Nanoparticles in Drug Delivery: From History to Therapeutic Applications
Abstract
:1. Introduction
2. History
3. Recent Approaches Used in Drug Carriage System for Treatment of Various Diseases
3.1. Brain Drug Delivery System and Its Types
3.1.1. Role of Nanocarriers in Alzheimer’s Disease
Polymeric Nanoparticles
- I.
- The drug Tacrine was loaded on polymeric nanoparticles and administered through an intravenous route. It enhanced the concentration of tacrine inside the brain and also reduced the whole-dose quantity [115].
- II.
- Rivastigmine drug was loaded on polymeric nanoparticles and administered through an intravenous route. It enhanced learning and memory capacities [116].
Solid Lipid Nanoparticles (SLNPs)
- I.
- Piperine drug is loaded on solid lipid nanoparticles through an intraperitoneal route inside the brain to decrease plaques and masses and to increase AChE enzyme activity [118].
- II.
- Huperzine A improved cognitive functions. No main irritation was detected in rat skin when the drug was loaded on SLNPs in an in vitro study [119].
- I.
- II.
- III.
- IV.
Liposomes
- Curcumin–PEG derivative was loaded on liposomes and showed high affinity on senile plaques in an ex vivo experiment. Furthermore, in vitro it demonstrated the ability for Aβ aggregation and was taken inside by the BBB in a rat model [133].
- Folic acid was loaded on liposomes, administered through an intranasal route and absorbed through the nasal cavity [134].
Nanoemulsions
- I.
- Beta-Asarone was loaded on nanoemulsions, administered through an intranasal route, and enhanced bioavailability [130].
Micro Emulsion
- I.
- Tacrine was loaded on a microemulsion and improved memory. Such nanoparticles absorbed rapidly via the nose to the brain through an intranasal route [135].
Liquid Crystals
- I.
- T. divaricate was loaded on liquid crystals and injected through a transdermal route. It increased permanency of the drug in designs and also increased skin infusion and retention [136].
3.1.2. Role of Nanocarriers in Parkinson’s Disease (PD)
Ropinirole (RP)
3.2. Mechanism of Nanoparticles’ Brain Drug Delivery (across BBB)
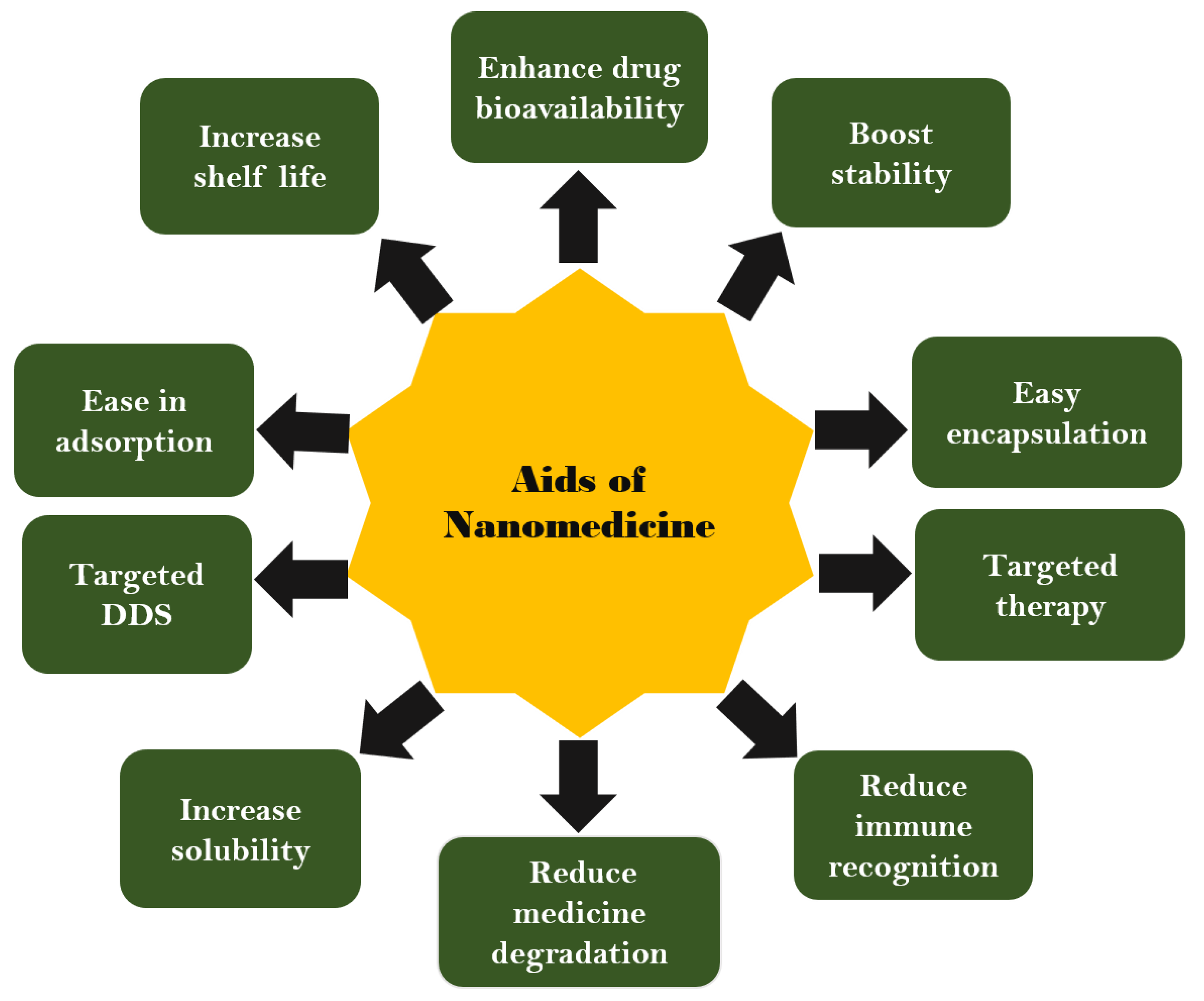
3.3. Advantages and Disadvantages of Nanomedicines
4. Nanocarriers Role in Major Cancers
4.1. Brain Cancer
4.2. Breast Cancer
4.3. Lung Cancer
5. Drug Delivery Approach in Heart Diseases
6. Drug Delivery Approach in Skin Diseases
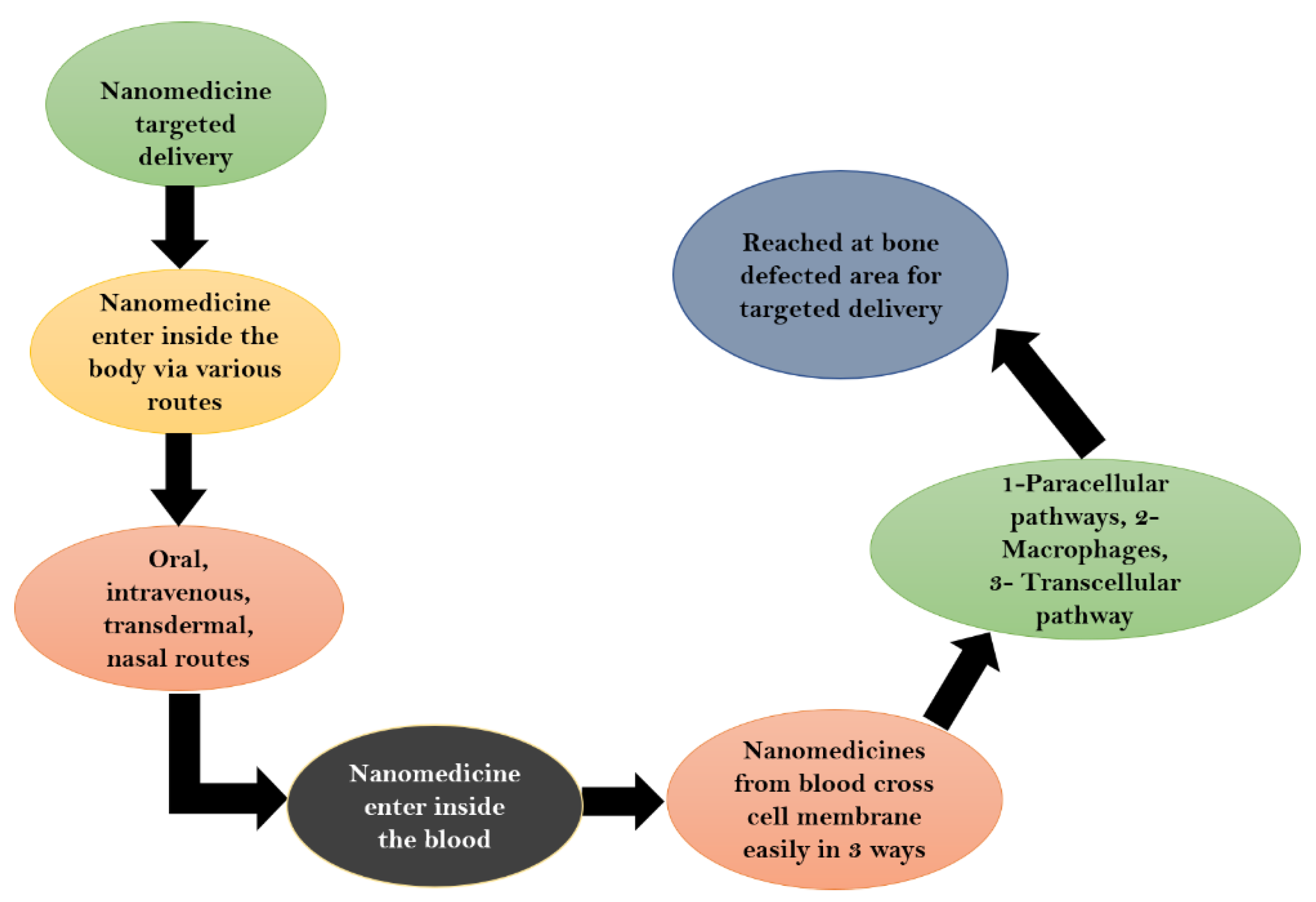
7. Drug Delivery Approach in Bone Diseases
Mechanism of Drug Delivery
8. Drug Delivery Approach in Blood Diseases
9. Future Challenges of Nanomedicines
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.S. Applicatio of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly (Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Builders, P.F.; Arhewoh, M.I. Pharmaceutical applications of native starch in conventional drug delivery. Starch-Stärke 2016, 9–10, 864–873. [Google Scholar] [CrossRef]
- Alshammari, M.K.; Alshehri, M.M.; Alshehri, A.M.; Alshlali, O.M.; Mahzari, A.M.; Almalki, H.H.; Kulaybi, O.Y.; Alghazwni, M.K.; Kamal, M.; Imran, M. Camptothecin loaded nano-delivery systems in the cancer therapeutic domains: A critical examination of the literature. J. Drug Deliv. Sci. Technol. 2022, 79, 104034. [Google Scholar] [CrossRef]
- Lai, H.; Liu, S.; Yan, J.; Xing, F.; Xiao, P. Facile Fabrication of Biobased Hydrogel from Natural Resources: L-Cysteine, Itaconic Anhydride, and Chitosan. ACS Sustain. Chem. Eng. 2020, 8, 4941–4947. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Willi, J.; Vielba-Gomez, F.; Gatti, F.; Tibbitt, M.W. Environment Controls Biomolecule Release from Dynamic Covalent Hydrogels. Biomacromolecules 2021, 22, 146–157. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Peppas, N.A. Chronobiology, drug delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2018, 9–10, 828–851. [Google Scholar]
- Jamieson, L.E.; Byrne, H.J. Vibrational spectroscopy as a tool for studying drug-cell interaction: Could high throughput vibrational spectroscopic screening improve drug development. Vib. Spectrosc. 2017, 91, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Drug delivery systems—An overview. Drug Deliv. Syst. 2008, 437, 1–50. [Google Scholar]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A new paradigm for drug development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Su, Y.; Xie, Z.; Kim, G.B.; Dong, C.; Yang, J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS Biomater. Sci. Eng. 2005, 4, 201–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Prasad, R.D.; Charmode, N.; Shrivastav, O.P.; Prasad, S.R.; Moghe, A.; Sarvalkar, P.D.; Prasad, N.R. A review on concept of nanotechnology in veterinary medicine. ES Food Agrofor. 2021, 4, 28–60. [Google Scholar] [CrossRef]
- Lateef, A.; Darwesh, O.M.; Matter, I.A. Microbial nanobiotechnology: The melting pot of microbiology, microbial technology and nanotechnology. In Microbial Nanobiotechnology; Springer: Singapore, 2021; pp. 1–19. [Google Scholar]
- Mansor, N.I.; Nordin, N.; Mohamed, F.; Ling, K.H.; Rosli, R.; Hassan, Z. Crossing the blood-brain barrier: A review on drug delivery strategies for treatment of the central nervous system diseases. Curr. Drug Deliv. 2019, 16, 698–711. [Google Scholar] [CrossRef]
- Mughal, T.A.; Ali, S.; Hassan, A.; Kazmi, S.A.R.; Saleem, M.Z.; Shakir, H.A.; Nazer, S.; Farooq, M.A.; Awan, M.Z.; Khan, M.A.; et al. Phytochemical screening, antimicrobial activity, in vitro and in vivo antioxidant activity of Berberis lycium Royle root bark extract. Braz. J. Biol. 2021, 84. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; dos Santos Ramos, M.A.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Astruc, D. Introduction to Nanomedicine. Molecules 2016, 21, 4. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nano-Enabled Med. Appl. 2021, 61–91. [Google Scholar] [CrossRef]
- Cleal, K.; He, L.; Watson, P.D.; Jones, A.T. Endocytosis, intracellular traffic and fate of cell penetrating peptide-based conjugates and nanoparticles. Curr. Pharm. Des. 2013, 19, 2878–2894. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Jafari, S.; Khosroushahi, A.Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z. Nanomedicines as emerging platform for simultaneous delivery of cancer therapeutics: New developments in overcoming drug resistance and optimizing anticancer efficacy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1015–1024. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Cho, H.-Y. Tumor homing reactive oxygen species nanoparticle for enhanced cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 23909–23918. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Acharya, S.; Sahoo, S.K. Recent trends of nano-medicinal approaches in clinics. Int. J. Pharm. 2018, 538, 263–278. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 2013, 42, 7289–7325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, W.; Nam, J.; Moon, J.J.; Kim, B.Y. Immunomodulating nanomedicine for cancer therapy. Nano Lett. 2018, 18, 6655–6659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef] [PubMed]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-based nanomedicine platforms for drug delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J. Phase II study of pegylated liposomal doxorubicin (CaelyxTM) as induction chemotherapy for patients with squamous cell cancer of the head and neck. Eur. J. Cancer 2001, 37, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Couvreur, P.; Vauthier, C. Poly alkyl cyanoacrylate nanoparticles as drug carrier: Present state and perspectives. J. Control. Release 1991, 17, 187–198. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Chauvierre, C.; Brigger, I.; Couvreur, P. Drug delivery to resistant tumors: The potential of poly (alkyl cyanoacrylate) nanoparticles. J. Control. Release 2003, 93, 151–160. [Google Scholar] [CrossRef]
- Shinto, Y.; Uchida, A.; Korkusuz, F.; Araki, N.; Ono, K. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J. Bone Joint Surg. Br. 1992, 74, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Esterhai, J.L., Jr.; Bednar, J.; Kimmelman, C.P. Gentamicin-induced ototoxicity complicating treatment of chronic osteomyelitis. Clin. Orthop. 1986, 209, 185–188. [Google Scholar] [CrossRef]
