Ag-Decorated Iron Oxides-Silica Magnetic Nanocomposites with Antimicrobial and Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Ag-Decorated IONPs
2.3. Structural and Morphologic Characterization
2.4. Optical Properties
2.5. Magnetic Properties
2.6. Photocatalytic Activity
2.7. Antimicrobial Activity
3. Results and Discussion
3.1. Morphology and Structure
3.2. Textural Analysis
3.3. Magnetic Properties
3.4. Optical Properties
3.5. Antimicrobial Activities
3.6. Nanosorption and Photocatalytic Activities
- Formation of the MB de-methylated intermediates, called Azure A, Azure B, and thionine acetate, with the preservation of the chromophore poly-heterocyclic group and the release of Cl− (most ionized, in the independent state).
- Breaking the poly-heterocyclic linkages into central, followed by side, aromatic rings, with the formation of fragments and possible residual single-ring structure products.
- Degradation of resulting fragments into various intermediate species (aniline, phenol, carboxylate species, R-NH3+, etc.).
- Degradation of intermediate species, until final products, which are volatile low molecular weight and less toxic products (CO2, H2O, SO42−, and NO3−).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belessiotis, G.V.; Falara, P.P.; Ibrahim, I.; Kontos, A.G. Magnetic Metal Oxide-Based Photocatalysts with Integrated Silver for Water Treatment. Materials 2022, 15, 4629. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.C.L.; Mortari, S.R.; Vizzotto, B.S.; Chuy, G.; Santos, C.; Brum, L.F.W.; Silva, W.L. Iron oxide nanocatalyst with titanium and silver nanoparticles: Synthesis, characterization and photocatalytic activity on the degradation of Rhodamine B dye. Sci. Rep. 2020, 10, 3055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.P.; Reis, C.P.; Robalo, T.T.; Melo Jorge, M.E.; Ferreira, P.; Gonçalves, J.; Hajalilou, A.; Cruz, M.M. Assisted Synthesis of Coated Iron Oxide Nanoparticles for Magnetic Hyperthermia. Nanomaterials 2022, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Musat, V.; Stanica, N.; Anghel, E.M.; Atkinson, I.; Culita, D.C.; Polosan, S.; Crintea, L.; Cantaragiu Ceoromila, A.; Buruiana, C.-T.; Carp, O. Magnetic Core-Shell Iron Oxides-Based Nanophotocatalysts and Nanoadsorbents for Multifunctional Thin Films. Membranes 2022, 12, 466. [Google Scholar] [CrossRef]
- Patil, S.R. 18-Metal oxide-based composites as photocatalysts. In Advances in Metal Oxides and Their Composites for Emerging Applications; Delekar, S.D., Ed.; Metal Oxide Series; Elsevier: Amsterdam, The Netherlands, 2022; pp. 633–672. [Google Scholar] [CrossRef]
- Balu, S.; Chuaicham, C.; Balakumar, V.; Rajendran, S.; Sasaki, K.; Sekar, K.; Maruthapillai, A. Recent development on core-shell photo(electro)catalysts for elimination of organic compounds from pharmaceutical wastewater. Chemosphere 2022, 298, 134311. [Google Scholar] [CrossRef]
- Hirad, A.H.; Ansari, S.A.; Ali, M.A.E.; Egeh, M.A. Microwave-mediated synthesis of iron oxide nanoparticles: Photocatalytic, antimicrobial and their cytotoxicity assessment. Proc. Biochem. 2022, 118, 205–214. [Google Scholar] [CrossRef]
