Carbon Surface-Influenced Heterogeneity of Ni and Co Catalytic Sites as a Factor Affecting the Efficiency of Oxygen Reduction Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
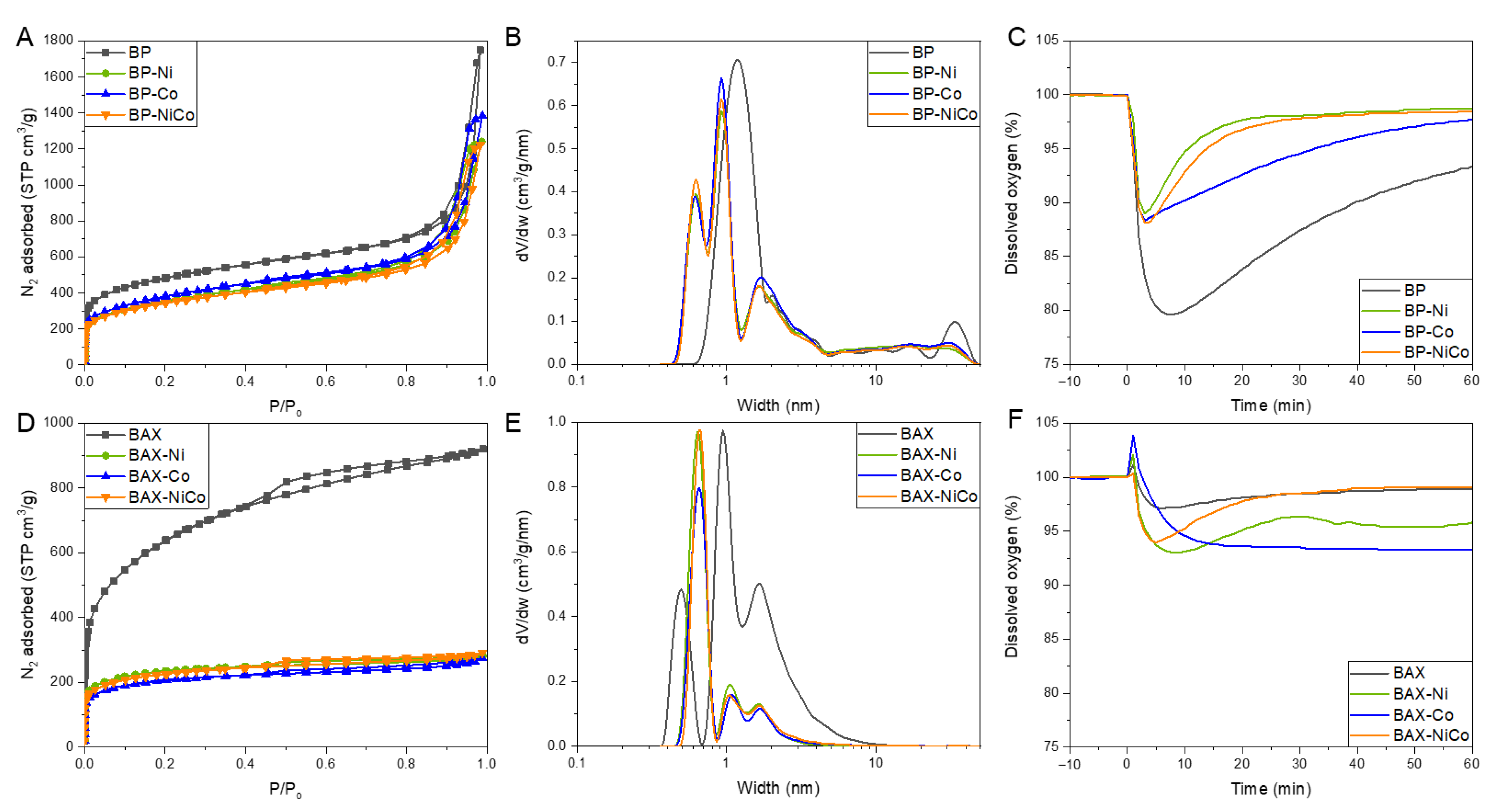
2.2.1. Surface Characterization
2.2.2. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reactions: Nanostructure. Activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Mun, B.S.L.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Yang, Z.; Nie, H.; Chen, X.A.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Wang, Y. Carbon nitride in energy conversion and storage: Recent advances and future prospects. ChemSusChem 2015, 8, 931–946. [Google Scholar] [CrossRef]
- Han, J.; Bian, J.; Sun, C. Recent advances in single-atom electrocatalysts for oxygen reduction reaction. Research 2020, 2020, 9512763. [Google Scholar] [CrossRef]
- Gan, Z.Z.; Sheng, Z.M.; Huang, H.; Dai, X.Y.; Niu, R.L.; Jia, R.P. Highly mesoporous Fe and N Co-doped graphitic catalysts prepared from short-time synthesis of precursor towards highly efficient oxygen reduction. Sustain. Energy Fuels 2019, 3, 3335–3343. [Google Scholar] [CrossRef]
- Yi, J.D.; Xu, R.; Wu, Q.; Zhang, T.; Zang, K.T.; Luo, J.; Liang, Y.L.; Huang, Y.B.; Cao, R. Atomically dispersed iron-nitrogen active sites within porphyrinic triazine-based frameworks for oxygen reduction reaction in both alkaline and acidic media. ACS Energy Lett. 2018, 3, 883–889. [Google Scholar] [CrossRef]
- Jorge, A.B.; Jervis, R.; Periasamy, A.P.; Qiao, M.; Feng, J.; Tran, L.N.; Titirici, M.-M. 3D carbon materials for efficient oxygen and hydrogen electrocatalysis. Adv. Energy Mater. 2020, 10, 1902494. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, e1500564. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pan, X.; Cao, X.; Hu, P.; Bao, X. Oxygen reduction reaction mechanism on nitrogen-doped graphene: A density functional theory study. J. Catal. 2011, 282, 183–190. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Yang, W.; Jia, J. Recent advances in carbon-based electrocatalysts for oxygen reduction reaction. Chin. Chem. Lett. 2020, 31, 626–634. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Yao, X. Defects on carbons for electrocatalytic oxygen reduction. Chem. Soc. Rev. 2018, 47, 7628–7658. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, W.; Wang, L.; Yue, Q.; Xu, S.; Wang, H.; Liu, J. Synthesis of three-dimensional flowerlike nitrogen-doped carbons by a copyrolysis route and the effect of nitrogen species on the electrocatalytic activity in oxygen reduction reaction. Carbon 2013, 54, 249–257. [Google Scholar] [CrossRef]