- Couvreur, P.; Puisieux, F. Nano-and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993, 10, 141–162. [Google Scholar] [CrossRef]
- Hwang, S.R.; Byun, Y. Advances in oral macromolecular drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.J.; Lukowski, G.; Kröber, R.; Damaschun, G.; Dittgen, M. Acrylic acid copolymer nanoparticles for drug delivery: Structural characterization of nanoparticles by small-angle x-ray scattering. Colloid Polym. Sci. 1994, 272, 755–769. [Google Scholar] [CrossRef]
- Lukowski, G.; Müller, R.H.; Müller, B.W.; Dittgen, M. Acrylic acid copolymer nanoparticles for drug delivery. Part II: Characterization of nanoparticles surface-modified by adsorption of ethoxylated surfactants. Colloid Polym. Sci. 1993, 271, 100–105. [Google Scholar] [CrossRef]
- Fresta, M.; Puglisi, G.; Giammona, G.; Cavallaro, G.; Micali, N.; Furneri, P.M. Pefloxacine mesilate-and ofloxacin-loaded poly ethyl cyanoacrylate nanoparticles: Characterization of the colloidal drug carrier formulation. J. Pharm. Sci. 1995, 74, 895–902. [Google Scholar] [CrossRef]
- Cavallaro, G.; Fresta, M.; Giammona, G.; Puglisi, G.; Villari, A. Entrapment of β-lactams antibiotics in polyethylcyanoacrylate nanoparticles: Studies on the possible in vivo application of this colloidal delivery system. Int. J. Pharm. 1994, 111, 31–41. [Google Scholar] [CrossRef]
- Pardridge, W.M. Physiologic-based strategies for protein drug delivery to the brain. J. Control. Release 1996, 39, 281–286. [Google Scholar] [CrossRef]
- Partridge, W.M. Drug and gene targeting to the brain via blood–brain barrier receptor-mediated transport systems. Int. Congr. Ser. 2005, 1277, 49–62. [Google Scholar] [CrossRef]
- Labhasetwar, V.; Song, C.; Levy, R.J. Nanoparticle drug delivery system for restenosis. Adv. Drug Deliv. Rev. 1997, 24, 63–85. [Google Scholar] [CrossRef]
- Labhasetwar, V.; Underwood, T.; Schwendeman, S.P.; Levy, R.J. Iontophoresis for modulation of cardiac drug delivery in dogs. Proc. Natl. Acad. Sci. USA 1995, 92, 2612–2616. [Google Scholar] [CrossRef] [Green Version]
- Kwon, G.S. Diblock copolymer nanoparticles for drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1998, 5, 481–512. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Fernández-Urrusuno, R.; Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- May, J.P.; Li, S.-D. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013, 10, 511–527. [Google Scholar] [CrossRef]
- Calvo, P. PEGylated poly cyanoacrylate nanoparticles as vector for drug delivery in prion diseases. J. Neurosci. Methods 2001, 111, 151–155. [Google Scholar] [CrossRef]
- Collinge, J. Molecular neurology of prion disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 906–919. [Google Scholar] [CrossRef]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin-and transferrin-receptor-antibody6modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bio reduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Targeting intracellular targets. Curr. Drug Deliv. 2004, 1, 235–247. [Google Scholar] [CrossRef]
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front Biosci. 2005, 9, 1864–7336. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, G.F.; Kingston, D.G.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Hattori, Y.; Maitani, Y. Folate-linked lipid-based nanoparticle for targeted gene delivery. Curr. Drug Deliv. 2005, 2, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Low, P.S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 2002, 54, 675–693. [Google Scholar] [CrossRef]
- Xiao, S. Preparation of folate-conjugated starch nanoparticles and its application to tumor-targeted drug delivery vector. Chin. Sci. Bull. 2006, 51, 1693–1697. [Google Scholar] [CrossRef]
- Yu, D.; Xiao, S.; Tong, C.; Chen, L.; Liu, X. Dialdehyde starch nanoparticles: Preparation and application in drug carrier. Chin. Sci. Bull. 2007, 52, 2913–2918. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Multi-Functional Gold Nanoparticles for Drug Delivery. In Bio-Applications of Nanoparticles; Chan, W.C.W., Ed.; Springer: New York, NY, USA, 2007; pp. 48–56. [Google Scholar]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazori, T.; Khoshayand, M.R.; Azizi, E.; Yazdizade, P.; Nomani, A.; Haririan, I. Evaluation of Alginate/Chitosan nanoparticles as antisense delivery vector: Formulation, optimization and in vitro characterization. Carbohydr. Polym. 2009, 77, 599–606. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D.C.; Neufeld, R.J. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2847. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Cancer-Cell-Specific Induction of Apoptosis Using Mesoporous Silica Nanoparticles as Drug-Delivery Vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.; Sun, C.; Wang, J.; Zhang, M. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir 2005, 21, 8858–8864. [Google Scholar] [CrossRef]
- Alhaddad, A. Nanodiamond as a vector for siRNA delivery to Ewing sarcoma cells. Phys. Q.-Bio. 2011, 21, 3087–3095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengesha, A.E.; Youan, B.C. Nano diamonds for drug delivery systems. In Diamond-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 186–205. [Google Scholar]
- Arjunan, N.K.; Murugan, K.; Rejeeth, C.; Madhiyazhagan, P.; Barnard, D.R. Green Synthesis of Silver Nanoparticles for the Control of Mosquito Vectors of Malaria, Filariasis, and Dengue. Vector-Borne Zoonotic Dis. 2012, 12, 262–268. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Brown, P.K.; Qureshi, A.T.; Moll, A.N.; Hayes, D.J.; Monroe, W.T. Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing. ACS Nano 2013, 7, 2948–2959. [Google Scholar] [CrossRef]
- Minelli, C.; Lowe, S.B.; Stevens, M.M. Engineering nanocomposite materials for cancer therapy. Small 2010, 21, 2336–2357. [Google Scholar] [CrossRef]
- Rajeshkumar, S. Synthesis of silver nanoparticles using fresh bark of Pongamia pinnata and characterization of its antibacterial activity against gram positive and gram negative pathogens. Resour.-Effic. Technol. 2016, 2, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Beg, M. Green synthesis of silver nanoparticles using Pongamia pinnata seed: Characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J. Mol. Recognit. 2017, 30, e2565. [Google Scholar] [CrossRef] [PubMed]
- Urbán, P.; Ranucci, E.; Fernàndez-Busquets, X. Polyamidoamine nanoparticles as nanocarriers for the drug delivery to malaria parasite stages in the mosquito vector. Nanomed 2015, 10, 3401–3414. [Google Scholar] [CrossRef] [PubMed]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Kulbacka, J. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [Green Version]
- Ju, Z.; Sun, W. Drug delivery vectors based on filamentous bacteriophages and phage-mimetic nanoparticles. Drug Deliv. 2017, 24, 1898–1908. [Google Scholar] [CrossRef] [Green Version]
- Jahromi, M.A.M.; Zangabad, P.S.; Basri, S.M.M.; Zangabad, K.S.; Ghamarypour, A.; Aref, A.R. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar]
- Aljabali, A.A. Innovative Applications of Plant Viruses in Drug Targeting and Molecular Imaging—A Review. Curr. Med. Imaging 2021, 17, 491–506. [Google Scholar] [CrossRef]
- Slita, A.; Egorova, A.; Casals, E.; Kiselev, A.; Rosenholm, J.M. Characterization of modified mesoporous silica nanoparticles as vectors for siRNA delivery. Asian J. Pharm. Sci. 2018, 13, 592–599. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Pathak, C.; Vaidya, F.U.; Pandey, S.M. Mechanism for development of nanobased drug delivery system. Appl. Target. Nano Drugs Deliv. Syst. 2019, 1, 35–67. [Google Scholar]
- Ghaz-Jahanian, M.A.; Abbaspour-Aghdam, F.; Anarjan, N.; Berenjian, A.; Jafarizadeh-Malmiri, H. Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Mol. Biotechnol. 2015, 157, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Assa, F. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2017, 37, 492–509. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, F.X.; Zhang, L.; Yang, W.; Wang, H.X. A pH-sensitive drug delivery system based on folic acid-targeted HBP-modified mesoporous silica nanoparticles for cancer therapy. Colloids Surf. Physicochem. Eng. Asp. 2020, 590, 124470. [Google Scholar] [CrossRef]
- Shafiei, N.; Nasrollahzadeh, M.; Iravani, S. Green Synthesis of Silica and Silicon Nanoparticles and Their Biomedical and Catalytic Applications. Comments Inorg. Chem. 2021, 41, 317–372. [Google Scholar] [CrossRef]
