 138, 36–39. [Google Scholar] [CrossRef]
- Wang, W.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.P.; Huotari, S.; Yu, M.; Geng, B. Mass-Production of Mesoporous MnCo2O4 Spinels with Manganese(IV)- and Cobalt(II)-Rich Surfaces for Superior Bifunctional Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Ma, R.; Gao, C.; Liu, L.; Hu, L.; Zhu, J.; Sun, T.; Tang, Y.; Liu, D.; et al. A glass-ceramic with accelerated surface reconstruction toward the efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 2021, 60, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Olowoyo, J.O.; Kriek, R.J. Recent progress on bimetallic-based spinels as electrocatalysts for the oxygen evolution reaction. Small. 2022, 18, 2203125–2203174. [Google Scholar] [CrossRef]
- Li, J.; Stephanopoulos, M.F.; Xia, Y. Introduction: Heterogeneous single-atom catalysis. Chem. Rev. 2020, 120, 11699–11702. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, C.; Wang, P.; Zeng, W.; Zhu, J.; Li, Y.; Peng, W.; Liu, Q.; Xu, H.; Zhao, Y.; et al. Polymetallic phosphides evolved from MOF and LDH dual-precursors for robust oxygen evolution reaction in alkaline and seawater media. Mater. Today Phys. 2022, 60, 100684–100694. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Sun, D.; Liu, J.; Zhang, Q.; Li, X.; Fu, H.; Liu, M.; Xu, J.; Jiang, G.; Lu, Y. Enhanced Electrocatalytic Water Oxidation of Ultrathin Porous Co3O4 Nanosheets by Physically Mixing with Au Nanoparticles. Nanomaterials 2022, 12, 4419. https://doi.org/10.3390/nano12244419
Hu C, Sun D, Liu J, Zhang Q, Li X, Fu H, Liu M, Xu J, Jiang G, Lu Y. Enhanced Electrocatalytic Water Oxidation of Ultrathin Porous Co3O4 Nanosheets by Physically Mixing with Au Nanoparticles. Nanomaterials. 2022; 12(24):4419. https://doi.org/10.3390/nano12244419
Chicago/Turabian StyleHu, Changhe, Dejuan Sun, Jie Liu, Qi Zhang, Xiao Li, Huhui Fu, M. Liu, Jiayue Xu, Guojian Jiang, and Yalin Lu. 2022. "Enhanced Electrocatalytic Water Oxidation of Ultrathin Porous Co3O4 Nanosheets by Physically Mixing with Au Nanoparticles" Nanomaterials 12, no. 24: 4419. https://doi.org/10.3390/nano12244419