- Liang, L.; Zheng, Y.; Chen, J.; Liu, J.; Hulicova-Jurcakova, D.; Jaroniec, M.; Qiao, S.Z. Facile oxygen reduction on a three-dimensionally ordered macroporous graphitic C3N4/carbon composite electrocatalyst. Angew. Chem. Int. Ed. 2012, 51, 3892–3896. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhi, L.; Tang, Z.; Feng, X.; Maier, J.; Müllen, K. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 6, 7084–7091. [Google Scholar] [CrossRef] [PubMed]
- von Deak, D.; Biddinger, E.J.; Luthman, K.A.; Ozkan, U.S. The effect of phosphorus in nitrogen-containing carbon nanostructures on oxygen reduction in PEM fuel cells. Carbon 2010, 48, 3637–3639. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.-B.; Dai, L. BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef]
- Florent, M.; Wallace, R.; Bandosz, T.J. Oxygen electroreduction on nanoporous carbons: Textural features vs nitrogen and boron catalytic centers. ChemCatChem 2019, 11, 851–860. [Google Scholar] [CrossRef]
- Quilez-Bermejo, J.; Morallon, E.; Cazorla Amoros, D. Metal-free heteroatom doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Li, D.; Jia, Y.; Chang, G.; Chen, J.; Liu, H.; Wang, J.; Hu, Y.; Xia, Y.; Yang, D.; Yao, X. A defect-driven metal-free electrocatalyst for oxygen reduction in acidic electrolyte. Chem 2018, 4, 2345–2356. [Google Scholar] [CrossRef]
- Morais, R.G.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Glucose-derived carbon materials with tailored properties as electrocatalysts for the oxygen reduction reaction. Beilstein J. Nanotechnol. 2019, 10, 1089–1102. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Relationships between texture, surface chemistry and performance of N-doped carbon xerogels in the oxygen reduction reaction. Appl.Surf. Sci. 2021, 548, 149242. [Google Scholar] [CrossRef]
- Gabe, A.; Ruiz-Rosas, R.; González-Gaitán, C.; Morallón, E.; Cazorla-Amorós, D. Modeling of oxygen reduction reaction in porous carbon materials in alkaline medium. Effect of microporosity. J. Power Sources 2019, 412, 451–464. [Google Scholar] [CrossRef]