- Shariatinia, Z. Inorganic Material-Based Nanocarriers for Delivery of Biomolecules. Nanoeng. Biomater. Biomed. Appl. 2022, 2, 245–293. [Google Scholar]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery. Adv. Healthc. Mater. 2021, 10, 2001812. [Google Scholar] [CrossRef]
- Thi, T.T.; Suys, E.J.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. The latest strategies in the fight against the COVID-19 pandemic: The role of metal and metal oxide nanoparticles. New J. Chem. 2021, 45, 6167–6179. [Google Scholar] [CrossRef]
- Kelleni, M.T. Resveratrol-zinc nanoparticles or pterostilbene-zinc: Potential COVID-19 mono and adjuvant therapy. Biomed. Pharmacother. 2021, 139, 111626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Zhong, H.; Niu, S.; Ding, S.; Lv, S. Iridium oxide nanoparticles-based theranostic probe for in vivo tumor imaging and synergistic chem/photothermal treatments of cancer cells. Chem. Eng. J. 2022, 430, 132–675. [Google Scholar] [CrossRef]
- Cao, S.; Deng, Y.; Zhang, L.; Aleahmad, M. Chitosan nanoparticles, as biological macromolecule-based drug delivery systems to improve the healing potential of artificial neural guidance channels: A review. Int. J. Biol. Macromol. 2022, 201, 569–579. [Google Scholar] [CrossRef]
- Fricke, I.B.; Schelhaas, S.; Zinnhardt, B.; Viel, T.; Hermann, S.; Couillard-Després, S.; Jacobs, A.H. In vivo bioluminescence imaging of neurogenesis—The role of the blood brain barrier in an experimental model of Parkinson’s disease. Eur. J. Neurosci. 2017, 45, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: How to ‘open’ the blood brain barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Sahni, J.K.; Doggui, S.; Ali, J.; Baboota, S.; Dao, L.; Ramassamy, C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J. Control. Release 2012, 152, 208–231. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Muhamad, S.; Pecze, L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017, 16, 73–782. [Google Scholar] [CrossRef]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, C.; Lyu, Y.; Chen, H.; Jiang, G.; Gao, X. Biomimetic drug-delivery systems for the management of brain diseases. Biomater. Sci. 2020, 8, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticles system for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dalwadi, G.; Benson, H.A.E. Drug delivery across the blood—Brain barrier. Cur. Drug Deliv 2004, 1, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Fundaro, A.; Cavalli, R.; Bagoni, A.; Vighetto, D.; Zara, G.P.; Gasco, M.R. Non-stealth and stealth solid lipid nanoparticles(sln) carrying doxorubicin: Pharmacokinetic and tissue distribution after IV administration to rats. Pharm. Res. 2000, 42, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwarlu, V.; Manjunath, K. Preparation, characterization and in vitro release kinetics of clozapine Solid Lipid Nanoparticles. J. Control. Release 2004, 65, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, C.; Kayser, O.; Muller, R.H. Lipase degradation of Dynasan 114 & 116 SLN-effect of surfactant, storage time & crystallinity. Int. J. Pharm. 2002, 237, 119–128. [Google Scholar]
- Cavalli, R.; Caputo, O.; Carlotti, M.E.; Trotta, M.; Scarnecchia, C.; Gasco, M.R. Sterilization and freeze drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 1997, 148, 47–54. [Google Scholar] [CrossRef]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Olbrich, C.; Gessner, A.; Schroder, W.; Kayser, O.; Muller, R.H. Lipid drug conjugate nanoparticles of the hydrophilic drug diminazine-cytotoxicity testing and mouse serum adsorption. J. Control. Release 2004, 96, 425–435. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.S.; Muller, R.H. Solid lipid nanoparticles for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 96, 83–96. [Google Scholar] [CrossRef]
- Zimmermann, E.; Müller, R.H.; Mader, K. Influence of different parameters on reconstitution of lyophilized SLN. Int. J. Pharm. 2000, 196, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Physicochemical characterization of polyacrylic nanoparticles. Int. J. Pharm. 1983, 65, 43–58. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Rühl, D.; Runge, S.A. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. Int. J. Pharm. 1996, 144, 115–121. [Google Scholar] [CrossRef]
- Lai, F.; Fadda, A.M.; Sinico, C. Liposomes for brain delivery. Expert Opin. Drug Deliv. 2013, 10, 1003–1022. [Google Scholar] [CrossRef]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal drug delivery systems: An update review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef]
- Leonor Pinzon-Daza, M.; Campia, I.; Kopecka, J.; Garzón, R.; Ghigo, D.; Rigant, C. Nanoparticle-and liposome-carried drugs: New strategies for active targeting and drug delivery across blood-brain barrier. Curr. Drug Metab. 2013, 14, 625–640. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A promising nanocarrier for natural drug delivery through blood-brain barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, M.K.; Radhakrishnan, A.K.; et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson’s disease. Int. J. Pharm. 2020, 579, 119148. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated Progress of Nanocarrier-Based Intranasal Drug Delivery Systems for Treatment of Brain Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 5, 433–467. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of different nanocarriers for encapsulation of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 3468–3497. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008, 70, 75–84. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Khan, M.; Khan, R.A.; Ahmed, B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J. Drug Target. 2013, 21, 300–311. [Google Scholar] [CrossRef]
- Patel, P.A.; Patil, S.C.; Kalaria, D.R.; Kalia, Y.N.; Patravale, V.B. Comparative in vitro and in vivo evaluation of lipid based nanocarriers of Huperzine A. Int. J. Pharm. 2013, 446, 16–23. [Google Scholar] [CrossRef]
- Mourtas, S.; Lazar, A.N.; Markoutsa, E.; Duyckaerts, C.; Antimisiaris, S.G. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur. J. Med. Chem. 2014, 80, 175–183. [Google Scholar] [CrossRef]
- Ravouru, N.; Kondreddy, P.; Korakanchi, D.H.M. Formulation and Evaluation of Niosomal Nasal Drug Delivery System of Folic Acid for Brain Targeting. Curr. Drug Discov. Technol. 2013, 10, 270–282. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the blood-brain barrier: Advances in nanoparticle technology for drug delivery in neuro-oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano carrier drug delivery systems for the treatment of neuropsychiatric disorders: Advantages and limitations. Molecules 2020, 25, 5294. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.; Hamano, N.; Li, S.-D.; Chougule, M.; Shoyele, S.A.; Gupta, U.; Uddin, A.; et al. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin. Drug Deliv. 2018, 15, 589–617. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Chavda, K.; Vyas, B.; Patel, S. Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: Role of P-gp inhibition. Drug Deliv. Transl. Res. 2021, 11, 1166–1185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood–brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site-specific therapeutic delivery. Adv. Drug Deliv. Rev. 2018, 144, 57–77. [Google Scholar] [CrossRef]
- Jogani, V.V.; Shah, P.J.; Mishra, P.; Mishra, A.K.; Misra, A.R. Intranasal Mucoadhesive Microemulsion of Tacrine to Improve Brain Targeting. Alzheimer Dis. Assoc. Disord. 2008, 22, 116–124. [Google Scholar] [CrossRef]
- Chaiyana, W.; Rades, T.; Okonogi, S. Characterization and in vitro permeation study of microemulsions and liquid crystalline systems containing the anticholinesterase alkaloidal extract from Tabernaemontana divaricata. Int. J. Pharm. 2013, 452, 201–210. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s disease and parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Ishihara, L.; Brayne, C. A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol. Scand. 2006, 113, 211–220. [Google Scholar] [CrossRef]
- Kyle, S.; Saha, S. Nanotechnology for the detection and therapy of stroke. Adv. Healthc. Mater. 2014, 3, 1703–1720. [Google Scholar] [CrossRef]
