Magnetite Nanoparticles as Solar Photo-Fenton Catalysts for the Degradation of the 5-Fluorouracil Cytostatic Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe3O4 Nanoparticles
2.2. Characterization Techniques
2.3. Degradation Study by Solar Photo-Fenton Process
3. Results and Discussion
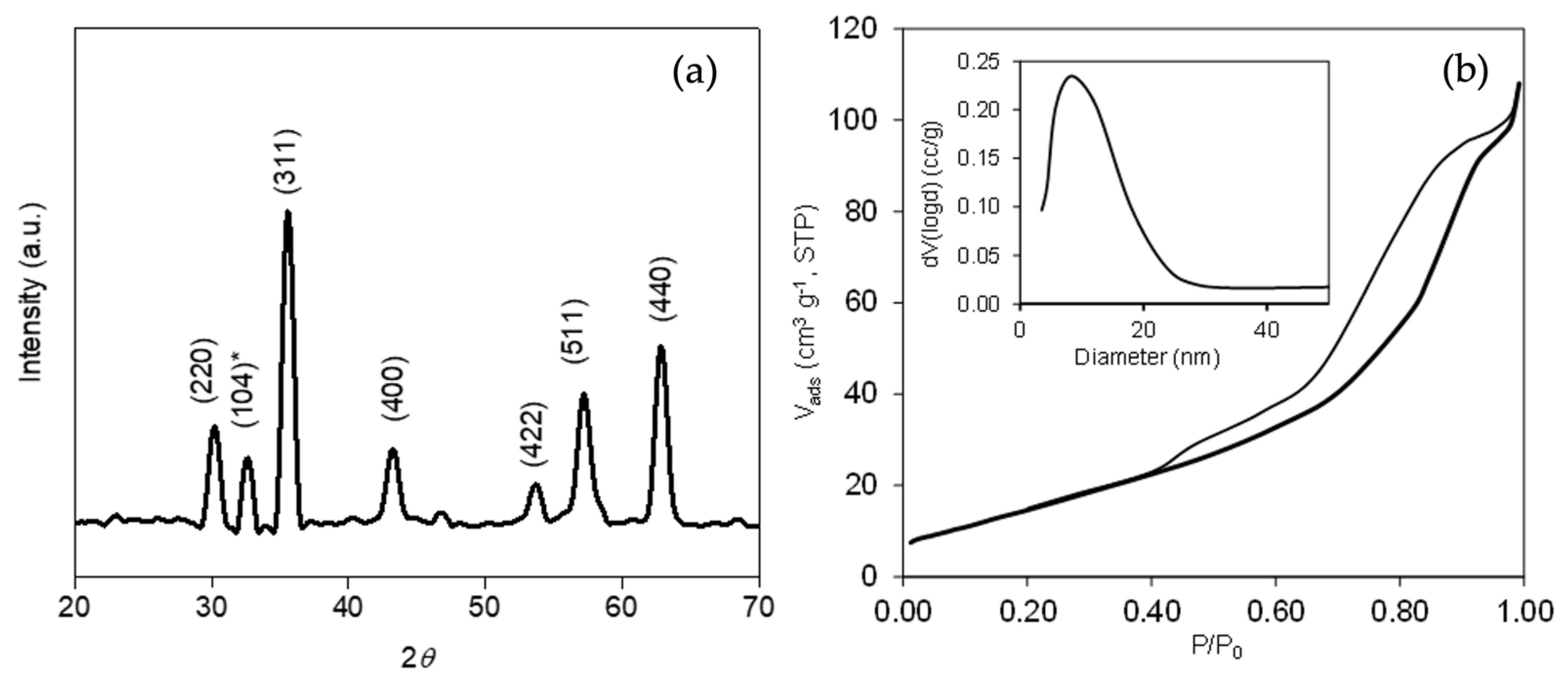
3.1. Materials Characterization
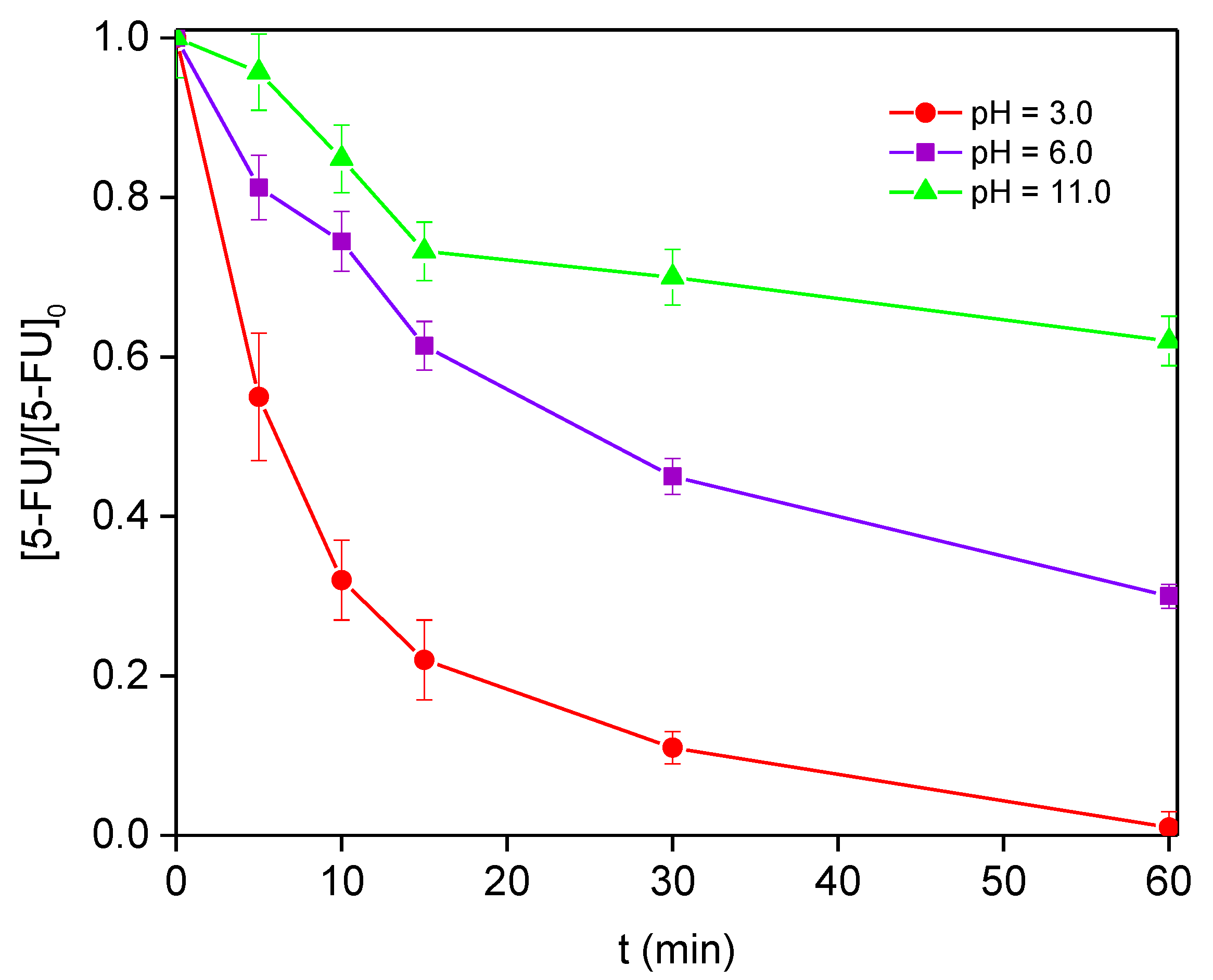
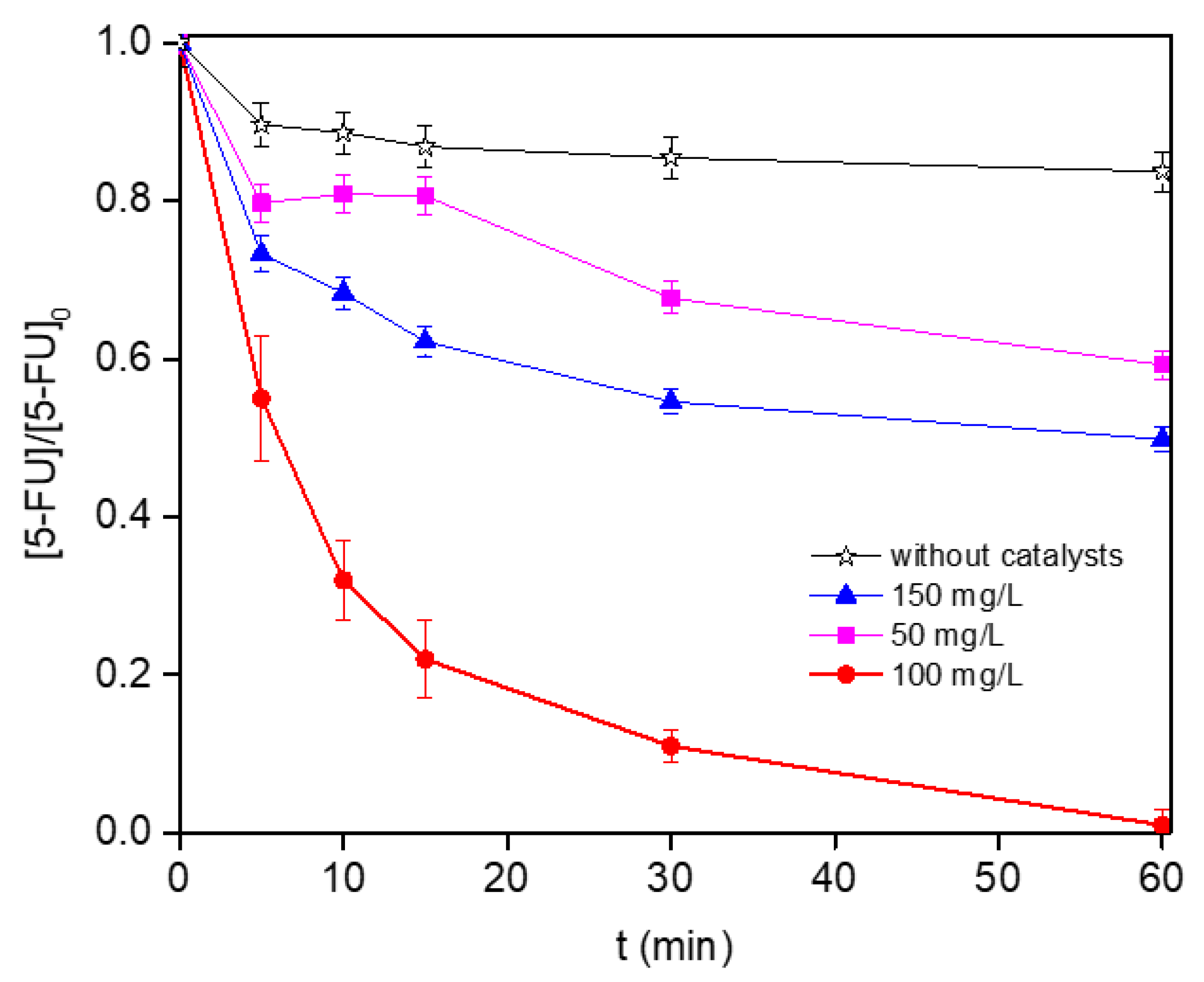
3.2. Degradation of 5-FU by Solar Photo-Fenton Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Vinoth Kumar, R.; Barbosa, M.O.; Ribeiro, A.R.; Morales-Torres, S.; Pereira, M.F.R.; Silva, A.M.T. Advanced oxidation technologies combined with direct contact membrane distillation for treatment of secondary municipal wastewater. Process Saf. Environ. Prot. 2020, 140, 111–123. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opris, O.; Gutoiu, S.; Leostean, C.; Lazar, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 nanocomposite for ibuprofen and paracetamol removal from aqueous solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mehta, D.; Dhangar, K.; Kumar, M. Environmental fate, distribution and state-of-the-art removal of antineoplastic drugs: A comprehensive insight. Chem. Eng. J. 2021, 407, 127–184. [Google Scholar] [CrossRef]
- Kümmerer, K.; Haiß, A.; Schuster, A.; Hein, A.; Ebert, I. Antineoplastic compounds in the environment—Substances of special concern. Environ. Sci. Pollut. Res. 2016, 23, 14791–14804. [Google Scholar] [CrossRef]
- Garcia-Costa, A.; Alves, A.; Madeira, L.; Santos, M. Oxidation processes for cytostatic drugs elimination in aqueous phase: A critical review. J. Environ. Chem. Eng. 2020, 9, 104709. [Google Scholar] [CrossRef]
- Zorrilla-Veloz, R.I.; Stelzer, T.; López-Mejías, V. Measurement and correlation of the solubility of 5-fluorouracil in pure and binary solvents. J. Chem. Eng. Data 2018, 63, 3809–3817. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Antonopoulou, M.; Sykiotou, M.; Εvgenidou, Ε.; Konstantinou, I.; Lambropoulou, D.A. Photo-Fenton and Fenton-like processes for the treatment of the antineoplastic drug 5-fluorouracil under simulated solar radiation. Environ. Sci. Pollut. Res. 2017, 24, 4791–4800. [Google Scholar] [CrossRef]