- Eisenberg, D.; Prinsen, P.; Geels, N.J.; Stroek, W.; Yan, N.; Hua, B.; Kuo, J.-L.; Rothenberg, G. The evolution of hierarchical porosity in self templated nitrogen-doped carbon and its effects on oxygen reduction electrocatalysis. RCS Adv. 2016, 6, 80398–80407. [Google Scholar] [CrossRef]
- Bandosz, T.J. Exploring the silent aspect of carbon nanopores. Nanomaterials 2021, 11, 407. [Google Scholar] [CrossRef]
- Bandosz, T.J. Revealing the impact of small pores on oxygen reduction on carbon electrocatalysts: A journey through recent findings. Carbon 2022, 188, 289–304. [Google Scholar] [CrossRef]
- Seredych, M.; Szczurek, A.; Fierro, V.; Celzard, A.; Bandosz, T.J. Electrochemical reduction of oxygen on hydrophobic ultramicroporous PolyHIPE carbon. ACS Catal. 2016, 6, 5618–5628. [Google Scholar] [CrossRef]
- Encalada, J.; Savaram, K.; Travlou, N.A.; Li, W.; Li, Q.; Delgado-Sánchez, C.; Fierro, V.; Celzard, A.; He, H.; Bandosz, T.J. Combined effect of porosity and surface chemistry on the electrochemical reduction of oxygen on cellular vitreous carbon foam catalyst. ACS Catal. 2017, 7, 7466–7478. [Google Scholar] [CrossRef]
- Barrera, D.; Florent, M.; Sapag, K.; Bandosz, T.J. Insight into the mechanism of oxygen reduction reaction on micro/mesoporous carbons: Ultramicropores versus nitrogen-containing catalytic centers in ordered pore structure. ACS Appl. Energy Mater. 2019, 2, 7412–7424. [Google Scholar] [CrossRef]
- Barrera, D.; Florent, M.; Kulko, M.; Bandosz, T.J. Ultramicropore-influenced mechanism of oxygen electroreduction on metal-free carbon catalysts. J. Mater. Chem. A 2019, 7, 27110–27123. [Google Scholar] [CrossRef]
- de Falco, G.; Florent, M.; Jagiello, J.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Bandosz, T.J. Alternative view of oxygen reduction on porous carbon electrocatalysts: The substance of complex oxygen-surface interactions. iScience 2021, 24, 102216. [Google Scholar] [CrossRef]
- de Falco, G.; Florent, M.; Bandosz, T.J. Oxygen adsorption in pores promotes its reduction on metal-free carbon catalysts: A case of carbon blacks. Carbon 2022, 189, 230–239. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. 2018, 1804297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Wang, J.; Yin, G.; Shao, Y.; Zhang, S.; Wang, Z.; Gao, Y. Effect of carbon black support corrosion on the durability of Pt/C catalyst. J. Power Sources 2007, 171, 331–339. [Google Scholar] [CrossRef]