- Ghazy, E.; Rahdar, A.; Barani, M.; Kyzas, G.Z. Nanomaterials for Parkinson disease: Recent progress. J. Mol. Struct. 2021, 1231, 129698. [Google Scholar] [CrossRef]
- Dudhipala, N.; Gorre, T. Neuroprotective Effect of Ropinirole Lipid Nanoparticles Enriched Hydrogel for Parkinson’s Disease: In Vitro, Ex Vivo, Pharmacokinetic and Pharmacodynamic Evaluation. Pharmaceutics 2020, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Barcia, E.; Boeva, L.; García-García, L.; Slowing, K.; Fernández-Carballido, A.; Casanova, Y. Nanotechnology-based drug delivery of ropinirole for Parkinson’s disease. Drug Deliv. 2017, 24, 1112–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef]
- Jha, A.; Mukhopadhaya, K. Supporting Diagnosis and Treatment. In Alzheimer’s Disease; Springer: Berlin/Heidelberg, Germany, 2021; pp. 65–85. [Google Scholar]
- McMahon, D.; O’Reilly, M.A.; Hynynen, K. Therapeutic Agent Delivery across the Blood–Brain Barrier Using Focused Ultrasound. Annu. Rev. Biomed. Eng. 2021, 23, 89–113. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Mendez, G.; Ozpinar, A.; Raskin, J.; Gultekin, S.H.; Ross, D.A. Case comparison and literature review of glioblastoma: A tale of two tumors. Surg. Neurol. Int. 2014, 5, 121. [Google Scholar]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and Other Malignant Gliomas: A Clinical Review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Prasad, B.L.V. Cytotoxicity of sophorolipid -gellan gum- gold nanoparticle conjugates and their doxorubicin loaded derivatives towards human glioma and human glioma stem cell lines. Nanoscale 2011, 3, 575–580. [Google Scholar]
- Mazur, J.; Roy, K.; Kanwar, J.R. Recent advances in nanomedicine and survivin targeting in brain cancers. Nanomedicine 2018, 13, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zheng, X.; Pang, X.; Pang, Z.; Zhao, J.; Zhang, Z. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 2016, 7, 34038–34051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erin, N.; Kale, Ş.; Tanrıöver, G.; Köksoy, S.; Duymuş, Ö.; Korcum, A.F. Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Res. Treat 2013, 139, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, Z.; Cao, S.; Xi, Z.; Zhang, S.; Pang, Z. Behavior and anti-glioma effect of lapatinib-incorporated lipoprotein-like nanoparticles. Nanotechnology 2012, 23, 435101. [Google Scholar] [CrossRef] [PubMed]
- Bonde, G.V.; Yadav, S.K.; Chauhan, S.; Mittal, P.; Ajmal, G.; Thokala, S.; Mishra, B. Lapatinib nano-delivery systems: A promising future for breast cancer treatment. Expert Opin. Drug Deliv. 2018, 15, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Bigdeli, B.; Jalili-baleh, L.; Baharifar, H.; Akrami, M.; Dehghani, S. Curcumin-lipoic acid conjugate as a promising anticancer agent on the surface of gold-iron oxide nanocomposites: A pH-sensitive targeted drug delivery system for brain cancer theranostics. Eur. J. Pharm. Sci. 2018, 114, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Sohn, S.; Kwon, H.J.; Kim, S.U.; Kim, M.J.; Lee, S.J.; Choi, K.S. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007, 67, 6314–6324. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.; Singh, R.P.; Sonali; Kumari, L.; Sharma, G.; Koch, B. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Eng. C 2017, 74, 167–176. [Google Scholar] [CrossRef]
- Muthu, M.S.; Avinash Kulkarni, S.; Liu, Y.; Feng, S.-S. Development of docetaxel-loaded vitamin E TPGS micelles: Formulation optimization, effects on brain cancer cells and biodistribution in rats. Nano Diam. 2012, 7, 353–364. [Google Scholar] [CrossRef]
- Lara-Velazquez, M.; Alkharboosh, R.; Norton, E.S.; Ramirez-Loera, C.; Freeman, W.D.; Guerrero-Cazares, H. Chitosan-Based Non-viral Gene and Drug Delivery Systems for Brain Cancer. Front. Neurol. 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Trapani, A.; Laquintana, V.; Lopedota, A.; Trapani, G. Recent advances in medicinal chemistry and pharmaceutical technology-strategies for drug delivery to the brain. Curr. Top. Med. Chem. 2009, 9, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Shinde, G.; Shiyani, S.; Shelke, S.; Chouthe, R.; Kulkarni, D.; Marvaniya, K. Enhanced brain targeting efficiency using 5-FU (fluorouracil) lipid–drug conjugated nanoparticles in brain cancer therapy. Prog. Biomater. 2020, 9, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Clares, B.; Morales, M.E.; Gallardo, V.; Ruiz, M.A. Lipid-based drug delivery systems for cancer treatment. Curr. Drug Targets 2011, 12, 1151–1165. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Soliman, A.S. Cancer in the global health era: Opportunities for the Middle East and Asia. Asia Pac. J. Public Health 2010, 22, 75S–82S. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Çelikgün, S.; Koç, T.; Tuncer, E.; Özer, H.; Nur, N. Cancer Map between 2010–2019 Sivas City. Int. J. Acad Med. Pharm. 2021, 3, 273–276. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2020, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P. Treatment of breast cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar] [PubMed]
- Jin, K.-T.; Lu, Z.-B.; Chen, J.-Y.; Liu, Y.-Y.; Lan, H.-R.; Dong, H.-Y. Recent trends in nanocarrier-based targeted chemotherapy: Selective delivery of anticancer drugs for effective lung, colon, cervical, and breast cancer treatment. J. Nanomater. 2020, 2020, 9184284. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Saiful Yazan, L.; Che Abdullah, C.A. A review on current nanomaterials and their drug conjugate for targeted breast cancer treatment. Int. J. Nanomed. 2017, 12, 2373–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yassemi, A.; Kashanian, S.; Zhaleh, H. Folic acid receptor-targeted solid lipid nanoparticles to enhance cytotoxicity of letrozole through induction of caspase-3 dependent-apoptosis for breast cancer treatment. Pharm. Dev. Technol. 2020, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, J.; Yuan, X.; Wang, J.; Zhang, L. Folic acid grafted and tertiary amino based pH-responsive pentablock polymeric micelles for targeting anticancer drug delivery. Mater. Sci. Eng. 2018, 82, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Tezcan, O.; Li, D.; Beztsinna, N.; Lou, B.; Etrych, T.; Ulbrich, K.; Metselaar, J.M.; Lammers, T.; Hennink, W.E. Overcoming multidrug resistance using folate receptor-targeted and pH-responsive polymeric nanogels containing covalently entrapped doxorubicin. Nanoscale 2017, 9, 10404–10419. [Google Scholar] [CrossRef] [Green Version]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018, 12, 1578. [Google Scholar] [CrossRef] [Green Version]
- Riganti, C.; Gazzano, E.; Gulino, G.R.; Volante, M.; Ghigo, D.; Kopecka, J. Two repeated low doses of doxorubicin are more effective than a single high dose against tumors overexpressing P-glycoprotein. Cancer Lett. 2015, 23, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of anti-HER2-targeted doxorubicin–core-shell chitosan nanoparticles for the treatment of human breast cancer. Int. J. Nanomed. 2019, 14, 4105–4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of doxorubicin—Core Shell chitosan nanoparticles to treat Cancer. In Proceedings of the 2011 International Conference on Biomedical Engineering and Technology, Kuala Lumpur, Malaysia, 4–5 June 2011. [Google Scholar]
- Di, H.; Wu, H.; Gao, Y.; Li, W.; Zou, D.; Dong, C. Doxorubicin-and cisplatin-loaded nanostructured lipid carriers for breast cancer combination chemotherapy. Drug Dev. Ind. Pharm. 2016, 42, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Namdari, M.; Cheraghi, M.; Negahdari, B.; Eatemadi, A.; Daraee, H. Recent advances in magnetoliposome for heart drug delivery. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1051–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looga, R. Reflex cardiovascular responses to lung inflation: A review. Respir. Physiol. 1997, 12, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mouton, C.; Hidalgo, A.; Cruz, A.; Pérez-Gil, J. The Lord of the Lungs: The essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur. J. Pharm. Biopharm. 2016, 144, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Jud, C.; Clift, M.; Petri-Fink, A.; Rothen-Rutishauser, B. Nanomaterials and the human lung: What is known and what must be deciphered to realize their potential advantages? Swiss Med. Wkly. 2013, 143, w13758. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Poland, C.A. Inhaled nanoparticles and lung cancer—What we can learn from conventional particle toxicology. Swiss Med. Wkly. 2012, 142, w13547. [Google Scholar] [CrossRef] [Green Version]