- Governo, M.; Santos, M.S.F.; Alves, A.; Madeira, L.M. Degradation of the cytostatic 5-fluorouracil in water by Fenton and photo-assisted oxidation processes. Environ. Sci. Pollut. Res. 2017, 24, 844–854. [Google Scholar] [CrossRef]
- Emídio, E.S.; Hammer, P.; Nogueira, R.F.P. Simultaneous degradation of the anticancer drugs 5-fluorouracil and cyclophosphamide using a heterogeneous photo-Fenton process based on copper-containing magnetites (Fe(3-x)Cu(x)O(4)). Chemosphere 2020, 241, 124990. [Google Scholar] [CrossRef]
- Chatzimpaloglou, A.; Christophoridis, C.; Fountoulakis, I.; Antonopoulou, M.; Vlastos, D.; Bais, A.; Fytianos, K. Photolytic and photocatalytic degradation of antineoplastic drug irinotecan. Kinetic study, identification of transformation products and toxicity evaluation. Chem. Eng. J. 2021, 405, 126866. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontàs, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In situ growth and crystallization of TiO2 on polymeric membranes for the photocatalytic degradation of diclofenac and 17α-ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Sanabria, P.; Scunderlick, D.; Wilde, M.L.; Lüdtke, D.S.; Sirtori, C. Solar photo-Fenton treatment of the anti-cancer drug anastrozole in different aqueous matrices at near-neutral pH: Transformation products identification, pathways proposal and in silico (Q)SAR risk assessment. Sci. Total Environ. 2021, 754, 142300. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.P.; da Rocha Sandim, L.; Bogo, D.; Barbosa, A.M.; Osugi, M.E.; Blanco, M.; de Oliveira, S.C.; de Fatima Cepa Matos, M.; Machulek, A., Jr.; Ferreira, V.S. Application of Fenton, photo-Fenton, solar photo-Fenton, and UV/H2O2 to degradation of the antineoplastic agent mitoxantrone and toxicological evaluation. Environ. Sci. Pollut. Res. 2013, 20, 2352–2361. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, X.; Huang, X.; Yang, H. Adsorption and heterogeneous Fenton catalytic performance for magnetic Fe3O4/reduced graphene oxide aerogel. J. Mater. Sci. 2020, 55, 15695–15708. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef] [Green Version]
- Pastrana-Martínez, L.M.; Pereira, N.; Lima, R.; Faria, J.L.; Gomes, H.T.; Silva, A.M.T. Degradation of diphenhydramine by photo-Fenton using magnetically recoverable iron oxide nanoparticles as catalyst. Chem. Eng. J. 2015, 261, 45–52. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on activated carbon and related materials. ChemSusChem 2011, 4, 1712–1730. [Google Scholar] [CrossRef]
- Zang, H.; Miao, C.; Shang, J.; Liu, Y.; Liu, J. Structural effects on the catalytic activity of carbon-supported magnetite nanocomposites in heterogeneous Fenton-like reactions. RSC Adv. 2018, 8, 16193–16201. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Arriaga, F.; Esplugas, S.; Giménez, J. Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res. 2010, 44, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; David, A.; Govil, T.; Rauniyar, S.; Rathinam, N.K.; Goh, K.M.; Sani, R.K. Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes 2020, 8, 747. [Google Scholar] [CrossRef]
- Peternele, W.S.; Monge Fuentes, V.; Fascineli, M.L.; Rodrigues da Silva, J.; Silva, R.C.; Lucci, C.M.; Bentes de Azevedo, R. Experimental investigation of the co-precipitation method: An approach to obtain magnetite and maghemite nanoparticles with improved properties. J. Nanomater. 2014, 2014, 682985. [Google Scholar] [CrossRef] [Green Version]
- Sulistyaningsih, T.; Santosa, S.; Siswanta, D.; Rusdiarso, B. Synthesis and characterization of magnetites obtained from mechanically and sonochemically assissted co-precipitation and reverse co-precipitation methods. Int. J. Mater. Mech. Manuf. 2017, 5, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Munasir, M.; Kusumawati, R. Synthesis and characterization of Fe3O4/rGO composite with wet-mixing (ex-situ) process. J. Phys. Conf. Ser. 2019, 1171, 012048. [Google Scholar] [CrossRef]