- Yang, H.; Ko, Y.; Lee, W.; Zuttel, A.; Kim, W. Nitrogen-doped carbon black supported Pt-M (M=Pd, Fe, Ni) alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Mater. Today Energy 2019, 13, 374–381. [Google Scholar] [CrossRef]
- Appleby, A.J.; Marie, J. Kinetics of oxygen reduction on carbon materials in alkaline solution. Electrochim. Acta 1979, 24, 195–202. [Google Scholar] [CrossRef]
- Fruehwald, H.M.; Ebralidze, I.I.; Melino, P.D.; Zenkina, O.V.; Easton, E.B. Probing the influence of the carbon support on the activity of Fe-N3/C model active sites for the oxygen reduction reaction. J. Electrochem. Soc. 2020, 167, 084520. [Google Scholar] [CrossRef]
- Tarasevich, M.R.; Mazin, P.V.; Kapustina, N.A. Kinetics and mechanism of oxygen electroreduction in acidic and neutral solutions on carbon black XC-72R modified by products of pyrolysis of cobalt 5,10,15,20-tetrakis(4-methoxyphenyl) porphyrin. Russ. J. Electrochem. 2011, 47, 923. [Google Scholar] [CrossRef]
- Zhutaeva, G.V.; Bogdanovskaya, V.A.; Davydova, E.S.; Kazanskii, L.P.; Tarasevich, M.R. Kinetics and mechanism of oxygen electroreduction on Vulcan XC72R carbon black modified by pyrolysis products of cobalt 5,10,15,20-tetrakis(4-methoxyphenyl) porphyrine in a broad pH interval. J. Solid State Electrochem. 2014, 18, 1319–1334. [Google Scholar] [CrossRef]
- Oh, J.; Park, S.; Jang, D.; Shin, Y.; Lim, D.; Park, S. Metal-free N-doped carbon black s as excellent electro catalysts for oxygen reduction reactions. Carbon 2019, 45, 481–487. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J. Tungsten nitride nanocrystals on nitrogen-doped carbon black as efficient electrocatalysts for oxygen reduction reactions. Chem. Commun. 2015, 51, 572–575. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, G.; Liu, P.; Han, S.; Han, J.; Ye, F.; Song, W.; Lan, B.; Sun, M.; Yu, L. Nitrogen doped Ketjenblack carbon supported Co3O4 nanoparticles as a synergistic electrocatalyst for oxygen reduction reactions. Frontiers Chem. 2019, 7, 766. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wand, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalyst for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Cui, X.; Huang, Y.; Yao, H.; Chen, Y.; Tian, H.; Meng, G.; Chen, C.; Chang, Z.; Shi, J. N-doped carbon electrocatalyts: Marked ORR activity in acidic media without the contribution from metal sites? Angew. Chem. 2022, 61, e2o2116290. [Google Scholar] [CrossRef] [PubMed]