- Ningsih, L.A.; Yoshid, M.; Sakai, A.; Lin, K.-Y.A.; Wu, K.C.W.; Catherine, H.N.; Ahamad, T.; Hu, C. Ag-modified TiO2/SiO2/Fe3O4 sphere with core-shell structure for photo-assisted reduction of 4-nitrophenol. Environ. Res. 2022, 214, 113690. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid. Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Kang, H.J.H.; Ali, R.F.; Paul, M.T.Y.; Radford, M.J.; Andreu, I.; Leea, A.W.H.; Gates, B.D. Tunable functionalization of silica coated iron oxide nanoparticles achieved through a silanol–alcohol condensation reaction. Chem. Commun. 2019, 55, 10452–10455. [Google Scholar] [CrossRef]
- Aguda, O.N.; Lateef, A. Recent advances in functionalization of nanotextiles: A strategy to combat harmful microorganisms and emerging pathogens in the 21st century. Helyon 2022, 8, e09761. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Wozniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization—The way for advanced applications of smart materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Stefan, M.; Pana, O.; Leostean, C.; Bele, C.; Silipas, D.; Senila, M.; Gautron, E. Synthesis and characterization of Fe3O4-TiO2 core-shell nanoparticles. J. Appl. Phys. 2014, 116, 114312. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Henley, S.J.; Emerson, N.G.; Silva, S.R.P. From 1D and 2D ZnO nanostructures to 3D hierarchical structures with enhanced gas sensing properties. Nanoscale 2014, 6, 235–247. [Google Scholar] [CrossRef]
- Bușilă, M.; Mușat, V.; Textor, T.; Mahltig, B. Synthesis and characterization of antimicrobial textile finishing based on Ag:ZnO nanoparticles/chitosan biocomposites. RSC Adv. 2015, 5, 21562–21571. [Google Scholar] [CrossRef]
- Li, J.W.; Yang, L.W.; Zhou, Z.F.; Chu, P.K.; Wang, X.H.; Zhou, J.; Li, L.T.; Sun, C.Q. Bandgap Modulation in ZnO by Size, Pressure, and Temperature. J. Phys. Chem. C 2010, 114, 13370–13374. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Manabeng, M.; Mwankemwa, B.S.; Ocaya, R.O.; Motaung, T.E.; Malevu, T.D. A Review of the Impact of Zinc Oxide Nanostructure Morphology on Perovskite Solar Cell Performance. Processes 2022, 10, 1803. [Google Scholar] [CrossRef]
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Ibañez, A.B.; Panda, P.K.; Santana, G.; Vega, H.A.; Suar, M.; Rodelo, C.G.; Kaushik, A.K.; et al. Bio-acceptable 0D and 1D ZnO nanostructures for cancer diagnostics and treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Shingange, K.; Tshabalala, Z.P.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Motaung, D.E.; Mhlongo, G.H. 0D to 3D ZnO nanostructures and their luminescence, magnetic and sensing properties: Influence of pH and annealing. Mater. Res. Bull. 2017, 85, 52–63. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Noorden, A.F.A.; Tan, M.L.P.; Jamaluddin, H.; Hamid, F.A.; Ahmad, M.K.; Hashim, U.; Ahmad, M.R.; Sultan, S.M. Review—Three Dimensional Zinc Oxide Nanostructures as an Active Site Platform for Biosensor: Recent Trend in Healthcare Diagnosis. J. Electrochem. Soc. 2020, 167, 137501. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef] [Green Version]