- Kuzmov, A.; Minko, T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef]
- Azarmi, S.; Roa, W.H.; Löbenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Nie, X.; Xu, Q.; Jiao, F.; Li, W. Efficient Delivery of Antitumor Drug to the Nuclei of Tumor Cells by Amphiphilic Biodegradable Poly(L-Aspartic Acid-co-Lactic Acid)/DPPE Co-Polymer Nanoparticles. Small 2012, 8, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, T.; Chen, C.; Liu, Y. Right or left: The role of nanoparticles in pulmonary diseases. Int. J. Mol. Sci. 2014, 15, 17577–17600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouameur, D.; Cheng, H.; Opoku-Damoah, Y.; Sun, B.; Dong, Q.; Han, Y.; Zhou, J.; Ding, Y. Stimuli-responsive gel-micelles with flexible modulation of drug release for maximized antitumor efficacy. Nano Res. 2018, 11, 4245–4264. [Google Scholar] [CrossRef]
- Kamat, C.D.; Shmueli, R.B.; Connis, N.; Rudin, C.M.; Green, J.J.; Hann, C.L. Poly(β-amino ester) Nanoparticle Delivery of TP53 Has Activity against Small Cell Lung Cancer In Vitro and In Vivo. Mol. Cancer Ther. 2013, 12, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, R.A.; Serra, A.; Coelho, J.F.; Faneca, H. Poly(β-amino ester)-based gene delivery systems: From discovery to therapeutic applications. J. Control. Release 2019, 310, 155–187. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y. Lung carcinoma therapy using epidermal growth factor receptor-targeted lipid polymeric nanoparticles co-loaded with cisplatin and doxorubicin. Oncol. Rep. 2019, 42, 2087–2096. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Guo, Z.; Chen, J.; Lin, L.; Wu, J. Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J. Control. Release 2019, 295, 153–163. [Google Scholar] [CrossRef]
- Tan, S.; Wang, G. Redox-responsive and pH-sensitive nanoparticles enhanced stability and anticancer ability of erlotinib to treat lung cancer in vivo. Drug Des. Devel. Ther. 2017, 11, 3519–3529. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, H.; Zeng, X.; Guo, W.; Jin, Y.; Wang, S. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B 2019, 9, 167–176. [Google Scholar] [CrossRef]
- Rehman, S.; Nabi, B.; Pottoo, F.H.; Baboota, S.; Ali, J. Nanoparticle based gene therapy approach: A pioneering rebellion in the management of psychiatric disorders. Curr. Gene Ther. 2020, 20, 164–173. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Ghorbani, M.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Targeted hyaluronic acid-based lipid nanoparticle for apigenin delivery to induce Nrf2-dependent apoptosis in lung cancer cells. J. Drug Deliv. Sci. Technol. 2019, 49, 268–276. [Google Scholar] [CrossRef]
- Chan, M.; Huang, W.; Wang, J.; Liu, R.; Hsiao, M. Next-Generation Cancer-Specific Hybrid Theranostic Nanomaterials: MAGE-A3 NIR Persistent Luminescence Nanoparticles Conjugated to Afatinib for In Situ Suppression of Lung Adenocarcinoma Growth and Metastasis. Adv. Sci. 2020, 7, 1903741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Jia, E. Ovarian cancer targeted hyaluronic acid-based nanoparticle system for paclitaxel delivery to overcome drug resistance. Drug Deliv. 2016, 23, 1810–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadighian, S.; Rostamizadeh, K.; Hosseini-Monfared, H.; Hamidi, M. Doxorubicin-conjugated core–shell magnetite nanoparticles as dual-targeting carriers for anticancer drug delivery. Colloids Surf. B Biointerfaces 2014, 117, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sui, J.; Ma, M.; Hu, J.; Sun, Y.; Yang, L. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J. Control. Release 2020, 326, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent advances in epsilon-poly-L-lysine and L-lysine-based dendrimer synthesis, modification, and biomedical applications. Front. Chem. 2021, 9, 169. [Google Scholar] [CrossRef]
- Shi, C.; He, Y.; Feng, X.; Fu, D. ε-Polylysine and next-generation dendrigraft poly-L-lysine: Chemistry, activity, and applications in biopharmaceuticals. J. Biomater. Sci. Polym. Ed. 2015, 26, 1343–1356. [Google Scholar] [CrossRef]
- Devi, L.; Gupta, R.; Jain, S.K.; Singh, S.; Kesharwani, P. Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of Gemcitabine with natural polysaccharides for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101–565. [Google Scholar] [CrossRef]
- Kush, P.; Bajaj, T.; Kaur, M.; Madan, J.; Jain, U.K.; Kumar, P.; Deep, A.; Kim, K.H. Biodistribution and pharmacokinetic study of gemcitabine hydrochloride loaded biocompatible iron-based metal organic framework. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2827–2841. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Reddy, B.; Rachamalla, S.S.; Sistla, R. Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int. J. Biol. Macromol. 2015, 80, 48–56. [Google Scholar] [CrossRef]
- Farid, R.M.; Gaafar, P.M.E.; Hazzah, H.A.; Helmy, M.W.; Abdallah, O.Y. Chemotherapeutic potential of L-carnosine from stimuli-responsive magnetic nanoparticles against breast cancer model. Nanomedicine 2020, 15, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Chew, D.P. Acute myocardial infarction. Lancet 2008, 372, 570–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, T.; Teli, S.; Rijal, J.; Bhat, H.; Raza, M.; Khoueiry, G.; Meghani, M.; Akhtar, M. Costantino Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Rev. Cardiovasc. Ther. 2013, 11, 55–59. [Google Scholar] [CrossRef]
- Everson-Rose, S.A.; Lewis, T.T. Psychosocial factors and cardiovascular diseases. Annu. Rev. Public Health 2005, 26, 469–500. [Google Scholar] [CrossRef]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The Clinical Profile and Pathophysiology of Atrial Fibrillation. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef]
- Tahere, T.; Zohreh, V.; Robabeh, M.; Mehrdad, N. Quality of Nursing Documentations in CCU by Hospital Information System (HIS). IJCCN 2012, 5, 53–62. [Google Scholar]
- Muñoz-Aguirre, P.; Flores, M.; Macias, N.; Quezada, A.D.; Denova-Gutiérrez, E.; Salmerón, J. The effect of vitamin D supplementation on serum lipids in postmenopausal women with diabetes: A randomized controlled trial. Clin. Nutr. Edinb. Scotl. 2015, 34, 799–804. [Google Scholar] [CrossRef]
- Bartels, K.; Karhausen, J.; Clambey, E.T.; Grenz, A.; Eltzschig, H.K. Perioperative organ injury. Anesthesiology 2013, 119, 1474–1489. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, H.; Laurent, S.; Liu, F.; Arsalani, N.; Elst, L.V.; Muller, R.N. Magnetoliposomes as multimodal contrast agents for molecular imaging and cancer nanotheragnostics. Nanomedicine 2011, 6, 529–544. [Google Scholar] [CrossRef]
- Soenen, S.J.; Velde, G.V.; Ketkar-Atre, A.; Himmelreich, U.; De Cuyper, M. Magnetoliposomes as magnetic resonance imaging contrast agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Banai, S.; Chorny, M.; Gertz, S.D.; Fishbein, I.; Gao, J.; Perez, L. Locally delivered nanoencapsulated tyrphostin (AGL-2043) reduces neointima formation in balloon-injured rat carotid and stented porcine coronary arteries. Biomaterials 2005, 26, 451–461. [Google Scholar] [CrossRef] [PubMed]
- McDowell, G.; Slevin, M.; Krupinski, J. Nanotechnology for the treatment of coronary in stent restenosis: A clinical perspective. Vasc. Cell. 2011, 3, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Pan, H.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy. Adv. Chronic Kidney Dis. 2013, 20, 466–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheraghi, M.; Negahdari, B.; Daraee, H.; Eatemadi, A. Heart targeted nanoliposomal/nanoparticles drug delivery: An updated review. Biomed. Pharmacother. 2017, 86, 316–323. [Google Scholar] [CrossRef]
- Yin, X.; Fu, Y.; Yutani, C.; Ikeda, Y.; Enjyoji, K.; Kato, H. HVJ-AVE liposome-mediated Tissue Factor Pathway Inhibitor (TFPI) gene transfer with recombinant TFPI (rTFPI) irrigation attenuates restenosis in atherosclerotic arteries. Int. J. Cardiol. 2009, 135, 245–248. [Google Scholar] [CrossRef]