- Bagotzky, V.S.; Shumilova, N.A.; Samoilov, G.P.; Khrushcheva, E.I. Electrochemical oxygen reduction on nickel electrodes in alkaline solutions—II. Electrochim. Acta 1972, 17, 1625–1635. [Google Scholar] [CrossRef]
- Bagotzky, V.S.; Shumilova, N.A.; Khrushcheva, E.I. Electrochemical oxygen reduction on oxide catalysts. Electrochim. Acta 1976, 21, 919–924. [Google Scholar] [CrossRef]
- Ouyang, C.; Ni, B.; Sun, Z.; Zhuang, J.; Xiao, H.; Wang, X. Boosting the ORR performance of modified carbon black via C–O bonds. Chem. Sci. 2019, 10, 2118–2123. [Google Scholar] [CrossRef]
- Xie, S.; Huang, S.; Wei, W.; Yang, X.; Liu, Y.; Lu, X.; Tong, Y. Chitosan Waste-Derived Co and N Co-doped Carbon Electrocatalyst for Efficient Oxygen Reduction Reaction. ChemElectroChem 2015, 2, 1806–1812. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, X.-X.; Ding, X.-L.; Kong, H.-C.; Sha, H.-D.; Lin, H.; Wen, W.; Shen, G.; Guo, Z.; Ma, Z.-F.; et al. Effects of cobalt precursor on pyrolyzed carbon-supported cobalt-polypyrrole as electrocatalyst toward oxygen reduction reaction. Nanoscale Rese. Lett. 2013, 8, 478. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, M.; Feng, C.H. A doping-adsorption-pyrolysis strategy for constructing atomically dispersed cobalt sites anchored on a N-doped carbon framework as an efficient bifunctional electrocatalyst for hydrogen evolution and oxygen reduction. RSC Adv. 2022, 12, 20578. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhen, L.; Yang, J.; Li, Y.; Wan, X.; Liu, X.; Zhang, X.; Yu, R.; Shui, J. Carbon black-supported FM–N–C (FM = Fe, Co, and Ni) single-atom catalysts synthesized by the self-catalysis of oxygen-coordinated ferrous metal atoms. J. Mater. Chem. A 2020, 8, 13166–13172. [Google Scholar] [CrossRef]
- Morais, R.G.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Fe, Co, N-doped carbon nanotubes as bifunctional oxygen electrocatalysts. Appl. Surf. Sci. 2022, 572, 151459. [Google Scholar] [CrossRef]