- Banihashemi, M.; Dalali, N.; Sehati, N.; Farajmand, B. Decoration of Fe3O4@SiO2@ZnO as a high performance nanosorbent on a stir bar microextraction device for preconcentration and determination of cadmium in real water samples. Microchem. J. 2020, 154, 104599. [Google Scholar] [CrossRef]
- Darabi, R.R.; Jahanshahi, M.; Peyravi, M. A support assisted by photocatalytic Fe3O4/ZnO nanocomposite for thin-film forward osmosis membrane. Chem. Eng. Res. Des. 2018, 133, 11–25. [Google Scholar] [CrossRef]
- Shabib, F.; Fazaeli, R.; Aliyan, H.; Richeson, D. Hierarchical mesoporous plasmonic Pd-FeO/NiFe-LDH composites: Characterization, and kinetic study of a photodegradation catalyst for aqueous metoclopramide. Environ. Technol. Innov. 2022, 27, 102515. [Google Scholar] [CrossRef]
- Alburaih, H.A.; Aman, S.; Ahmad, N.; Ejaz, S.R.; Khosa, R.Y.; Abid, A.G.; Manzoor, S.; Farid, H.M.T.; Waheed, M.S.; Taha, T.A. Synergistic photodegradation of methylene blue by Sm doped Fe2O3 photocatalyst under sunlight. Chin. J. Phys. 2022. [Google Scholar] [CrossRef]
- Chang, C.-W.; Wu, H.-T.; Huang, S.-H.; Chen, C.-K.; Un, I.-W.; Yen, T.-J. Single-crystalline heterostructure of ZnO nanowire arrays on large Ag microplates and its photocatalytic activity. Acta Mater. 2013, 61, 6993–6999. [Google Scholar] [CrossRef]
- Yilmaz, M.; Mengelizadeh, N.; Saloot, M.K.; Shahbaksh, S.; Balarak, D. Facile synthesis of Fe3O4/ZnO/GO photocatalysts for decolorization of acid blue 113 under solar, visible and UV lights. Mater. Sci. Semicond. Process. 2022, 144, 106593. [Google Scholar] [CrossRef]
- Harikumar, B.; Kokilavani, S.; Khan, S. Magnetically separable N/S doped Fe3O4 embedded on MoO3 nanorods for photodegradation of cefixime, Cr(VI) reduction, and its genotoxicity study. Chem. Eng. J. 2022, 446, 137273. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Chen, Y.; Fan, H.; et al. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloys Compds. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, B.; Zhang, Y.; Zhang, N.; Yang, Z. Dopant segregation and CO adsorption on doped Fe3O4 (1 1 1) surfaces: A first-principle study. J. Catal. 2018, 364, 291–296. [Google Scholar] [CrossRef]
- Reddy, G.K.; Gunasekera, K.; Boolchand, P.; Dong, J.; Smirniotis, P.G. High Temperature Water Gas Shift Reaction over Nanocrystalline Copper Codoped-Modified Ferrites. J. Phys. Chem. C 2011, 115, 7586–7595. [Google Scholar] [CrossRef]
- Pimpliskar, P.V.; Motekar, S.C.; Umarji, G.G.; Lee, W.; Arbuj, S.S. Synthesis of silver-loaded ZnO nanorods and their enhanced photocatalytic activity and photoconductivity study. Photochem. Photobiol. Sci. 2019, 18, 1503–1511. [Google Scholar] [CrossRef]
- Uma, K.; Arjun, N.; Pan, G.-T.; Yang, T.C.-K. The photodeposition of surface plasmon Ag metal on SiO2@α-Fe2O3 nanocomposites sphere for enhancement of the photo-Fenton behavior. Appl. Surf. Sci. 2017, 425, 377–383. [Google Scholar] [CrossRef]