- Haeri, A.; Sadeghian, S.; Rabbani, S.; Shirani, S.; Anvari, M.S.; Dadashzadeh, S. Physicochemical characteristics of liposomes are decisive for their antirestenosis efficacy following local delivery. Nanomed 2017, 12, 131–145. [Google Scholar] [CrossRef]
- Oduk, Y.; Zhu, W.; Kannappan, R.; Zhao, M.; Borovjagin, A.V.; Oparil, S. VEGF nanoparticles repair the heart after myocardial infarction. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H278–H284. [Google Scholar] [CrossRef]
- Schwarz, E.R. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat—Angiogenesis and angioma formation. J. Am. Coll. Cardiol. 2000, 35, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Güngör, S.; Kahraman, E. Nanocarriers mediated cutaneous drug delivery. Eur. J. Pharm. Sci. 2021, 158, 105638. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 2, 20210011. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; Hussein, M.Z.; Bullo, S.; Arulselvan, P. Chlorambucil- iron oxide nanoparticles as a drug delivery system for leukemia cancer cells. Int. J. Nanomed. 2021, 16, 6205. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef] [PubMed]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Siraj, E.A. Targeted drug delivery—From magic bullet to nanomedicine: Principles, challenges, and future perspectives. J. Multidiscip. Healthc. 2021, 14, 1711. [Google Scholar] [CrossRef]


| Year | Types of NPs | Drug Delivery Approaches | Diseases | Applications | Characterization | References |
|---|---|---|---|---|---|---|
| 1991 | Poly-alkyl-cyanoacrylate nanoparticles | Carrier that delivers drug to target specific site. | Cancer | Cancer chemotherapy and intracellular antibiotherapy. | Scanning electron microscope (SEM) | [37,38] |
| 1992 | Calcium hydroxyapatite ceramic (CHC) | Drug gentamicin placed in the porous blocks of calcium hydroxyapatite antibiotics (CHA). | Chronic osteomyelitis (animal model) | The bactericidal activity was retained and drug shows effective results. | No in vivo experiments performed | [39,40] |
| 1993 | Nano and micro particles | Micro-particulate system used for the administration of the drug. | Enhance oral immune system (immunization) | In vitro, self-diffusion, liberation due to erosion, pulsed delivery due to oscillating field. | In vitro experiments performed | [41,42] |
| 1994 | Acrylic acid copolymer NPs | Acrylic acid, acrylic amide, acrylic-butyl ester, and methacrylic methyl ester used as copolymer in drug delivery. | No | Help opsonin to reach specific target site and also enhance reticuloendothelial system. | Small angle X-ray scattering | [43,44] |
| 1995 | Poly-alkyl-cyanoacrylate (PECA) nanoparticles | Ofloxacin (OFX) and perfloxacine entrapped in PECA nanoparticles. OFX system more efficient than PFX system. | Bacterial diseases | The fluoro-quinolone-loaded nanoparticles enhance antimicrobial activity of the drug. | Freeze fracture electron microscopy, physicochemical characterization | [45,46] |
| 1996 | Protein and peptides-based NPs | Monoclonal antibodies, recombinant proteins transported to BBB by chimeric peptide approach. | Alzheimer’s disease | Avidin conjugate with BBB vector to transport all proteins across BBB. Vasoactive intestinal peptide cures brain diseases. | No characterization of physiologic-based strategy | [47,48] |
| 1997 | Nanoparticle | Nanoparticles as carrier to deliver drug to intra-arterial localization system. Cather based delivery | Restenosis (arterial reobstruction) | Easily penetrate into the arterial wall and without causing injury. Biocompatible and effective for restenosis treatment. | No | [49,50] |
| 1998 | Diblock copolymer nanoparticles | Micelles and nanosphere carry genes and hydrophobic drugs to target site. | No | Help to sustain drug rate. Solubilize, release, and protect drugs. Enhance retention time in the blood. | No | [51,52] |
| 1999 | Chitosan nanoparticles | Potential of chitosan nanoparticles to improve absorption of insulin through nasal cavity. | Diabetes | MicroAB assay used to determine insulin loading and release. | Zeta potential, laser doppler anemometry, photon correlation spectroscopy | [53,54] |
| 2000 | Liposome with hyperthermia as nanoparticles | Increased drug delivery to tumor. Hyperthermia helps liposome to work properly. | Ovarian carcinoma | Helpful in human cancer treatment. | Experiments performed | [55,56] |
| 2001 | PEGylated poly-cyano-acrylate nanoparticles | Efficient drug carrier to deliver therapeutic molecules in prion disease test. | Prion Diseases | Long retention time in blood as compared to non-PEGylated nanoparticles. Brain and spleen target tissues show uptake higher in scrapie-infected animals. | Experiments performed | [57,58] |
| 2002 | Transferrin mediated receptor endocytosis | Transferrin and transferrin receptor in drug and in gene transference via the BBB. | Cancer and Brain diseases | Transferrin receptor interceded iron uptake; regulation of transferrin receptor expression, anticancer drugs site-specific to tumor cells. | No | [59,60] |
| 2003 | L-nanoparticles | Intravenous injection of L-particles loaded with green dye shows hepatocellular carcinoma in humans. | I-Hepatitis B II-Hepatocellular carcinoma III-Hemophilia | Hepatitis B virus infects liver hepatocyte cells. L-nanoparticles deliver drugs or genes efficiently and specifically to the targeted hepatocyte cells in a mouse xenograft model. | No | [61,62] |
| 2004 | Colloidal gold nanoparticles | Colloidal gold nanoparticles used as vector to carry tumor necrosis factor (TNF) towards specific part of tumor in mice. | MC-38 carcinoma tumor | The designed vector PT-cAu-TNF bound on the surface of the gold NPs. Intravenous injection shows effective results in MC-38 carcinoma tumor. | TEM, dynamic light scatter, and differential centrifugal sedimentation, zeta potential | [63,64] |
| 2005 | Liposomes, nanoparticles | Vitamin Folic acid placed inside cationic liposomes and conjugate liposomes to folate ligand act as carrier and chemotherapeutics agents, and DNA attaches to the receptor-bearing cancer cells in vitro. | Cancer (human nasopharyngeal and prostate tumor) | Folate-associated, lipid-based nanoparticles transport DNA with high transfection efficacy and constraining tumor progress with intratumoral shot into human nasopharyngeal and prostate malignancy using an HSV-tk/GCV treatment system. | No | [65,66] |
| 2006 | Folate-conjugated starch nanoparticles (StNP’s) | Folate changed with PEG coupled to the exterior of starch NPs to attain the FA-PEG/StNPs. Doxorubicin loaded on FA-PEG/StNP. | Liver cancer | In vitro, FA-PEG/StNP targeted on liver cells BEL7404. It reduced DOX toxicity. This combination can be suitable for cancer targeting drug haulers in future. | AFM and zeta potential, UV Spectro-photometer characterize particle size determination | [67,68] |
| 2007 | Gold nanoparticles (AuNPs) | Drug and gene delivery approach to deliver drugs and genes by using gold nanoparticles. The transfection efficacy for beta galactosidase with various MMPCs. | Human nasopharyngeal carcinoma | Properties of drug transfer like reduced toxicity, treating acute diseases, uptake and release rate using fluorophore AuNPs provide added insight in future. | Fluorescence and bright-field microscopy | [69,70] |
| 2008 | PEGylated gold nanoparticles | Very effective drug transfers with AuNPs’ vector for in vivo photodynamic treatment in cancer. | Cancer | The diversity in medicine released in vitro in two-phase solution system. In vivo in cancer-bearing mice shows that the way of drug carriage is enormously well-planned, and submissive targeting prefers the tumor area. | TEM and image analysis, DLS measurement, UV-vis, and fluorescent spectrophotometer | [71,72] |
| 2009 | Alginate/ Chitosan (Alg/Chi) nanoparticles | Nanoparticles of alginate/chitosan polymers were arranged by pre-gel preparation method via drop-wise addition of several concentrations of CaCl2 to a definite concentration of sodium alginate. | No | Optimization of Alg/Chi NPs and preparation are areas of this research. Some parameters like ratio of Alg/Chi, ratio of CaCl2/Alginate and N/P can disturb size and loading ability of these particles. | Zeta potential, photon correlation spectroscopy, scattering particle size analyzer, FTIR analysis, DSC analysis | [73,74] |