- Kim, S.; Kato, S.; Ishizaki, T.; Li, O.L.; Kang, J. Transition metal (Fe, Co, Ni) nanoparticles on selective amino-N-doped carbon as high-performance oxygen reduction reaction electrocatalyst. Nanomaterials 2019, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.P.; Rachman, I.B.; Horino, H.; Rzeznicka, I.I. Bifunctional catalytic activity of γ-NiOOH toward oxygen reduction and oxygen evolution reactions in alkaline Solutions. Oxygen 2022, 2, 479–492. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A.; Tarlochan, F. Highly active and stable bi-functional nicoo2 catalyst for oxygen reduction and oxygen evolution reactions in alkaline medium. Int. J. Hydrog. Energy 2019, 44, 16603–16614. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Cheng, W.; Li, Y.; Zhou, W.; Su, H.; Zhao, X.; Yao, P.; Liu, Q. Metallic Ni3N quantum dots as a synergistic promoter for NiO nanosheet toward efficient oxygen reduction electrocatalysis. J. Phys. Chem. C 2019, 123, 8633–8639. [Google Scholar] [CrossRef]
- Abdelwahab, A.; Castelo-Quibén, J.; Vivo-Vilches, J.F.; Pérez-Cadenas, M.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Pérez-Cadena, A.F. Electrodes based on carbon aerogels partially graphitized by doping with transition metals for oxygen reduction reaction. Nanomaterials 2018, 8, 266. [Google Scholar] [CrossRef]
- Goubert-Renaudin, S.N.S.; Wieckowski, A. Ni and/or Co nanoparticles as catalysts for oxygen reduction (ORR) at room temperature. J. Electroanal. Chem. 2011, 652, 44–51. [Google Scholar] [CrossRef]
- Liu, X.; Park, M.; Kim, M.G.; Gupta, S.; Wu, G.; Choi, J. Integrating NiCo Alloys with their oxides as efficient bifunctional cathode catalysts for rechargeable zinc–air batteries. Angew. Chem. 2015, 54, 9654–9658. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, H.Y.; Jiang, C.; Zhang, T.H.; Zhan, R.; Li, X.; Li, J.F.; Tian, J.H.; Yang, R. NiCo alloy nanoparticles decorated on N-doped carbon nanofibers as highly active and durable oxygen electrocatalyst. Adv. Funct. Mater. 2018, 28, 1705094. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Tang, O.; Wang, E.; Qi, L.; Wang, S.; Sun, G. Amide-functionalized carbon supports for cobalt oxide toward oxygen reduction reaction in Zn-air battery. Appli. Catal. B 2014, 148–149, 212–220. [Google Scholar] [CrossRef]
- Florent, M.; Hashmi, R.; Bandosz, T.J. Extent of carbon surface oxygen affinity and its effects on the activity of metal-free carbon catalysts in oxygen reduction reaction: Interplay of porosity and N-, O- and S-enriched surface chemistry. Mater. Adv. 2022, 23, 8567–8578. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon slit pore model incorporating surface energetical heterogeneity and geometrical corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Zhao, C. Surface-oxidized carbon black as a catalyst for the water oxidation and alcohol oxidation reactions. Chem. Commun. 2016, 52, 6439–6442. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.-Z. Determination of the electron transfer number for the oxygen reduction reaction: From theory to experiment. ACS Catalysis 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- de Falco, G.; Florent, M.; De Rosa, A.; Bandosz, T.J. Proposing an unbiased oxygen reduction reaction onset potential determination by using a Savitzky-Golay differentiation procedure. J. Colloid Interface Sci. 2021, 586, 597–600. [Google Scholar] [CrossRef]
- Elghamry, I.; Alablan, A.S.; Alkhalifah, M.A.; Abdelsalam, M.E. High-performance organometallic catalyst based on nickel porphyrin/carbon fibre for the oxygen reduction reaction. J. Electrochem. Soc. 2021, 168, 016510. [Google Scholar] [CrossRef]
- Giddaerappa, N.; Shantharaja, M.H.; Lokesh, K.S. Tetraphenolphthalein cobalt (II) phthalocyanine polymer modified with multiwalled carbon nanotubes as an efficient catalyst for the oxygen reduction reaction. ACS Omega 2022, 7, 14291–14304. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.Q.; Shen, P.K.; Shao, Z.; Jiang, S.P. Boosting electrocatalytic activity of single atom catalysts supported on nitrogen-doped Carbon through N coordination environment engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef]
- Liu, D.; Tong, Y.; Yan, X.; Liang, J.; Dou, S.X. Recent advances in carbon-based bifunctional oxygen catalysts for zinc-air batteries. Batteries Supercaps 2019, 2, 743–765. [Google Scholar] [CrossRef]
- Wei, X.; Luo, X.; Wu, N.; Gu, W.; Lin, Y.; Zhu, C. Recent advances in synergistically enhanced single-atomic site catalysts for boosted oxygen reduction reaction. Nano Energy 2021, 84, 105817. [Google Scholar] [CrossRef]
- Rozario, A.; Silva e Silva, R.K.; Freitas, M.B.J.G. Recycling of nickel from NiOO/Ni(OH)2 electrodes of spent Ni-Cd batteries. J. Power. Sources 2006, 158, 754–759. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Florent, M.; Bandosz, T.J. Insight into the mechanism of perfluorooctanesulfonic acid adsorption on highly porous media: Sizes of hydrophobic pores and the extent of multilayer formation. Carbon 2022, 191, 535–545. [Google Scholar] [CrossRef]