- Long, L.; Ju, W.; Yang, H.-Y.; Li, Z. Dimensional Design for Surface-Enhanced Raman Spectroscopy. ACS Mater. Au 2022, 2, 552–575. [Google Scholar] [CrossRef]
- Han, D.; Yao, J.; Quan, Y.; Gao, M.; Yang, J. Plasmon-coupled Charge Transfer in FSZA Core-shell Microspheres with High SERS Activity and Pesticide Detection. Sci. Rep. 2019, 9, 13876. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.T.H.; Vu, X.H.; Dien, N.D.; Trang, T.T.; Chi, T.T.K.; Phuong, P.H.; Nghia, N.T. Ag nanoparticles on ZnO nanoplates as a hybrid SERS-active substrate for trace detection of methylene blue. RSC Adv. 2022, 12, 7850–7863. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Xu, Y.; Tian, H.; Wu, D.; Sun, Y. Synthesis of Fe3O4\SiO2\Ag nanoparticles and its application in surface-enhanced Raman scattering. J. Solid State Chem. 2010, 183, 2968–2973. [Google Scholar] [CrossRef]
- Thu, P.T.; Thinh, V.D.; Lam, V.D.; Bach, T.N.; Phong, L.T.H.; Tung, D.H.; Manh, D.H.; Van Khien, N.; Anh, T.X.; Le, N.T.H. Decorating of Ag and CuO on ZnO Nanowires by Plasma Electrolyte Oxidation Method for Enhanced Photocatalytic Efficiency. Catalysts 2022, 12, 801. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Cui, G.; Tian, Y.; Yan, S. Multifunctional magnetic core–shell dendritic mesoporous silica nanospheres decorated with tiny Ag nanoparticles as a highly active heterogeneous catalyst. Appl. Surf. Sci. 2016, 360, 252–262. [Google Scholar] [CrossRef]
- Godfrey, I.J.; Dent, A.J.; Parkin, I.P.; Maenosono, S.; Sankar, G. Following the Formation of Silver Nanoparticles Using In Situ X-ray Absorption Spectroscopy. ACS Omega 2020, 5, 13664–13671. [Google Scholar] [CrossRef]
- Spina, R.L.; Mehn, D.; Fumagalli, F.; Holland, M.; Reniero, F.; Rossi, F.; Gilliland, D. Synthesis of Citrate-Stabilized Silver Nanoparticles Modified by Thermal and pH Preconditioned Tannic Acid. Nanomaterials 2020, 10, 2031. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhao, Z.S.; Jiang, G.B. Coating Fe3O4 Magnetic Nanoparticles with Humic Acid for High Efficient Removal of Heavy Metals in Water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Wang, P.; Tang, Z.; Niu, H.; Cai, Y.; Wu, F.; Wang, H.; Meng, W.; Giesy, J.P. Surfactant-modified flowerlike layered double hydroxide-coated magnetic nanoparticles for preconcentration of phthalate esters from environmental water samples. J. Chromatogr. A 2015, 1414, 22–30. [Google Scholar] [CrossRef]
- Thottoli, Z.A.K.; Unni, A.K.A. Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles. J. Nanostructure Chem. 2013, 3, 56. [Google Scholar] [CrossRef]
- Upadhyay, S.; Parekh, K.; Pandey, B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J. Alloys Compd. 2016, 678, 478–485. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Mekhnache, M.; Drici, A.; Hamideche, L.S.; Benzarouk, H.; Amara, A.; Cattin, L.; Bernède, J.; Guerioune, M. Properties of ZnO thin films deposited on (glass, ITO and ZnO:Al) substrates. Superlattice. Microstruct. 2011, 49, 510–518. [Google Scholar] [CrossRef]