| 2010 | Mesoporous silica nanoparticles | Targeted carriage of chemotherapeutic mediator methotrexate (MTX) to tumor cells by means of poly (ethylene mine)-functionalized mesoporous silica small units as vectors for drug delivery. | Cancer | (a) Choice of adaptable surface functionalization; (b) High level of cell specificity and effective cellular uptake; (c) A slight grade of early seepage and the measured release of the medicine; (d) Low cytotoxicity of the transporter. | Scanning electron microscope (SEM) | [75,76] |
| 2011 | Nano diamond (ND) or diamond nanoparticles | Nano diamonds have ability to transport small interfering RNA into sarcoma (Ewing) cells. Was examined with evaluation of the route of in vivo anticancer nucleic acid drug transfer. | Ewing Sarcoma Cells (Cancer) | Well-organized delivery of oligonucleotide by a cationic nano-diamond nanoparticle: (i) Suitably robust adsorption of the biomolecule on the particle surface across the cell membrane deprived of damage of material; (ii) The severance of the compound on the time-scale of a cell division cycle. | FT-IR confirm the absorption of PAH on nano-diamonds and zeta potential | [77,78] |
| 2012 | Silver nanoparticles | This method was to design stable silver NP vector to make larvicides of mosquitos to destroy mosquitos’ life with drugs. | Malaria, Dengue fever, Filariasis | The leaf potage of Annona squamosa used as an active capping and reducing mediator for the fusion of silver nanoparticles. | Ultraviolet spectrophotometry, X-Ray diffraction, FT-IR, SEM | [79,80] |
| 2013 | Silver nanoparticle | Nanoparticles of noble metal show potential as photo-activated vectors for drug delivery. SNPs conjugated with thiol-terminated photo-liable DNA oligonucleotides. | Photo-activated gene silencing | Good consistency to nucleases, hybridization amplified action upon photo release, and effective cellular uptake as associated to commercial transfection vectors. | UV-spectrophotometer, fluorescent confocal microscopy | [81,82] |
| 2014 | Silver nanoparticles as drug-loading vector | Silver nanoparticles synthesized from plant Pongamia pinnata by green method. | Dengue | Medically active plant and earth eco-friendly. Larvicidal action of silver nanoparticles and leaf extract contrary to Aedes aegypti showed positive results. | UV-visible absorption spectrum, TEM, XRD, FTIR | [83,84] |
| 2015 | Polyamidoamine nanoparticles | Polyamidoamine nanoparticles work as nanocarrier and deliver anti-malarial drug to the targeted sites. It also works as nanomedicine. | Malaria | Union of doxorubicin and polymers increases drug solubility, enhances its blood half-life, decreases toxicity, and enhances targeting. | Fluorescence-assisted cell sorting, transmission electron microscopy, confocal immunofluorescence | [85,86] |
| 2016 | Solid Lipid nanoparticles (SLNP) | Electroporation and nanocarrier used to deliver drugs. In this study, SLNP laden with cyanine type IR-780, flavonoid derivatives, photosensitizer through solvent diffusion method. | Colon cancer | Drug transfer potential of therapeutics compressed with electroporation. | Confocal laser scanning microscopy (CLMS) for the estimation of F-actin AFM and DLS | [87,88] |
| 2017 | Filamentous bacteriophage and phage-mimetic nanoparticles | Delivery of drug and gene through phage particles. Phage can be chemically altered or genetically designed to load drugs and transfer foreign genes. | Bacterial and viral diseases | Filamentous bacteriophage used in the making of mark medicine transfer as virus-based delivery system. The bacteriophage uncovered with mark-definite peptides or antibodies can be bound with other carriers (such as liposomes, inorganic NPs) to make a unique transfer scheme. | No | [89,90] |
| 2018 | Mesoporous silica nanoparticles (MSNs) | Through electrostatic absorption, MSNs loaded with surface-hyper-branching polymerized poly (ethylene- mine) for loading siRNA. | No | The practice of non-viral vectors can solve most of these problems like short time, noxiousness while inorganic, and non-viral vectors, like MSNs, are also very affordable and vigorous. | Transmission electron microscopy, dynamic light scattering (DLS), and zeta potential involved in particle size determination | [91,92] |
| 2019 | Chitosan nanoparticles | Drug loaded on chitosan nanoparticles to deliver to targeting sites. All types of drug delivery sites involved. | No | Ocular drug delivery, vaccine delivery, perioral delivery, vaccine transfer, mucosal and nasal drug transfer, gene carriage, pulmonary drug delivery, buccal medicine distribution, vaccine transfer, and cancer treatment. | No | [93,94] |
| 2020 | Mesoporous silica NPs with folic acid (MSN−COOH-Tet-HBP-FA) | This approach is pH subtle drug delivery system built on folic-acid-targeted HBP to re-form/reshape the mesoporous silica nanoparticles. | Cancer | The hyper-branched polymer HBP encapsulates the drug particles in the mesopores as a lid, which progresses the permanency of the carrier material and permits the drug to attain “zero pre-release” within 20 h in a usual physiological atmosphere. | XRD, TEM, HNMR spectra, SEM, UV-analysis, Thermogravimetric analysis (TGA) | [95,96] |
| 2021 | Novel silver nanoparticles | In this approach, DNA or messenger RNA (mRNA) sequences are transported to the body to produce proteins, which copy disease antigens to arouse the immune response. | SARS-CoV-2 | The nucleic acid vaccines comprise cell-mediated and humoral immunity activation, affluence of strategy, quick malleability to altering pathogen strains, and customizable multi-antigen vaccines. To fight the SARS-CoV-2 epidemic and many other ailments, nucleic acid vaccines seem to be a hopeful way. | No | [97] |
| 2021 | 1-Lipid based nanoparticles 2-Metal and metal oxide NPs 3-Resveratrol-zinc NPs | These nanoparticles have crucial role in the COVID-19 success rate. Metals such as Au, Ag, Zn, Cu have potential in controlling coronavirus due to their discrete features. It is a drug delivered via carrier. It gives immuno-anti-inflammatory viral retort. | COVID-19 SARS-Cov-2 viral disease COVID-19 | It helped in the COVID-19 treatment vaccines, such as Doxil and Onpattro, and has a good success rate. Such NPs have been used in prevention like face masks, various immune sensors, and coatings on various things. Resveratrol-zinc nanoparticles possess a chief pharmacokinetic gain for COVID-19. | No COVID-19 mono and adjuvant therapy | [98,99,100] |
| 2022 | 1-Iridium oxide NPs 2-Chitosan nanoparticles | A nanoprobe was synthesized for in vivo fluorescence tomography of microRNA and coactive photothermal dealings of lump. It is a biotic macromolecule-based medicine transfer system to advance the curative potential of non-natural neural control networks. | Cancer Nervous breakdown | Nanoprobe helped in vivo in healing studies and continuously killed the lump growth. Theses neuroprotective mediators are merged into the structure of NGCs and delivered into brain via NPs. | No Nanocarriers are biocompatible, biodegradable, non-immunogenic, constant, and hold tunable properties | [101,102] |
| Nanomedicine Names | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Tacrine-loaded polymeric NPs | NPs are reserved in the brain for long time, biocompatible, low in cost, control drug release, and targeted conjugation with ligands | Slowly degradable, sometimes uncertain toxicity | [145] |
| Rivastigmine-loaded polymeric NPs | They increase drug concentration in the brain, avoid phagocytosis by RES | Increase oxidative stress, toxicity | [146] |
| Piperine-loaded SLNPs | Widely examined, fewer side effects of drugs, improved therapeutic effects and drug solubility | Low loading capacity, easily cleared by reticuloendothelial system | [147] |
| Folic-acid-loaded liposomes | Highly biocompatible and biodegradable, High stability and bioavailability, active surface targeted | Difficulty in binding with lipids, low stability and drug carriage rate | [148] |
| Beta-Asarone-loaded nanoemulsions | Improved bioavailability, capability to hydrolyze hydrophobic and hydrophilic drugs | Thermodynamically unstable, instant drug release | [149] |
| NP Name | NP Types | Drug Loaded on NPs | Cancer Type | Model | Action | Ref. |
|---|---|---|---|---|---|---|
| DOX-SL-GG AuNPs | Gold nanoparticles | Doxorubicin | Glioma and glioma stem cell lines | In vitro | Endocytosis occurs. Cytotoxic activity increased both on LN-229 glioma cells and HNGC-2 glioma stem cells. | [157,158] |