- López, J.; González, F.; Bonilla, F.; Zambrano, G.; Gomez, M. Synthesis and characterization of Fe3O4 magnetic nanofluid. Rev. Latinoam. Metal. Mater. 2010, 30, 60–66. [Google Scholar]
- Han, R.; Li, W.; Pan, W.; Zhu, M.; Zhou, D.; Li, F. 1D magnetic materials of Fe3O4 and Fe with high performance of microwave absorption fabricated by electrospinning method. Sci. Rep. 2014, 4, 7493. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; Ang, S.Y.; Ye, E.Y.; Sullivan, M.; Zhang, J.; Lin, M. Heterogeneous photo-Fenton reaction on hematite (α-Fe2O3) {104}, {113} and {001} surface facets. Phys. Chem. Chem. Phys. PCCP 2015, 17, 25333–25341. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption properties of magnetic magnetite nanoparticle for coexistent Cr(VI) and Cu(II) in mixed solution. Water 2020, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Rahmayanti, M. Synthesis of magnetite nanoparticles using reverse co-precipitation method with NH4OH as precipitating agent and its stability test at various pH. J. Sci. Technol. 2020, 9, 54–58. [Google Scholar] [CrossRef]
- De Oliveira Guidolin, T.; Possolli, N.M.; Polla, M.B.; Wermuth, T.B.; Franco de Oliveira, T.; Eller, S.; Klegues Montedo, O.R.; Arcaro, S.; Cechinel, M.A.P. Photocatalytic pathway on the degradation of methylene blue from aqueous solutions using magnetite nanoparticles. J. Clean. Prod. 2021, 318, 128556. [Google Scholar] [CrossRef]
- Vieira, Y.; Silvestri, S.; Leichtweis, J.; Jahn, S.L.; de Moraes Flores, É.M.; Dotto, G.L.; Foletto, E.L. New insights into the mechanism of heterogeneous activation of nano–magnetite by microwave irradiation for use as Fenton catalyst. J. Environ. Chem. Eng. 2020, 8, 103787. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green synthesis and characterization of magnetite (Fe3O4) nanoparticles using Chromolaena odorata root extract for smart nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Madeira, L.M. Wet peroxide oxidation of dye-containing wastewaters using nanosized Au supported on Al2O3. Catal. Today 2017, 280, 165–175. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M.; Mata, G.; Vicente, M.A.; Rojas-Cervantes, M.L.; López-Peinado, A.J.; Martín-Aranda, R.M. Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl. Catal. B Environ. 2007, 71, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Huang, W. Homogeneous and Heterogeneous Fenton and Photo-Fenton Processes: Impact of Iron Complexing Agent Ethylenediamine-N,N′-disuccinic Acid (EDDS). Ph.D. Thesis, Université Blaise Pascal-Clermont-Ferrand II, Clermont-Ferrand, France, 2012. [Google Scholar]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Rubio, D.; Nebot, E.; Casanueva, J.F.; Pulgarin, C. Comparative effect of simulated solar light, UV, UV/H2O2 and photo-Fenton treatment (UV–Vis/H2O2/Fe2+,3+) in the Escherichia coli inactivation in artificial seawater. Water Res. 2013, 47, 6367–6379. [Google Scholar] [CrossRef]
- Xu, M.; Wu, C.; Zhou, Y. 4. Advancements in the Fenton process for wastewater treatment. In Advanced Oxidation Processes—Applications, Trends, and Prospects; IntechOpen: London, UK, 2020; pp. 61–79. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. Int. Union Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall: New York, NY, USA, 2001. [Google Scholar]
- Newcombe, G.; Hayes, R.; Drikas, M. Granular activated carbon: Importance of surface properties in the adsorption of naturally occurring organics. Colloids Surf. A Physicochem. Eng. Asp. 1993, 78, 65–71. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Silva, A.M.T.; Faria, J.L. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Appl. Surf. Sci. 2018, 458, 839–848. [Google Scholar] [CrossRef]
- ISO 6332:1988. Water Quality: Determination of Iron. Spectrometric Method Using 1,10-phenanthroline; International Organization for Standardization: Geneva, Switzerland, 1982.