- Prasada Rao, T.; Santhoshkumar, M.C. Effect of thickness on structural, optical and electrical properties of nanostructured ZnO thin films by spray pyrolysis. Appl. Surf. Sci. 2009, 255, 4579. [Google Scholar] [CrossRef]
- Yakuphanoghu, F.; Sekerci, M.; Balaban, A. The effect of film thickness on the optical absorption edge and optical constants of the Cr(III) organic thin films. Opt. Mater. 2005, 27, 1369–1374. [Google Scholar] [CrossRef]
- Elyamny, S.; Eltarahony, M.; Abu-Serie, M.; Nabil, M.M.; Kashyout, A.E.-H.B. One-pot fabrication of Ag @Ag2O core–shell nanostructures for biosafe antimicrobial and antibioflm applications. Sci. Rep. 2021, 11, 22543. [Google Scholar] [CrossRef]
- Tagliazucchi, M. 1. Introduction to Chemically Modified Nanopores and Nanochannels. In Chemically Modified Nanopores and Nanochannels, 1st ed.; Tagliazucchi, M., Szleifer, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Dudric, R.; Souca, G.; Szatmari, A.; Szilard, T.; Nitica, S.; Iacovita, C.; Moldovan, A.I.; Stiufiuc, R.; Tetean, R.; Burzo, E. Magnetite Nanoparticles for Medical Applications. AIP Conf. Proc. 2020, 2218, 030014. [Google Scholar] [CrossRef]
- Mikhaylova, A.B.; Sirotinkin, V.P.; Fedotov, M.A.; Korneyev, V.P.; Shamray, B.F.; Kovalenko, L.V. Quantitative Determination of Content of Magnetite and Maghemite in Their Mixtures by X-Ray Diffraction Methods. Inorg. Mater. Appl. Res. 2016, 7, 130–136. [Google Scholar] [CrossRef]
- Zygieło, M.; Piotrowski, P.; Witkowski, M.; Cichowicz, G.; Szczytko, J.; Królikowska, A. Reduced Self-Aggregation and Improved Stability of Silica-Coated Fe3O4/Ag SERS-Active Nanotags Functionalized with 2-Mercaptoethanesulfonate. Front. Chem. 2021, 9, 697595. [Google Scholar] [CrossRef] [PubMed]
- Boucherit, N.; Hugot-Le Goff, A.; Joiret, S. Raman studies of corrosion films grown on Fe and Fe-6Mo in pitting conditions. Corros. Sci. 1991, 32, 497. [Google Scholar] [CrossRef]
- Cuenca, J.A.; Bugler, K.; Taylor, S.; Morgan, D.; Williams, P.; Bauer, J.; Porch, A. Study of the magnetite to maghemite transition using microwave permittivity and permeability measurements. J. Phys. Condens. Matter. 2016, 28, 106002. [Google Scholar] [CrossRef] [Green Version]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Zeferino, R.S.; Flores, M.B.; Pal, U. Photoluminescence and Raman Scattering in Ag-doped ZnO Nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Michałowska, A.; Kudelski, A. The First Silver-Based Plasmonic Nanomaterial for Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy with Magnetic Properties. Molecules 2022, 27, 3081. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.I.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO nanocomposite with highly performance photocatalytic activity for degradation methylene blue under visible light irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Lee, E.L.; Wachs, I.E. In Situ Raman Spectroscopy of SiO2-Supported Transition Metal Oxide Catalysts: An Isotopic 18O-16O Exchange Study. J. Phys. Chem. C 2008, 112, 112–6487. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (IUPAC Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Patescu, R.E.; Dragne, M.; Stanica, N.; Oprea, O. o-Vanillin functionalized mesoporous silica—Coated magnetite nanoparticles for efficient removal of Pb(II) from water. J. Solid State. Chem. 2016, 238, 311–320. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Khan, M.; Etim, U.J.; Naeem, M. Effective adsorptive performance of Fe3O4@SiO2 core shell spheres for methylene blue: Kinetics, isotherm and mechanism. J. Porous Mater. 2019, 26, 1465–1474. [Google Scholar] [CrossRef]
- Stoicescu, C.S.; Culita, D.; Stanica, N.; Papa, F.; State, R.N.; Munteanu, G. Temperature programmed reduction of a core-shell synthetic magnetite: Dependence on the heating rate of the reduction mechanism. Thermochim. Acta 2022, 709, 179146. [Google Scholar] [CrossRef]
- Amarjargal, A.; Tijing, L.D.; Im, I.-T.; Kim, C.S. Simultaneous preparation of Ag/Fe3O4 core–shell nanocomposites with enhanced magnetic moment and strong antibacterial and catalytic properties. Chem. Eng. J. 2013, 226, 243–254. [Google Scholar] [CrossRef]
- Shah, M.T.; Alveroglu, E. Facile synthesis of nanogels modified Fe3O4@Ag NPs for the efficient adsorption of bovine & human serum albumin. Mater. Sci. Eng. C 2021, 118, 111390. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Huang, L.; Wang, Y.; Li, S.; Ren., N. Improving edge quality and optical transmittance of Ag films on glass substrates by selective nanosecond pulsed laser ablation using various scanning methods. J. Mater. Sci. Mater. Electron. 2019, 30, 13729–13739. [Google Scholar] [CrossRef]
- Yulfriska, N.; Rianto, D.; Murti, F.; Darvina, Y.; Ramli, R. Optical Properties of Fe3O4 Thin Films Prepared from the Iron Sand by Spin Coating Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 335, 012010. [Google Scholar] [CrossRef] [Green Version]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Matusoiu, F.; Negrea, A.; Nemes, N.S.; Ianasi, C.; Ciopec, M.; Negrea, P.; Duteanu, N.; Ianasi, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial Perspectives of Active SiO2FexOy/ZnO Composites. Pharmaceutics 2022, 14, 2063. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Genet. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem. Eng. J. 2014, 251, 17–24. [Google Scholar] [CrossRef]
- Gabelica, I.; Curkovic, L.; Mandic, V.; Panžic, I.; Ljubas, D.; Zadro, K. Rapid Microwave-Assisted Synthesis of Fe3O4/SiO2/TiO2 Core-2-Layer-Shell Nanocomposite for Photocatalytic Degradation of Ciprofloxacin. Catalysts 2021, 11, 1136. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Tseng, W.J.; Chuang, Y.-C.; Chen, Y.-A. Mesoporous Fe3O4@Ag@TiO2 nanocomposite particles for magnetically recyclable photocatalysis and bactericide. Adv. Powder Technol. 2018, 29, 664–671. [Google Scholar] [CrossRef]
- Dagher, S.; Soliman, A.; Ziout, A.; Tit, N.; Hilal-Alnaqbi, A.; Khashan, S.; Alnaimat, F.; Abu Qudeiri, J. Photocatalytic removal of methylene blue using titania-and silica-coated magnetic nanoparticles. Mater. Res. Express 2018, 5, 065518. [Google Scholar] [CrossRef] [Green Version]