- Ozturk, S.; Xiao, Y.X.; Dietrich, D.; Giesen, B.; Barthel, J.; Ying, J.; Yang, X.Y.; Janiak, C. Nickel nanoparticles supported on a covalent triazine framework as electrocatalyst for oxygen evolution and oxygen reduction reactions. Beilstein J. Nanotechnol. 2020, 11, 770–781. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Liu, W.; Yu, B.; Liao, X.; Zhao, Y. Facet-Dependent Oxygen Reduction Reaction Activity on the Surfaces of Co3O4. Energy Environ. Mater. 2021, 4, 407–412. [Google Scholar] [CrossRef]
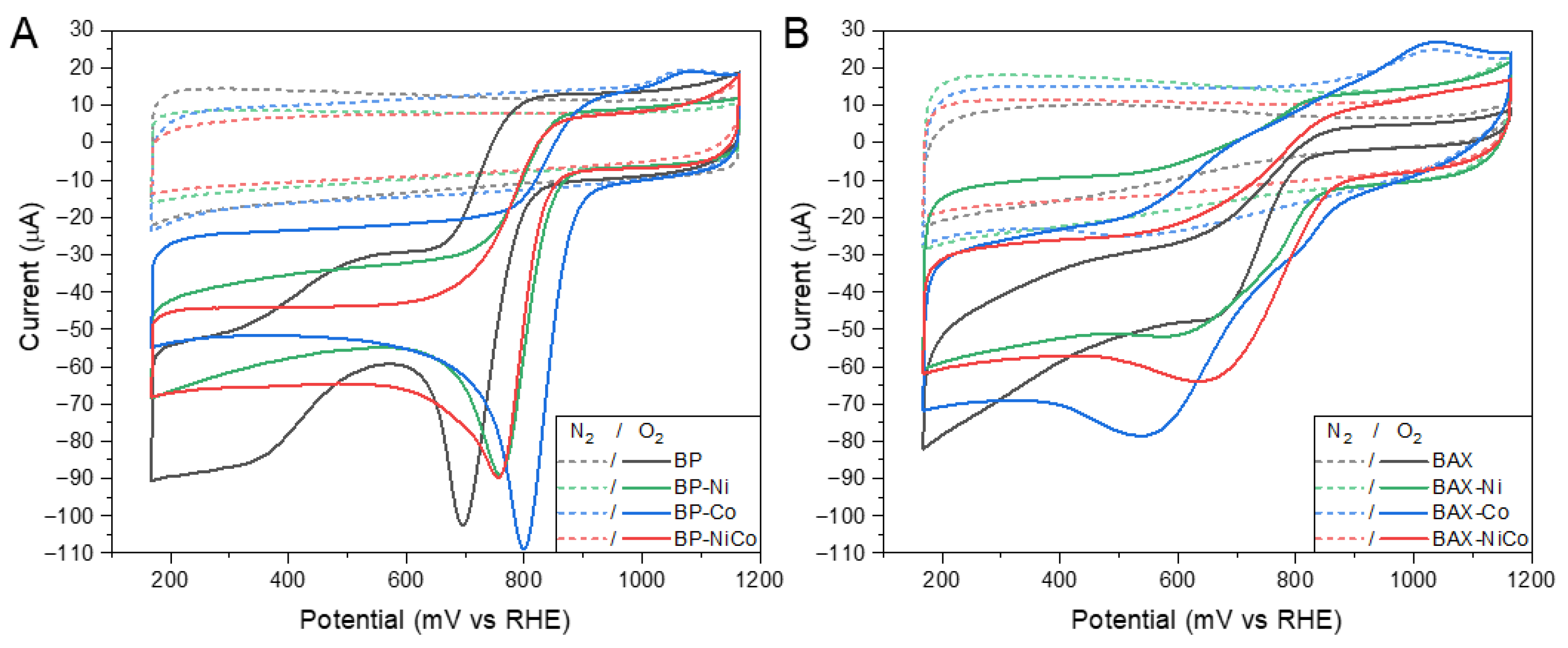
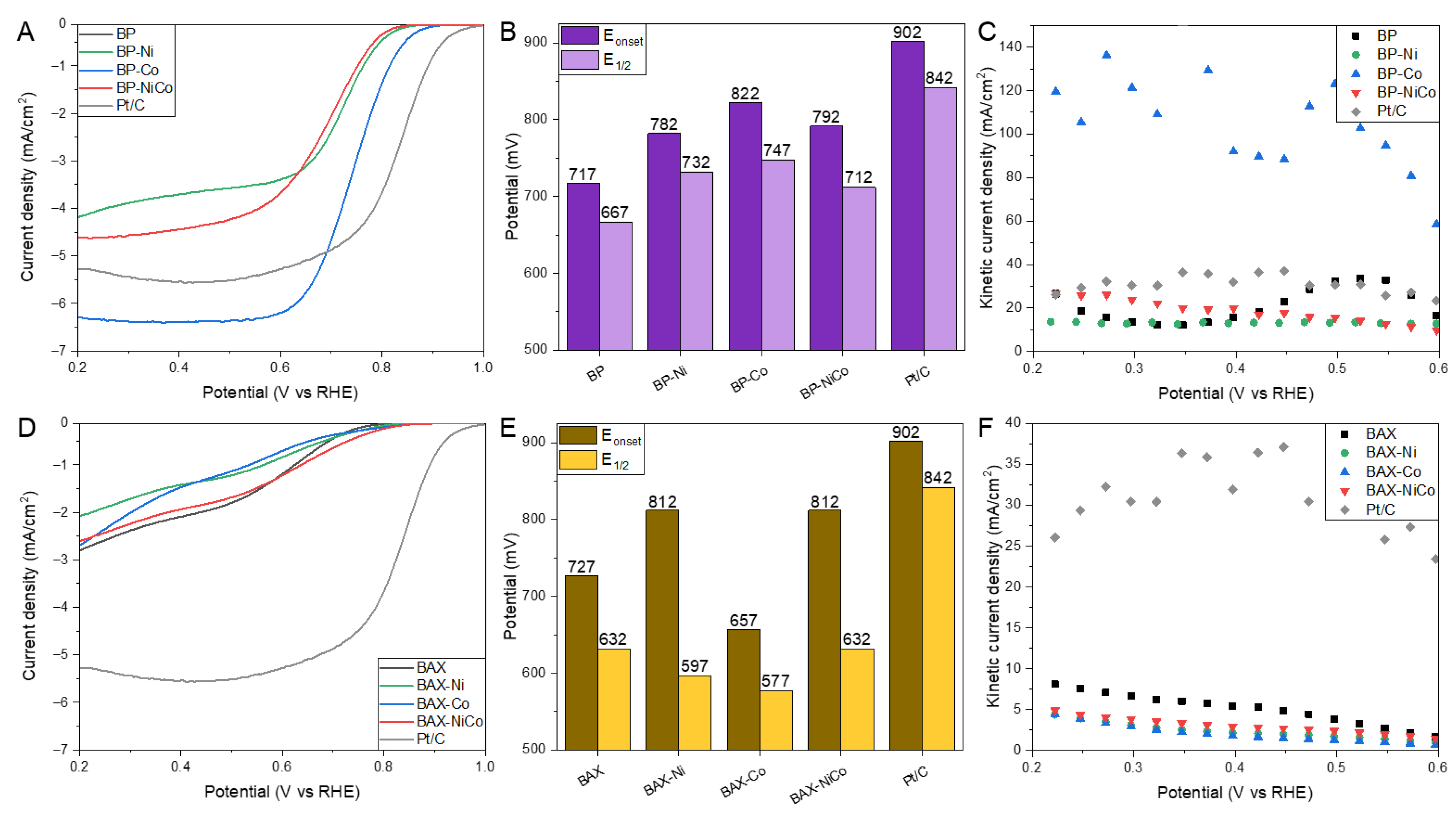
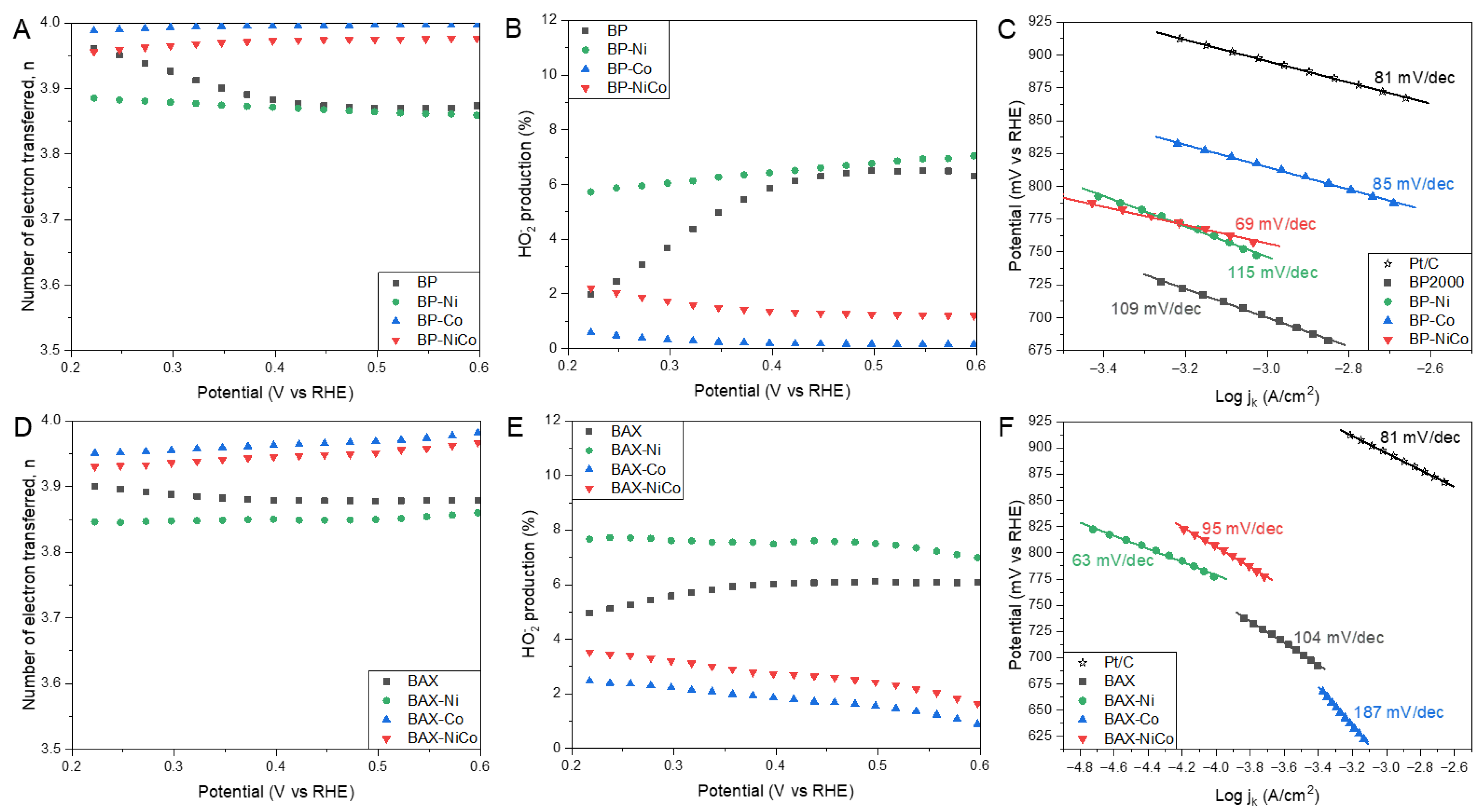
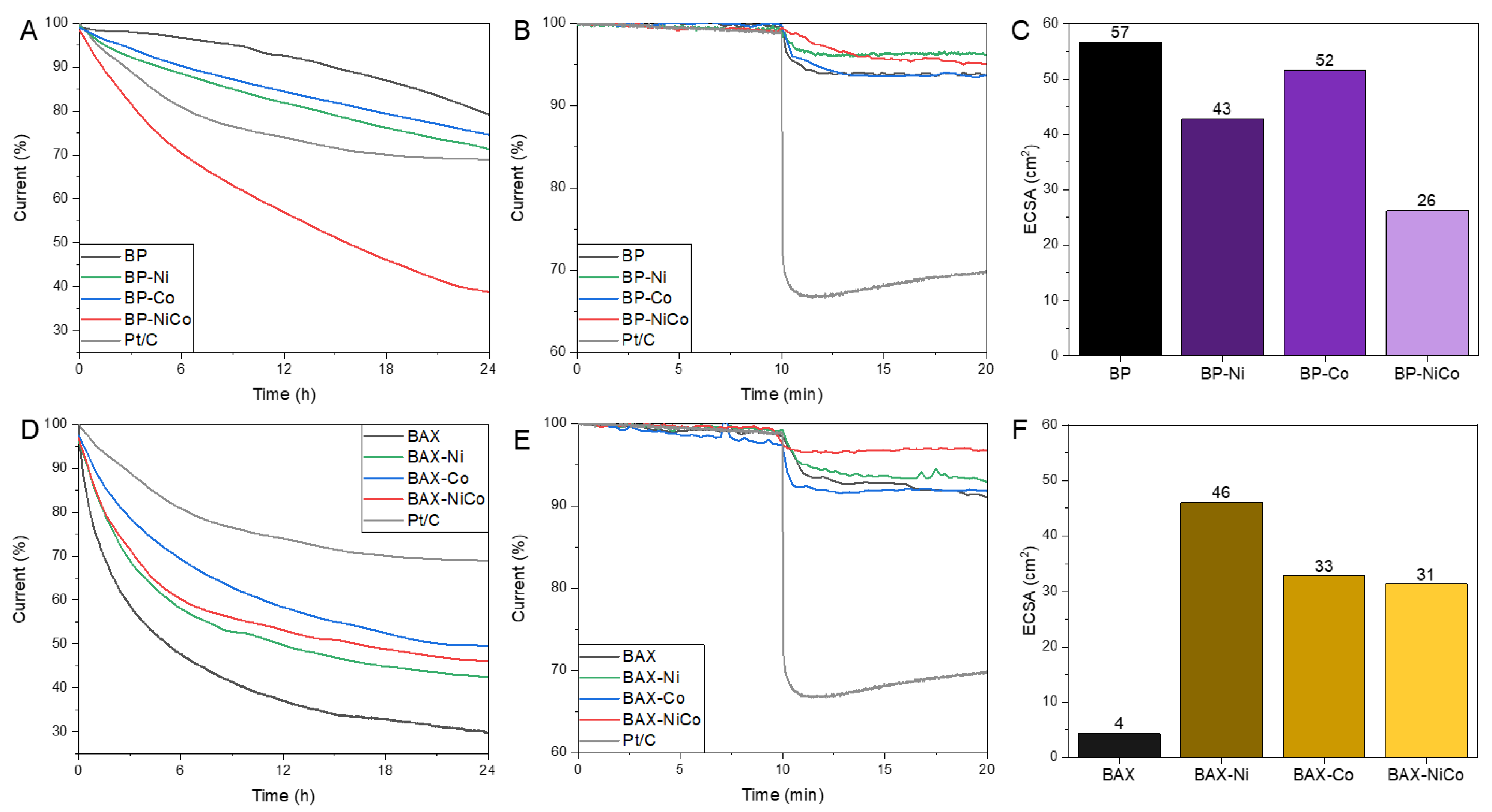
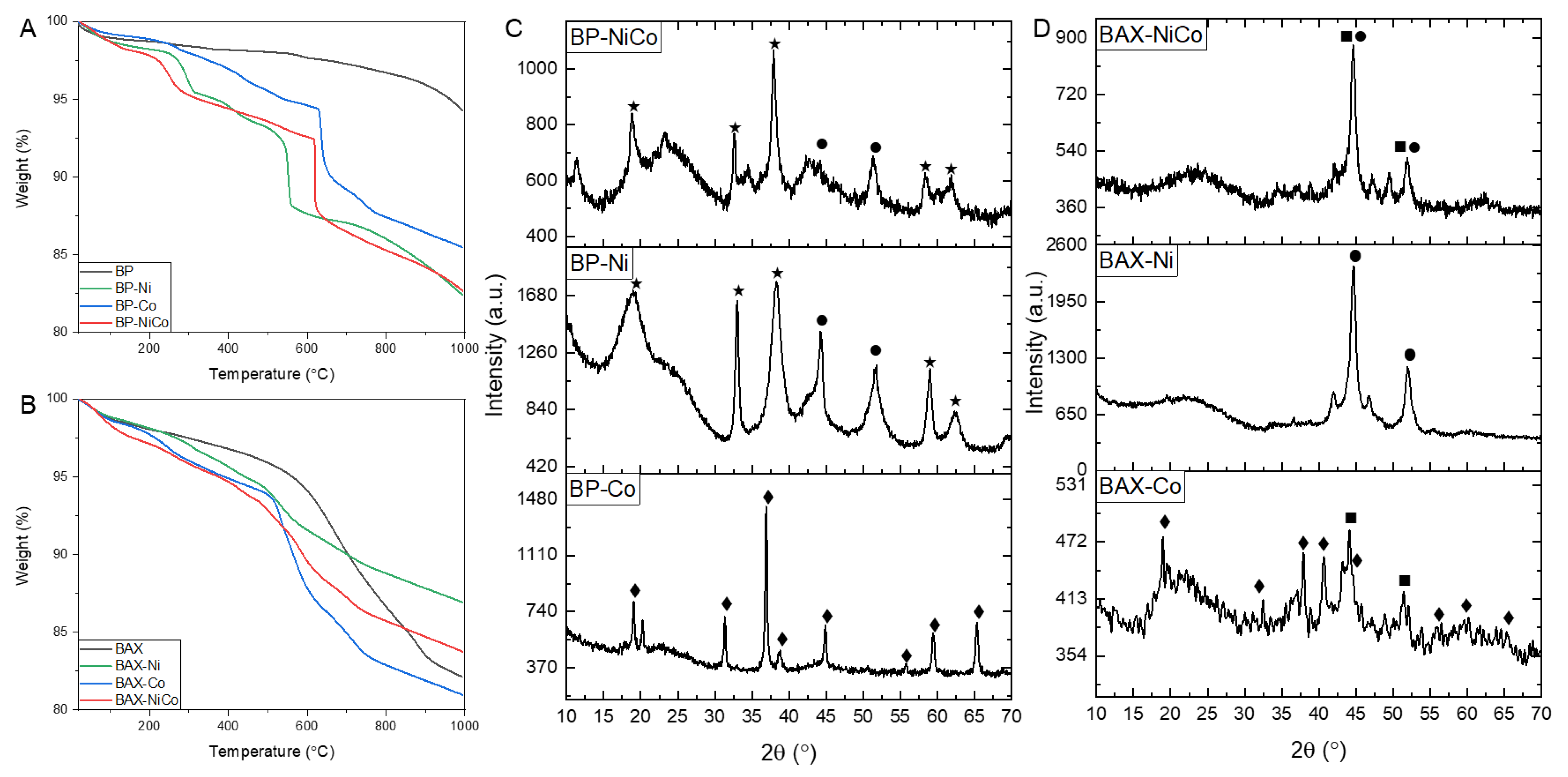
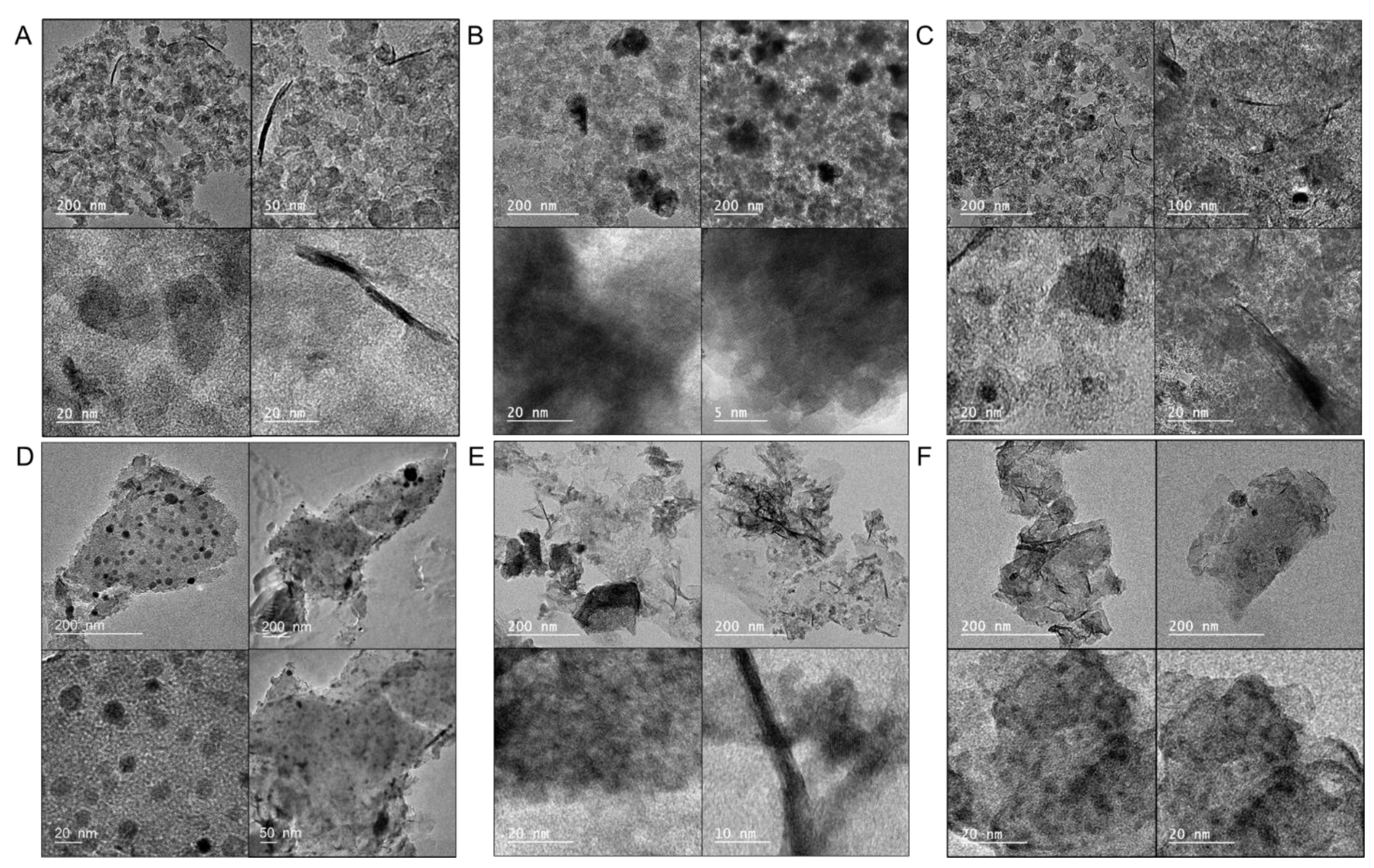
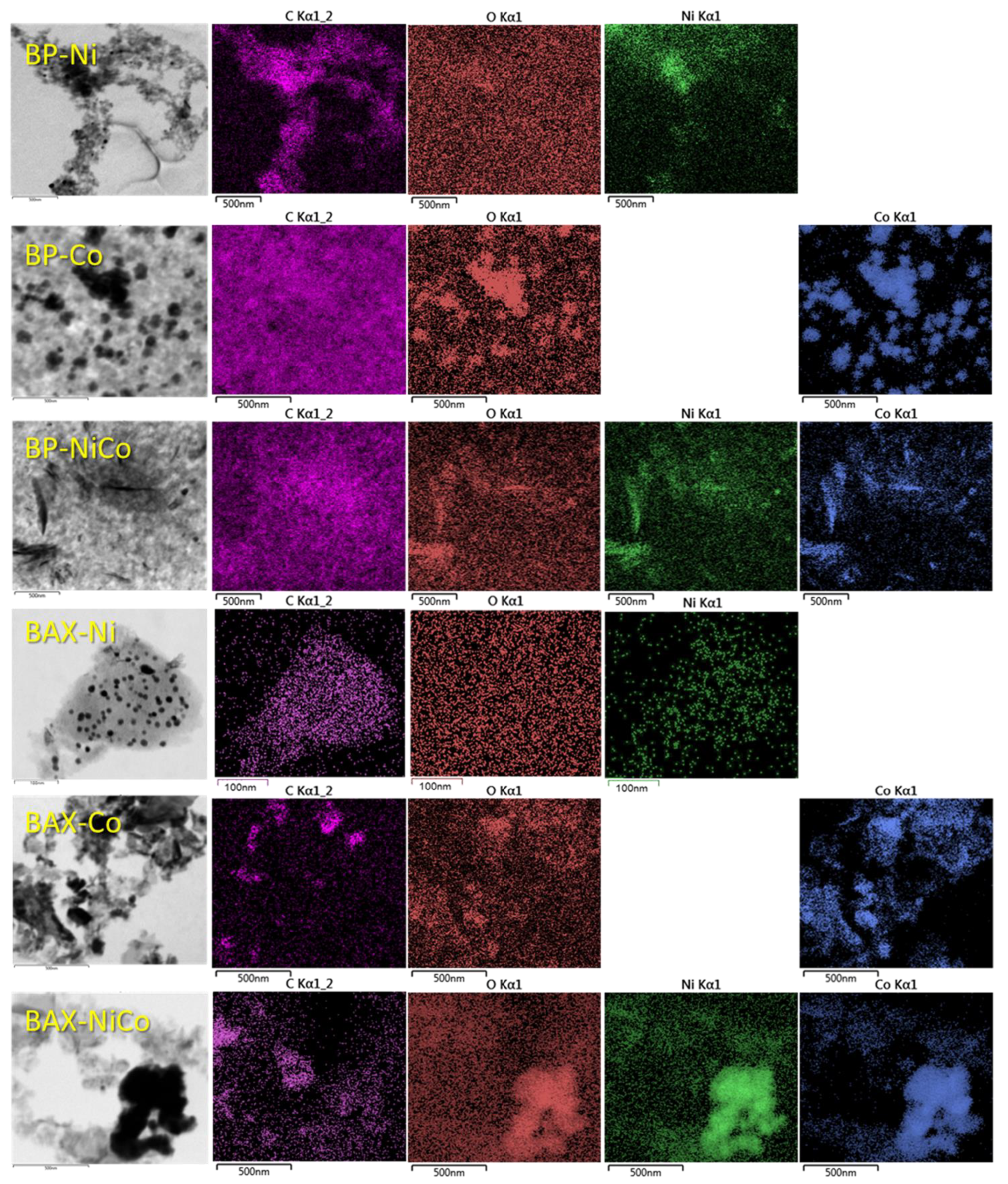

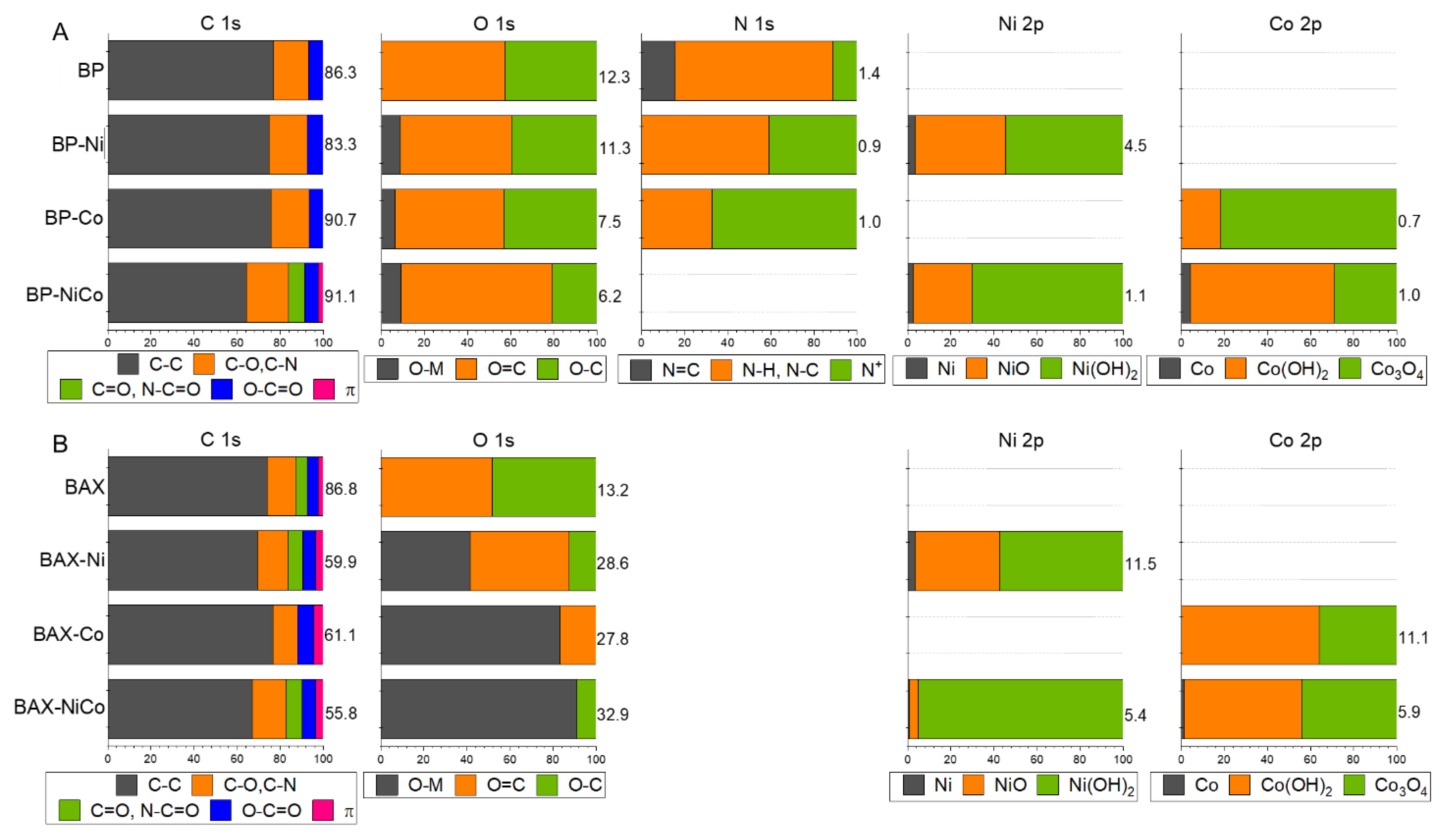
| Samples | SBET m2/g | Vtot cm3/g | V<0.7 cm3/g | Vmic cm3/g | Vmes cm3/g | Vmes/Vtot % |
|---|---|---|---|---|---|---|
| BP | 1609 | 2.705 | 0.001 | 0.532 | 2.173 | 80 |
| BP-Ni | 1201 | 1.919 | 0.063 | 0.361 | 1.558 | 81 |
| BP-Co | 1295 | 2.141 | 0.066 | 0.388 | 1.753 | 82 |
| BP-NiCo | 1161 | 1.889 | 0.067 | 0.356 | 1.533 | 81 |
| BAX | 2158 | 1.424 | 0.079 | 0.725 | 0.699 | 49 |
| BAX-Ni | 741 | 0.436 | 0.141 | 0.321 | 0.115 | 26 |
| BAX-Co | 658 | 0.425 | 0.113 | 0.272 | 0.153 | 36 |
| BAX-NiCo | 724 | 0.451 | 0.119 | 0.294 | 0.157 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florent, M.; Bandosz, T.J. Carbon Surface-Influenced Heterogeneity of Ni and Co Catalytic Sites as a Factor Affecting the Efficiency of Oxygen Reduction Reaction. Nanomaterials 2022, 12, 4432. https://doi.org/10.3390/nano12244432
Florent M, Bandosz TJ. Carbon Surface-Influenced Heterogeneity of Ni and Co Catalytic Sites as a Factor Affecting the Efficiency of Oxygen Reduction Reaction. Nanomaterials. 2022; 12(24):4432. https://doi.org/10.3390/nano12244432
Chicago/Turabian StyleFlorent, Marc, and Teresa J. Bandosz. 2022. "Carbon Surface-Influenced Heterogeneity of Ni and Co Catalytic Sites as a Factor Affecting the Efficiency of Oxygen Reduction Reaction" Nanomaterials 12, no. 24: 4432. https://doi.org/10.3390/nano12244432
APA StyleFlorent, M., & Bandosz, T. J. (2022). Carbon Surface-Influenced Heterogeneity of Ni and Co Catalytic Sites as a Factor Affecting the Efficiency of Oxygen Reduction Reaction. Nanomaterials, 12(24), 4432. https://doi.org/10.3390/nano12244432