| Lapatinib-loaded human serum albumin | Albumin-bound nanoparticle | Lapatinib | Brain metastasis | Murine model in vitro | Constrain movement, invasion and adhesion of high brain-metastatic 4T1 cells. | [159,160] |
| Lapatinib-incorporated lipoprotein like NPs | Lipoprotein-like nanoparticles | Lapatinib | Glioma | In vivo murine model | Both LTNPs (10 mg kg−1) and LTNPs (30 mg kg−1) significantly constrain the progress of U87 xenografts. | [161,162] |
| Gold–iron oxide nanocomposites | Curcumin–lipoic acid conjugate | Glutathione | Brain cancer | Cytotoxicity and apoptosis assay | Comparatively greater cytotoxicity against cancerous U87MG cells than standard astrocyte cells. | [163,164] |
| Tocopherol polyethylene glycol chitosan nanoparticles | Fabricated synergistic bioadhesive nanoparticles | Docetaxel | Brain cancer | Enhance cellular uptake and cytotoxicity | Synergistic influence of nanoparticles has increased the delivery of docetaxel into brain melanoma cells. | [165,166] |
| Chitosan or glycol chitosan (GCS) nanoparticles (NPs) | Methotrexate-loaded chitosan and glycol chitosan-based nanoparticles | Methotrexate (MTX) | C6 glioma cells | Cytotoxicity assay and cell lines | Nanoparticles show cytotoxicity against C6 cells line and are able to control MDCKII-MDR1 cell hindrance. | [167,168] |
| Lipid–drug-conjugated (LDC) nanoparticle | 5-FU (fluorouracil)nanoparticles | Fluorouracil | Brain cancer glioma cells | In vitro cytotoxic activity and human glioma cell lines in vivo | The effectiveness of 5-FU to medicate the brain malignancy is improved when it is designed with LDC nanoparticles. | [169,170] |
| Nanomaterial (Organic Nanomaterial) | Material Used | Drug Loaded with NPs | Animal Model | Disease | Description | Ref. |
|---|---|---|---|---|---|---|
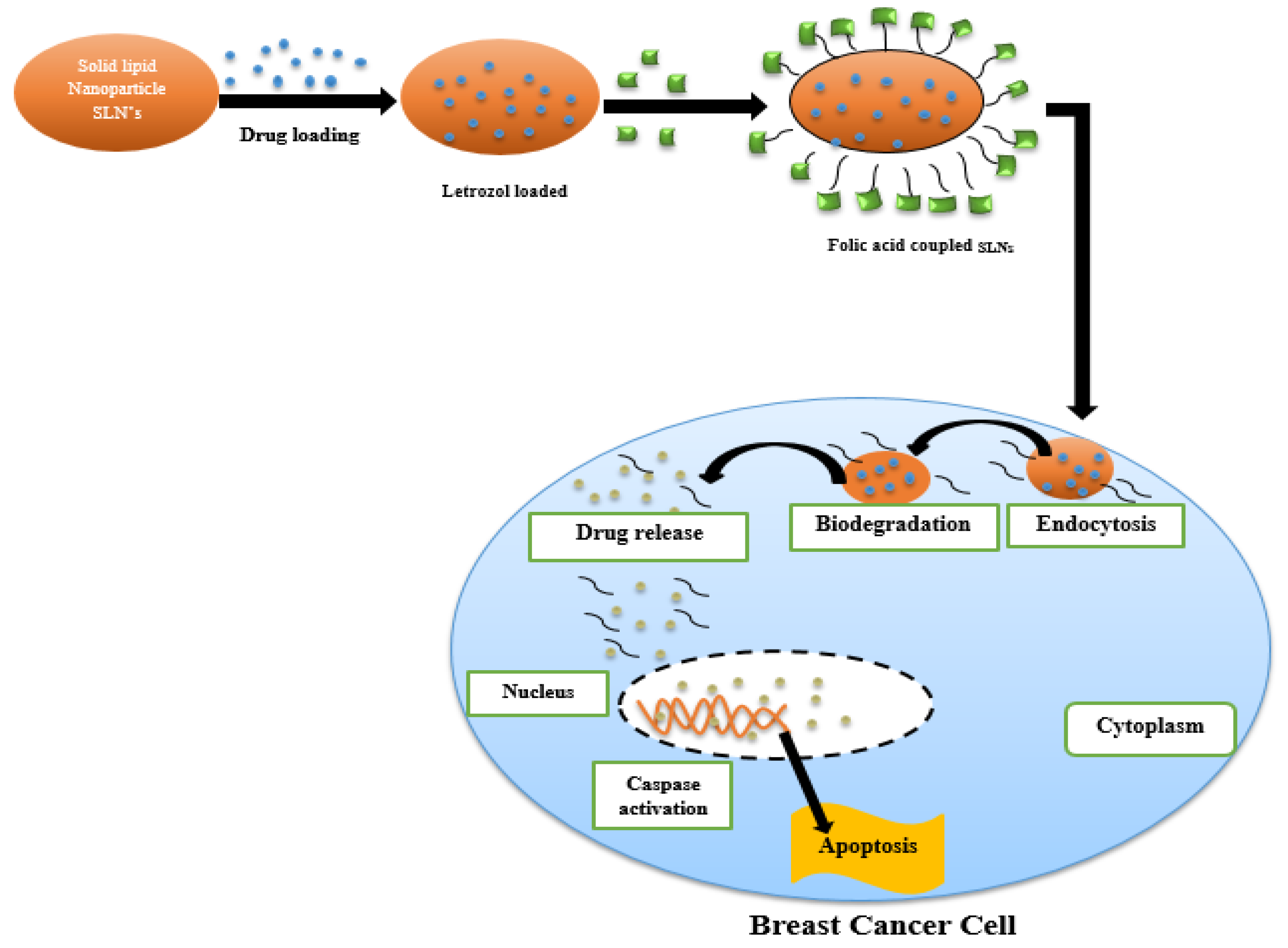
| Solid lipid nanoparticles (SLNPs) | Folic-acid-receptor-targeted solid lipid nanoparticles | Letrozol (LTZ) Folic acid | In-vitro MCF-7 cancer cell lines | Breast cancer | Lactate dehydrogenase (LDH) and 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays to check cell membrane damage. Caspase-3 activity and TUNEL assays were performed to confirm induced apoptosis. | [182,183] |
| Curcumin–Solid Lipid nanoparticles (CURC-SLNs) | CURC-loaded SLNs and doxorubicin p-glycoprotein (Pgp) | Doxorubicin (DOX) | In-vitro | Breast cancer | Curcumin-loaded SLNs 5–10 folds more effectively than curcumin in free form, increasing toxicity in Pgp-expressing triple negative breast cancer. | [184,185] |
| Copolymer-magnetite nanoparticles | doxorubicin–core-shell chitosan nanoparticles | Doxorubicin (DOX) | In-vitro | HER2-over-express in breast cancer | Anti-HER2-conjugated O-succinyl chitosan graft pluronic F127 copolymer nanoparticles are effective for the making of anticancer drug carriers. | [186,187] |
| Polymeric nanoparticles | PEGylated ε-poly-l-lysine polymeric nanoparticle | doxorubicin and lapatinib | In-vitro | MCF-7 breast cancer cell | Combination remedy by DMMA-P-DOX/LAP nanoparticles constrains the solid tumors to shrink or disappear completely in the MCF-7 tumor model. | [188,189] |
| Nanomaterial (Inorganic Nanomaterial) | Material Used | Drug Loaded on NPs | Animal Model | Disease | Description | Ref. |
| Colloidal gold nanoparticles Iron-based metal network | Gemcitabine-hydrochloride (GEM)-loaded colloidal gold nanoparticles | Gemcitabine | In vitro (MDA-MB-231) cell line | Human breast cancer adenocarcinoma | Gemcitabine-hydrochloride-loaded gold nanoparticles developed using gum acacia as a polysaccharides-based system. | [190,191] |
| Magnetic nanoparticles | L-carnosine-coated magnetic nanoparticles (CCMNPs) | L-carnosine | In vitro In vivo | Breast cancer | CCMNPs were targeted precisely, amassed in lump, showing noteworthy decrease in lump mass size with no general harmfulness. | [192,193] |
| Nanoparticles | Exposure Method | Animal Model | Description | Used for | Reference |
|---|---|---|---|---|---|
| Poly (L-aspartic acid co lactic acid)/DPPE copolymer nanoparticles | Intraperitoneal injection | Mouse xenograft model | DPPE co-polymer NPs laden with doxorubicin (DOX) | Lung melanoma | [200,201] |
| Poly (β-amino ester) nanoparticle (PBAE) | Intratumoral injection | Mouse xenograft model | PBAE polymers that self-assemble with DNA and evaluated for transfection effectiveness in the p53 mutant H446 SCLC cell line | Small cell lung cancer | [202,203] |
| Lipid polymeric nanoparticles | Intraperitoneal injection | Mice | The receptor factor (EGF) was co-designed with cisplatin plus doxorubicin | Lung carcinoma | [204] |
| Doxorubicin and cisplatin (CDDP) co-loaded nanoparticles | Pulmonary administration | Mouse model | Methoxy poly -poly (ethylenimine)-poly(l-glutamate) copolymers were manufactured as a transporter for the codelivery of DOX and CDDP | Metastatic lung melanoma | [205,206] |
| Redox-responsive plus pH-sensitive nanoparticles | Subcutaneous injection | Mouse xenograft model | PAA-ss-OA-modified Erlotinib (ETB)-loaded lipid nanoparticles (PAA-ETB-NPs) were made using the emulsification and solvent evaporation method | Non-small cell lung melanoma (NSCLC) | [207] |
| Nanoparticles/mesenchymal stem cell (MSC) | Injected by loading on NPs inside the body | Rabbit, mice, and monkey | MSC as lung-melanoma-targeted drug transfer transporters by loading nanoparticles (NPs) with anticancer medicine. MSC demonstrated a greater medicine ingestion ability than fibroblasts | Lung melanoma | [208,209] |
| Hyaluronic-acid-based lipid nanoparticle | Dialysis techniques used in in vitro study | No | Assessment of the capacity of hyaluronic-acid-based nanostructured lipid carriers (NLCs) to improve apigenin (APG) efficacy as Nrf2 inhibitor, in immediate administration with DTX in A549 NSCLC | Lung cancer | [210] |
| MAGE-A3 NIR insistent luminescence nanoparticles | In vitro activity | In vivo mouse model | Cancer-definite hybrid theranostics nanomaterials MAGE-A3 NIR insistent glow nanoparticles coupled to Afatinib for in situ conquest of lung adenocarcinoma | Non-small cell lung carcinoma | [211] |
| Hyaluronic-acid-based nanoparticle | In vivo In vitro | Mice used; in vitro assays used | Paclitaxel delivered via these NPs to cancerous cells to reduce or stop drug confrontation | Carcinoma | [212] |
| Nanocarriers | Experimental Model | Agents | Results | References |
|---|---|---|---|---|
| Polymeric (PLGA) nanoparticle | Balloon injured carotid and stented porcine coronary artery in rats | AG-1295 and AGL-2043 | Inhibition of restenosis | [224,225,226,227,228,229,230] |
| Perfluorocarbon nanoparticles | Human plasma lumps, hyperlipidemic animals | a3b integrins, surface-bound streptokinase, others | In vitro fibrinolysis and in vivo theranostics | [231,232,233,234,235] |
| Cationic nanoparticles | Clinical test, patients with 60 to 99% stricture in main arteries, confined supply via catheter (tube) | Vascular endothelial growth factor is involved to encode viral vector | Major improvement in myocardial perfusion | [236,237,238,239,240,241] |
| VEGF nanoparticles | Mice, murine myocardial infarction model | VEGF proangiogenic cytokine | Myocardial perfusion in coronary patients for heart repair | [242,243,244,245] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. https://doi.org/10.3390/nano12244494
Afzal O, Altamimi ASA, Nadeem MS, Alzarea SI, Almalki WH, Tariq A, Mubeen B, Murtaza BN, Iftikhar S, Riaz N, et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials. 2022; 12(24):4494. https://doi.org/10.3390/nano12244494
Chicago/Turabian StyleAfzal, Obaid, Abdulmalik S. A. Altamimi, Muhammad Shahid Nadeem, Sami I. Alzarea, Waleed Hassan Almalki, Aqsa Tariq, Bismillah Mubeen, Bibi Nazia Murtaza, Saima Iftikhar, Naeem Riaz, and et al. 2022. "Nanoparticles in Drug Delivery: From History to Therapeutic Applications" Nanomaterials 12, no. 24: 4494. https://doi.org/10.3390/nano12244494
APA StyleAfzal, O., Altamimi, A. S. A., Nadeem, M. S., Alzarea, S. I., Almalki, W. H., Tariq, A., Mubeen, B., Murtaza, B. N., Iftikhar, S., Riaz, N., & Kazmi, I. (2022). Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials, 12(24), 4494. https://doi.org/10.3390/nano12244494