| Parameter | pH | Catalyst Load (mg L−1) | H2O2 (mM) | X5-FU,1h (%) | XTOC,1h (%) | Fe-leached (mg L−1) |
|---|---|---|---|---|---|---|
| pH value | 3.0 | 100 | 58 | 100 | 76 | 1.97 |
| 6.0 | 100 | 58 | 70 | 48 | 0.40 | |
| 11.0 | 100 | 58 | 38 | 23 | 1.00 | |
| Catalyst load | 3.0 | 0 | 58 | 16 | 6 | - |
| 3.0 | 50 | 58 | 41 | 28 | 0.27 | |
| 3.0 | 100 | 58 | 100 | 76 | 1.97 | |
| 3.0 | 150 | 58 | 50.2 | 30 | 2.10 | |
| H2O2 concentration | 3.0 | 100 | 0 | 10 | 4 | - |
| 3.0 | 100 | 15 | 40 | 19 | 1.06 | |
| 3.0 | 100 | 30 | 55 | 28 | 1.71 | |
| 3.0 | 100 | 58 | 100 | 76 | 1.97 | |
| 3.0 | 100 | 82 | 30 | 11 | 0.73 |
| Blank Experiments | pH | Catalyst Load (mg L−1) | H2O2 (mM) | X5-FU,1h (%) | XTOC,1h (%) | Fe-leached (mg L−1) |
|---|---|---|---|---|---|---|
| Fe3O4/Solar | 3.0 | 100 | 0 | 10 | 4 | 0.5 |
| H2O2/Solar | 3.0 | 0 | 58 | 16 | 6 | - |
| Fe3O4/H2O2 | 3.0 | 100 | 58 | 80 | 26 | 0.8 |
| FeSO4/H2O2/Solar | 3.0 | 100 | 58 | 42 | 30 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Poyatos, L.T.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Magnetite Nanoparticles as Solar Photo-Fenton Catalysts for the Degradation of the 5-Fluorouracil Cytostatic Drug. Nanomaterials 2022, 12, 4438. https://doi.org/10.3390/nano12244438
Pérez-Poyatos LT, Morales-Torres S, Maldonado-Hódar FJ, Pastrana-Martínez LM. Magnetite Nanoparticles as Solar Photo-Fenton Catalysts for the Degradation of the 5-Fluorouracil Cytostatic Drug. Nanomaterials. 2022; 12(24):4438. https://doi.org/10.3390/nano12244438
Chicago/Turabian StylePérez-Poyatos, Lorena T., Sergio Morales-Torres, Francisco J. Maldonado-Hódar, and Luisa M. Pastrana-Martínez. 2022. "Magnetite Nanoparticles as Solar Photo-Fenton Catalysts for the Degradation of the 5-Fluorouracil Cytostatic Drug" Nanomaterials 12, no. 24: 4438. https://doi.org/10.3390/nano12244438
APA StylePérez-Poyatos, L. T., Morales-Torres, S., Maldonado-Hódar, F. J., & Pastrana-Martínez, L. M. (2022). Magnetite Nanoparticles as Solar Photo-Fenton Catalysts for the Degradation of the 5-Fluorouracil Cytostatic Drug. Nanomaterials, 12(24), 4438. https://doi.org/10.3390/nano12244438