- Ghasemy-Piranloo, F.; Bavarsiha, F.; Dadashian, S.; Rajabi, M. Synthesis of core/shell/shell Fe3O4/SiO2/ZnO nanostructure composite material with cubic magnetic cores and study of the photo-degradation ability of methylene blue. J. Aust. Ceram. Soc. 2020, 56, 507–515. [Google Scholar] [CrossRef]
- Loka, C.; Lee, K.-S. Enhanced Visible-Light-Driven Photocatalysis of Ag/Ag2O/ZnO Nanocomposite Heterostructures. Nanomaterials 2022, 12, 2528. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wei, B.; Wang, D.; Song, H.; Zhai, H.; Li, X. Synthesis of Fe3O4@SiO2@ZnO–Ag core–shell microspheres for the repeated photocatalytic degradation of rhodamine B under UV irradiation. J. Mol. Catal. A Chem. 2015, 406, 97–105. [Google Scholar] [CrossRef]
- Zahornyi, M.M.; Tyschenko, N.I.; Lobunets, T.F.; Kolomys, O.F.; Strelchuk, V.V.; Naumenko, K.S.; Biliavska, L.O.; Zahorodnia, S.D.; Lavrynenko, O.M.; Ievtushenko, A.I. The Ag Influence on the Surface States of TiO2, Optical Activity and Its Cytotoxicity. J. Nano-Electron. Phys. 2021, 13, 06009. [Google Scholar] [CrossRef]
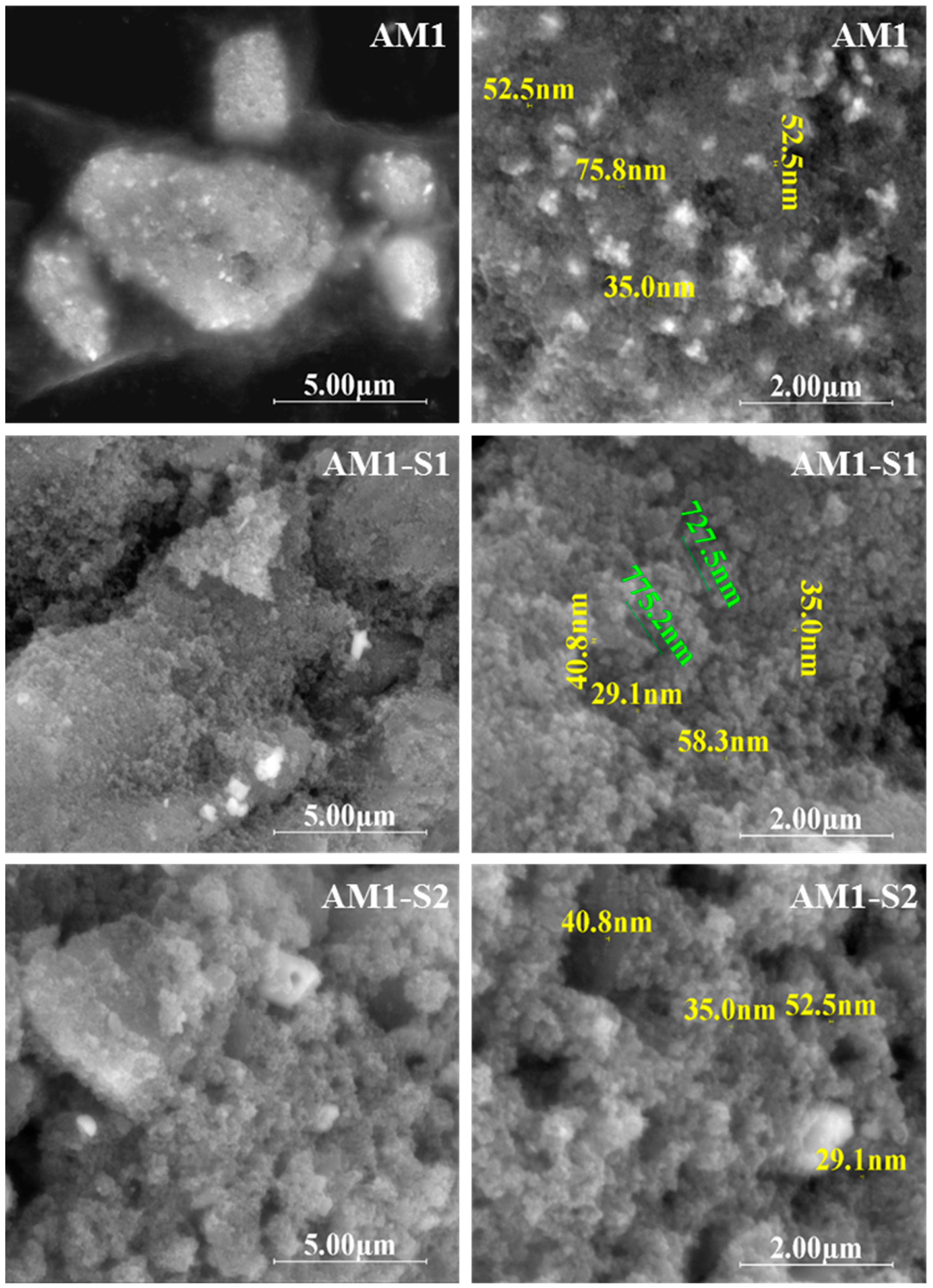

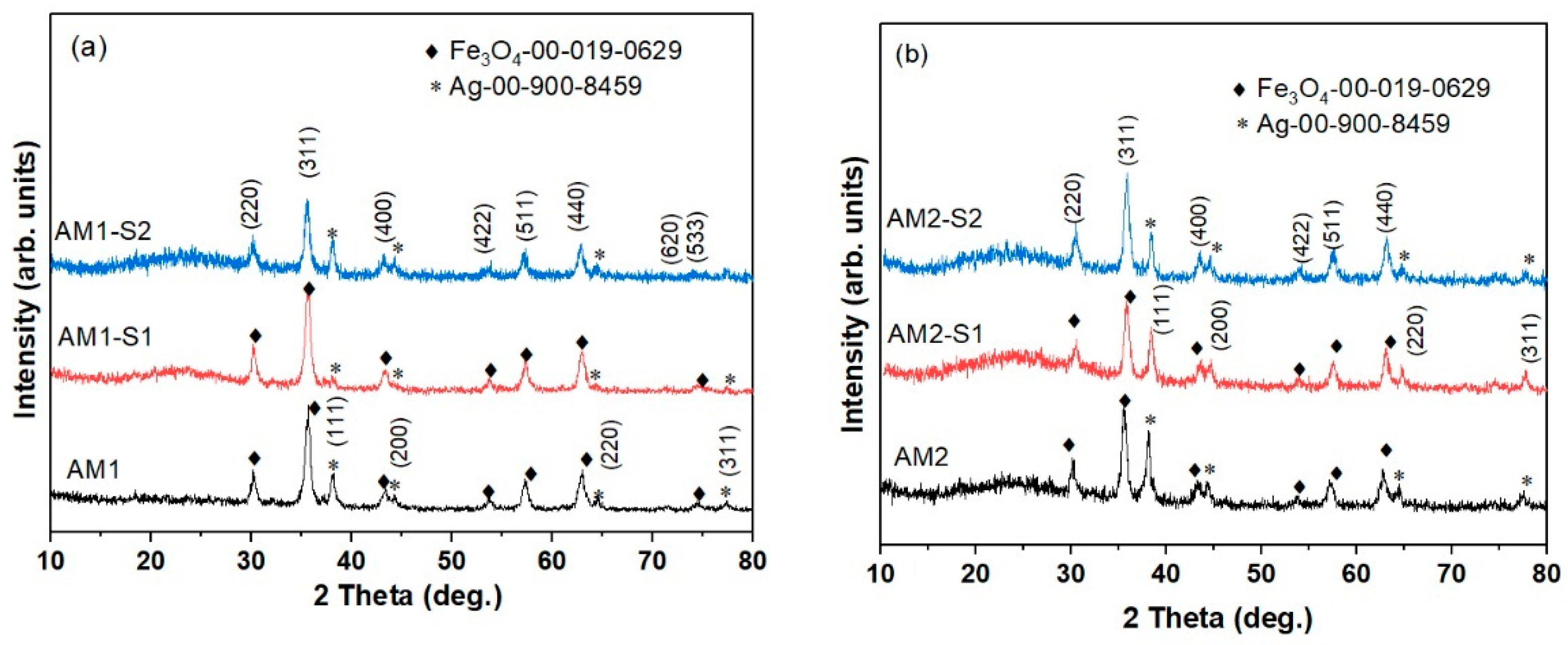
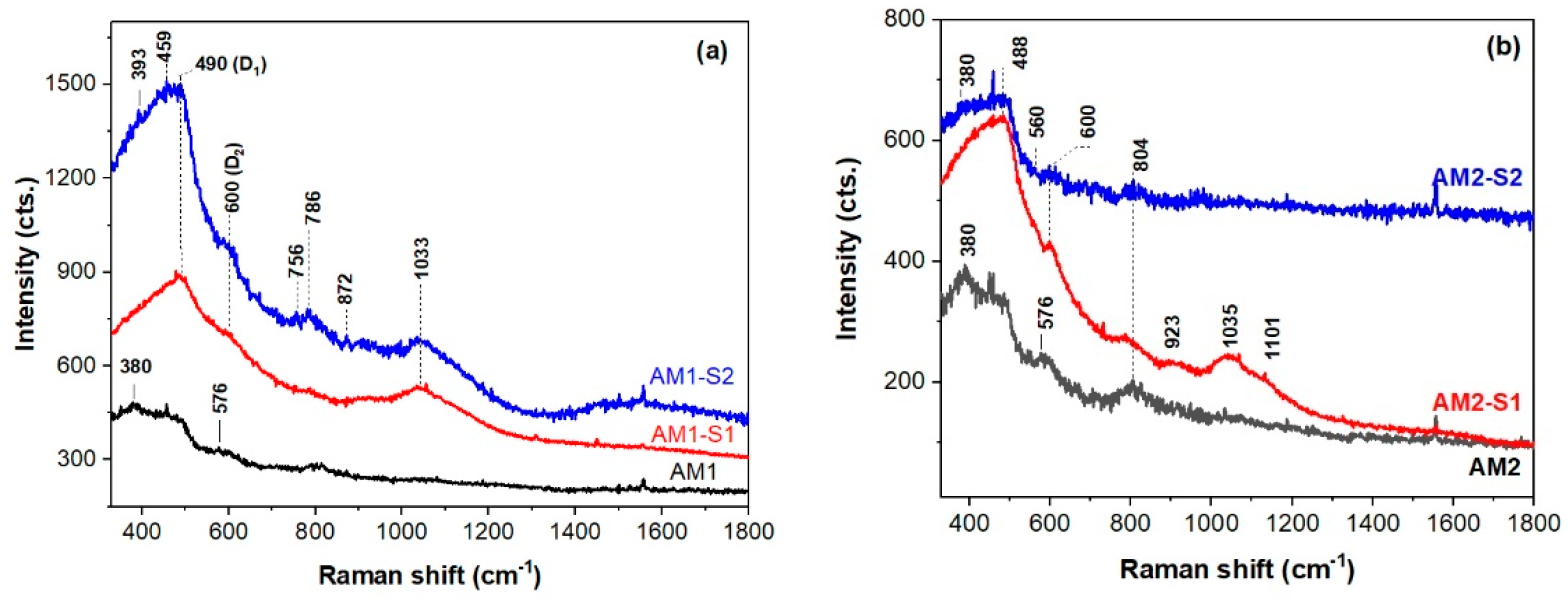
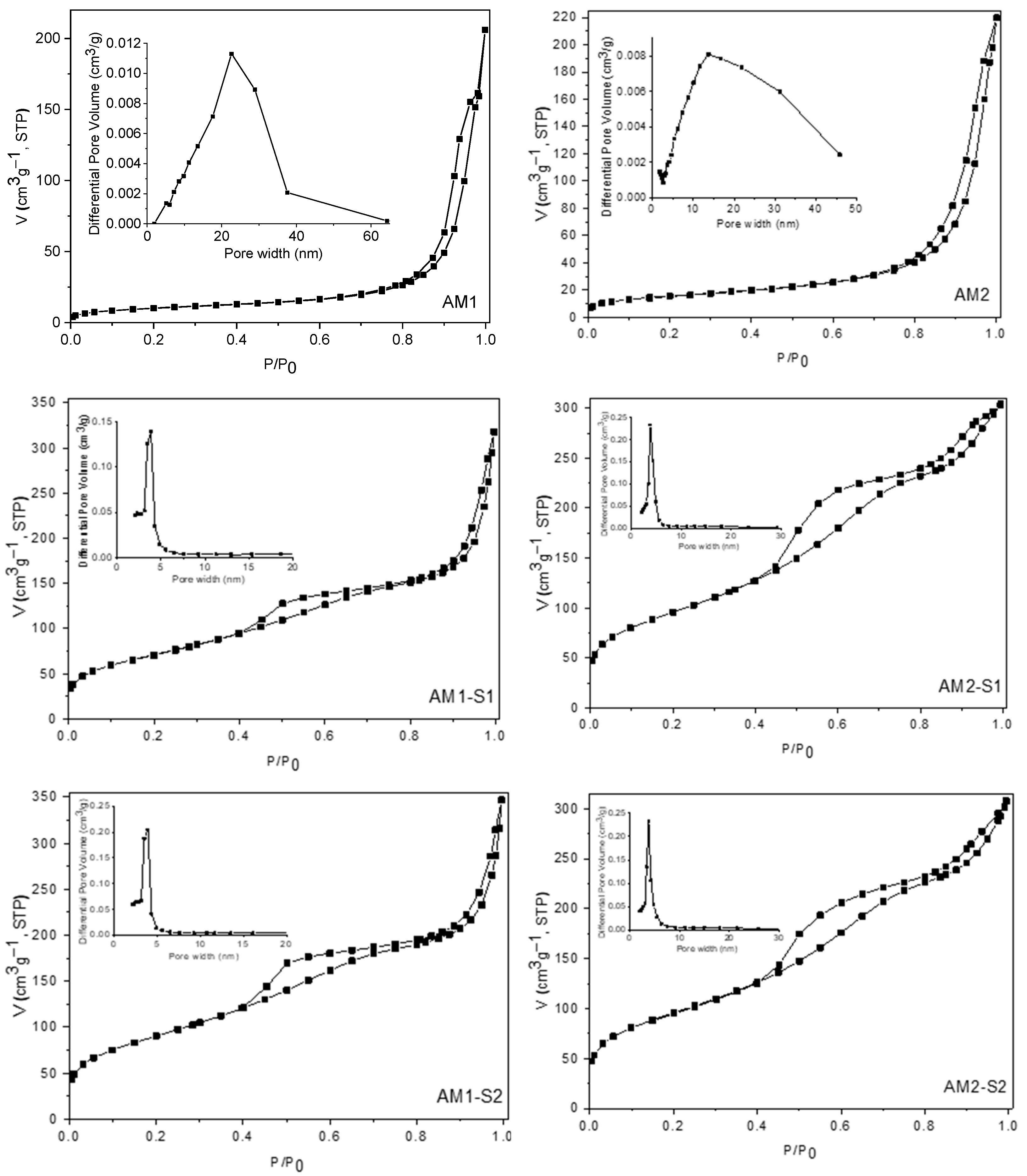
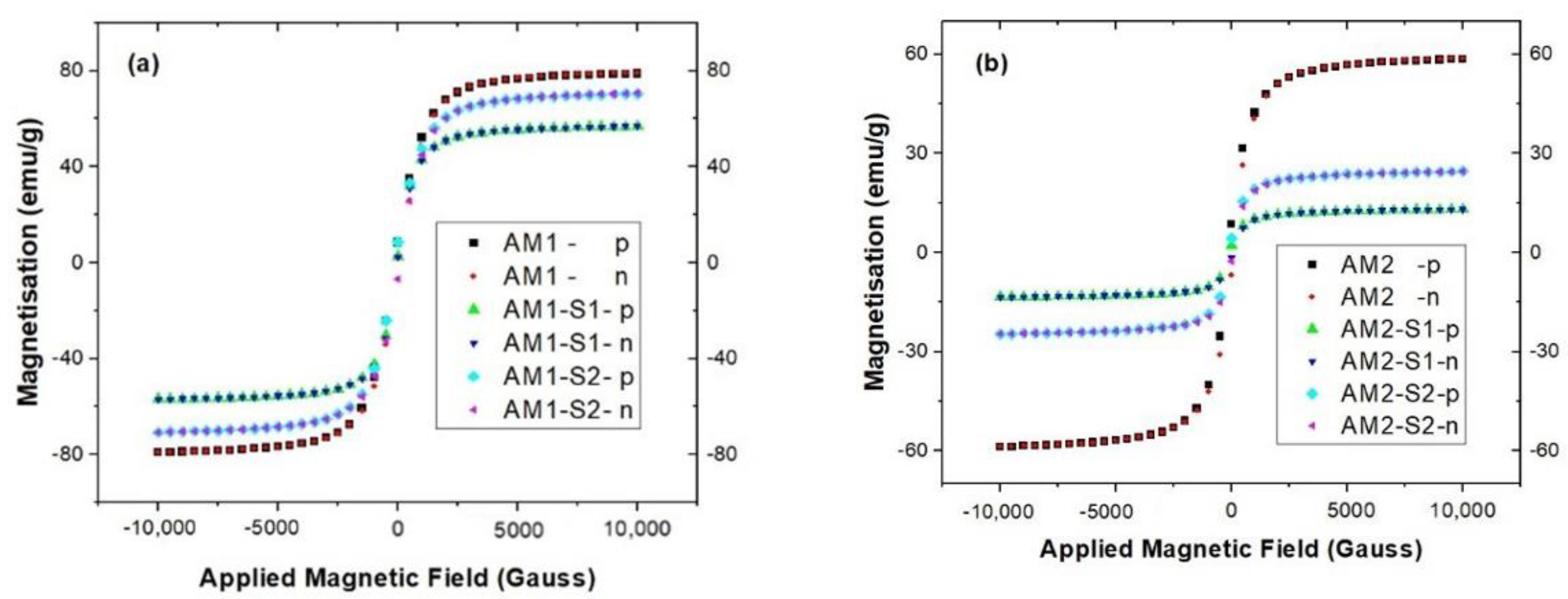
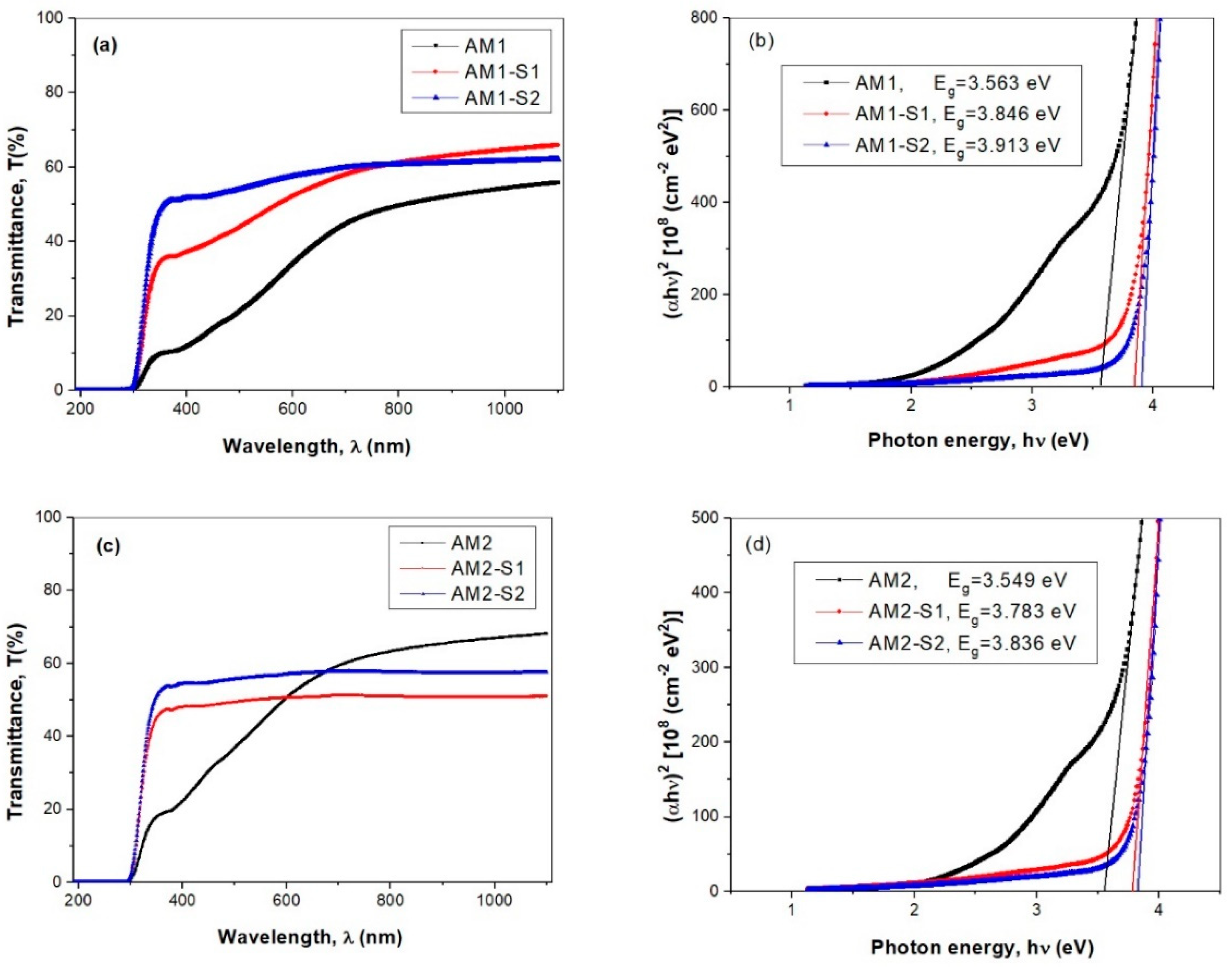
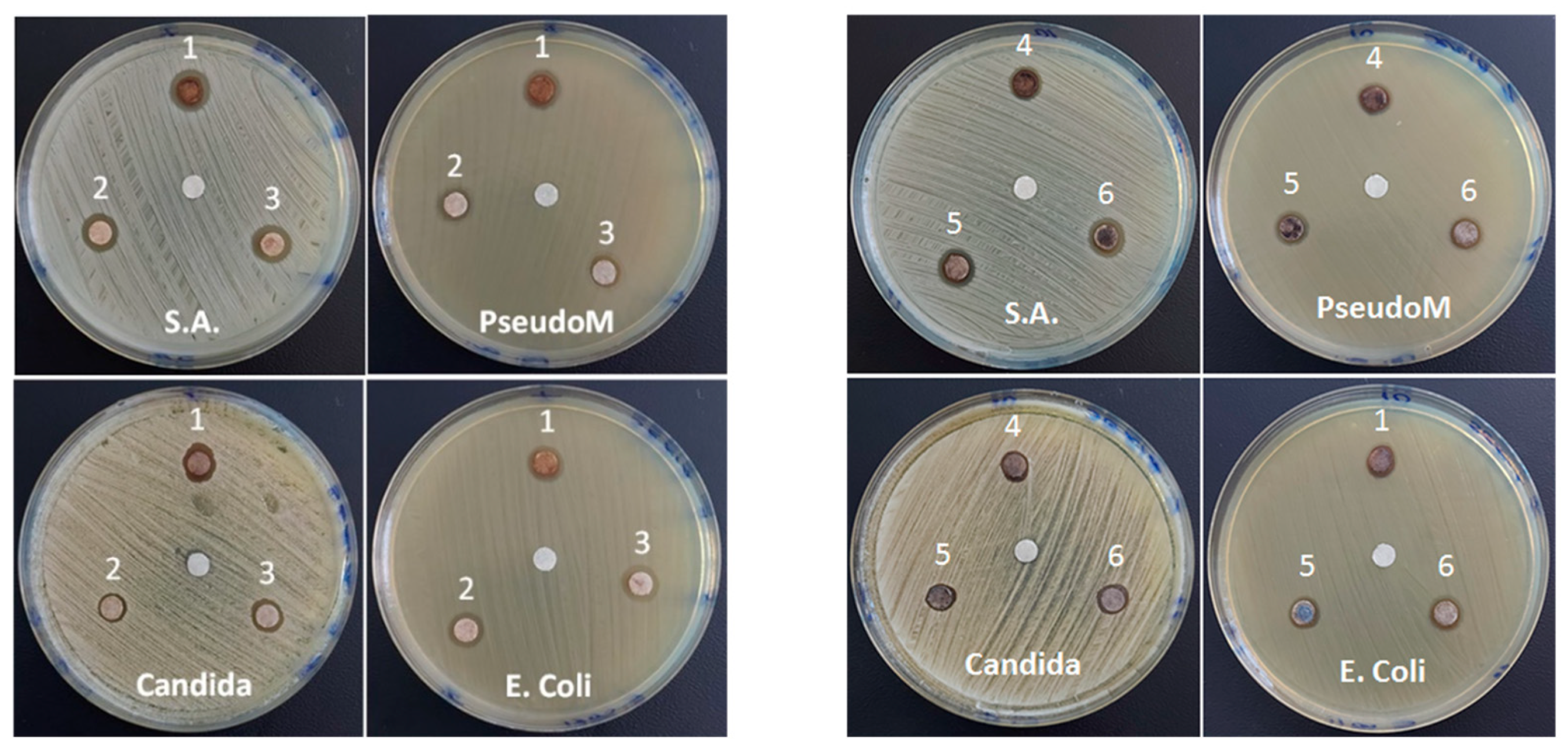
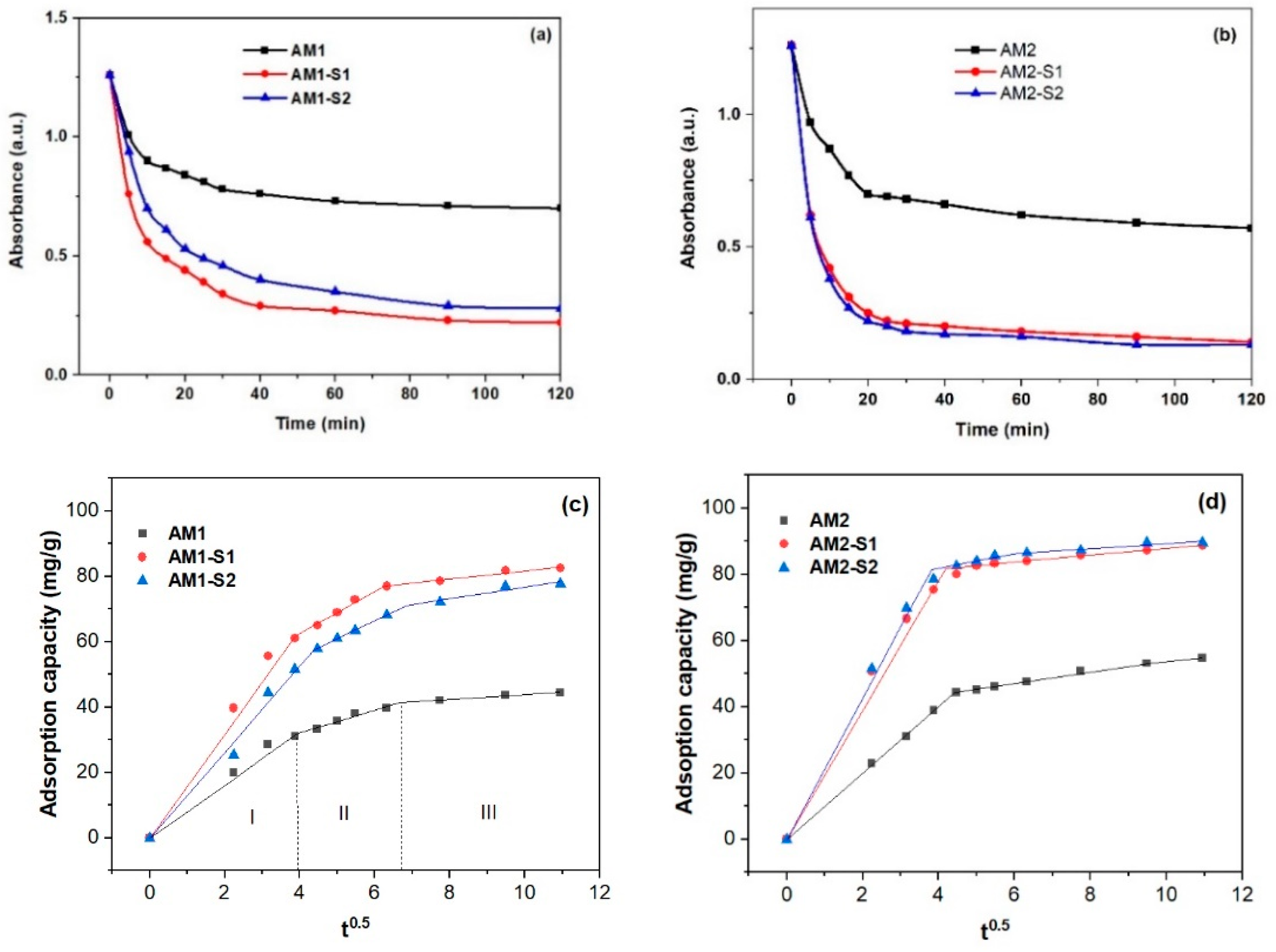


| Sample | XRD Results | Textural Parameters | Magnetic Parameters ** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases | wt% | 2θ (°) (311) | FWHM (°) | Lattice Parameter a = b = c (Å) | Crystallite Size (311) (Å) | Crystallite Size (WH) (Å) | SBET (m2g−1) | Pore Volume (cm3g−1) | Ms [emu/g] | μ/kB [K/Oe] | Quality Factor | |
| AM1 | Fe3O4 | 91.2 | 35.619 | 0.627 | 8.357 | 139 | 117 | 36.0 | 0.318 | 83.23 | 0.73 | 6.52 × 10−4 |
| Ag | 8.8 | - | - | - | - | - | ||||||
| AM1-S1 | Fe3O4 | 97.7 | 35.601 | 0.647 | 8.349 | 135 | 103 | 258.0 | 0.491 | 58.47 | 1.14 | 8.03 × 10−5 |
| Ag | 2.3 | - | - | - | - | - | ||||||
| AM1-S2 | Fe3O4 | 93 | 35.480 | 0.590 | 8.352 | 148 | 110 | 328.8 | 0.536 | 73.85 | 0.78 | 6.10 × 10−4 |
| Ag | 7 | - | - | - | - | - | ||||||
| AM2 | Fe3O4 | 81 | 35.507 | 0.609 | 8.368 | 143 | 126 | 55.7 | 0.290 | 60.57 | 0.947 | 6.80 × 10−4 |
| Ag | 19 | - | - | - | - | - | ||||||
| AM2-S1 | Fe3O4 | 84 | 35.473 | 0.624 | 8.371 | 140 | 119 | 344.6 | 0.471 | 13.41 | 1.29 | 9.52 × 10−4 |
| Ag | 16 | - | - | - | - | - | ||||||
| AM2-S2 | Fe3O4 | 92.6 | 35.481 | 0.588 | 8.387 | 148 | 127 | 342.5 | 0.475 | 24.87 | 1.29 | 7.20 × 10−4 |
| Ag | 7.4 | - | - | - | - | - | ||||||
| Reference (00-019-0629) | - | - | 35.42 | - | 8.396 | - | T[K] is temperature, H[Oe] is intensity of the applied field and [Oe] stands for [Gauss] | |||||
| Sample | AM1 | AM1-S1 | AM1-S2 | AM2 | AM2-S1 | AM2-S2 |
|---|---|---|---|---|---|---|
| kid (I) (mg/g h) | 8.0733 ± 0.31805 | 15.84282 ± 0.63744 | 13.12463 ± 0.35639 | 9.9987 ± 0.10022 | 19.49776 ± 0.79502 | 21.35053 ± 0.64258 |
| Kid (II) (mg/g h) | 3.44207 ± 0.56132 | 6.45414 ± 0.62699 | 5.52582 ± 0.10893 | 1.72125 ± 0.0643 | 1.16797 ± 0.18842 | 2.15124 ± 0.43609 |
| CII(mg g−1) | 18.39 | 36.70 | 33.30 | 36.67 | 76.79 | 73.28 |
| Kid (III) (mg/g h) | 0.7473 ± 0.10607 | 1.25483 ± 0.36674 | 1.76237 ± 2.7× 10-5 | 1.24082 ± 0.0818 | 0.98705 ± 0.04854 | 0.76131 ± 0.17163 |
| CIII(mg g−1) | 36.36 | 69.16 | 59.10 | 41.25 | 78.02 | 81.73 |
| Break point (min0.5) | 3.97 | 3.909 | 4.38 | 4.43 | 4.18 | 3.81 |
| 6.66 | 6.24 | 6.85 | 9.51 | 6.82 | 6.08 | |
| R2 (I) | 0.99383 | 0.99357 | 0.99706 | 0.9997 | 0.9965 | 0.99729 |
| R2 (II) | 0.9495 | 0.98148 | 0.9992 | 0.99722 | 0.97464 | 0.92406 |
| R2 (III) | 0.98025 | 0.9213 | 0.9999 | 0.9956 | 0.99759 | 0.90773 |
| Sample | K0 (min−1) | R2 | K1 (min−1) | R2 | K2 (min−1) | R2 |
|---|---|---|---|---|---|---|
| AM1 | 0.00622 ± 0.00146 | 0.64429 | 0.00827 ± 0.00187 | 0.66282 | 0.009 ± 0.00194 | 0.68356 |
| AM1-S1 | 0.01152 ± 0.00243 | 0.6925 | 0.02569 ± 0.00405 | 0.80105 | 0.06298 ± 0.0597 | 0.91747 |
| AM1-S2 | 0.01028 ± 0.002 | 0.7268 | 0.01944 ± 0.00285 | 0.82341 | 0.0355 ± 0.0322 | 0.92405 |
| AM2 | 0.00693 ± 0.0015 | 0.68064 | 0.00966 ± 0.00195 | 0.71114 | 0.01115 ± 0.00207 | 0.74435 |
| AM2-S1 | 0.0118 ± 0.00276 | 0.6467 | 0.02697 ± 0.00521 | 0.72787 | 0.06796 ± 0.00959 | 0.83391 |
| AM2-S2 | 0.01177 ± 0.00259 | 0.67295 | 0.02718 ± 0.00458 | 0.77922 | 0.07112 ± 0.00725 | 0.90586 |
| Equation [81] | Ct/C0 = 1 − (k0/C0)t | ln(Ct/C0) = k1t | (1/Ct − 1/C0) = k2t | |||
| Linear fit of data in Figure | Figure 9c,d | Figure 9e,f | Figure 9g,h |
| Sample | MB Photodegradation Efficiency (%) | Time (min.) | Rate Constant, k (min−1) | Reference |
|---|---|---|---|---|
| Fe3O4 | 89 | 140 | k0 = 0.0085 | [81] |
| Fe3O4-SiO2 | 97 | 140 | k0 = 0.102 | [81] |
| Fe3O4@Ag@TiO2 | 79.9 | 120 | k1 = 0.0120 | [80] |
| Fe3O4@ZnO | 88.5 | 120 | [63] | |
| Fe3O4@SiO2@ZnO cubes | 55 | 90 | k1 = 0.0074 | [82] |
| Ag-Ag2O-ZnO (VIS light) | 97.3 | 60 | k1 = 0.057 | [83] |
| Ag-Fe3O4@SiO2/ZnO | 90.47 | 120 | k2 = 0.07112 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muşat, V.; Crintea, L.; Anghel, E.-M.; Stănică, N.; Atkinson, I.; Culiţă, D.C.; Baroiu, L.; Țigău, N.; Cantaragiu Ceoromila, A.; Botezatu, A.-V.; et al. Ag-Decorated Iron Oxides-Silica Magnetic Nanocomposites with Antimicrobial and Photocatalytic Activity. Nanomaterials 2022, 12, 4452. https://doi.org/10.3390/nano12244452
Muşat V, Crintea L, Anghel E-M, Stănică N, Atkinson I, Culiţă DC, Baroiu L, Țigău N, Cantaragiu Ceoromila A, Botezatu A-V, et al. Ag-Decorated Iron Oxides-Silica Magnetic Nanocomposites with Antimicrobial and Photocatalytic Activity. Nanomaterials. 2022; 12(24):4452. https://doi.org/10.3390/nano12244452
Chicago/Turabian StyleMuşat, Viorica, Lenuța Crintea (Căpăţână), Elena-Maria Anghel, Nicolae Stănică, Irina Atkinson, Daniela Cristina Culiţă, Liliana Baroiu, Nicolae Țigău, Alina Cantaragiu Ceoromila, Andreea-Veronica Botezatu (Dediu), and et al. 2022. "Ag-Decorated Iron Oxides-Silica Magnetic Nanocomposites with Antimicrobial and Photocatalytic Activity" Nanomaterials 12, no. 24: 4452. https://doi.org/10.3390/nano12244452





