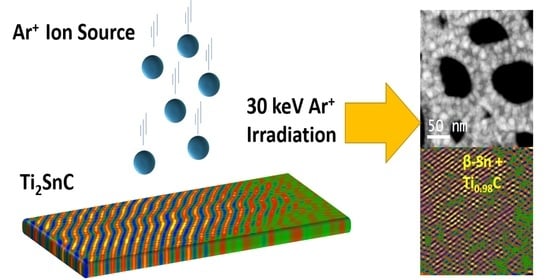
The Key Role of Tin (Sn) in Microstructure and Mechanical Properties of Ti2SnC (M2AX) Thin Nanocrystalline Films and Powdered Polycrystalline Samples
Abstract
:1. Introduction
2. Materials and Methods
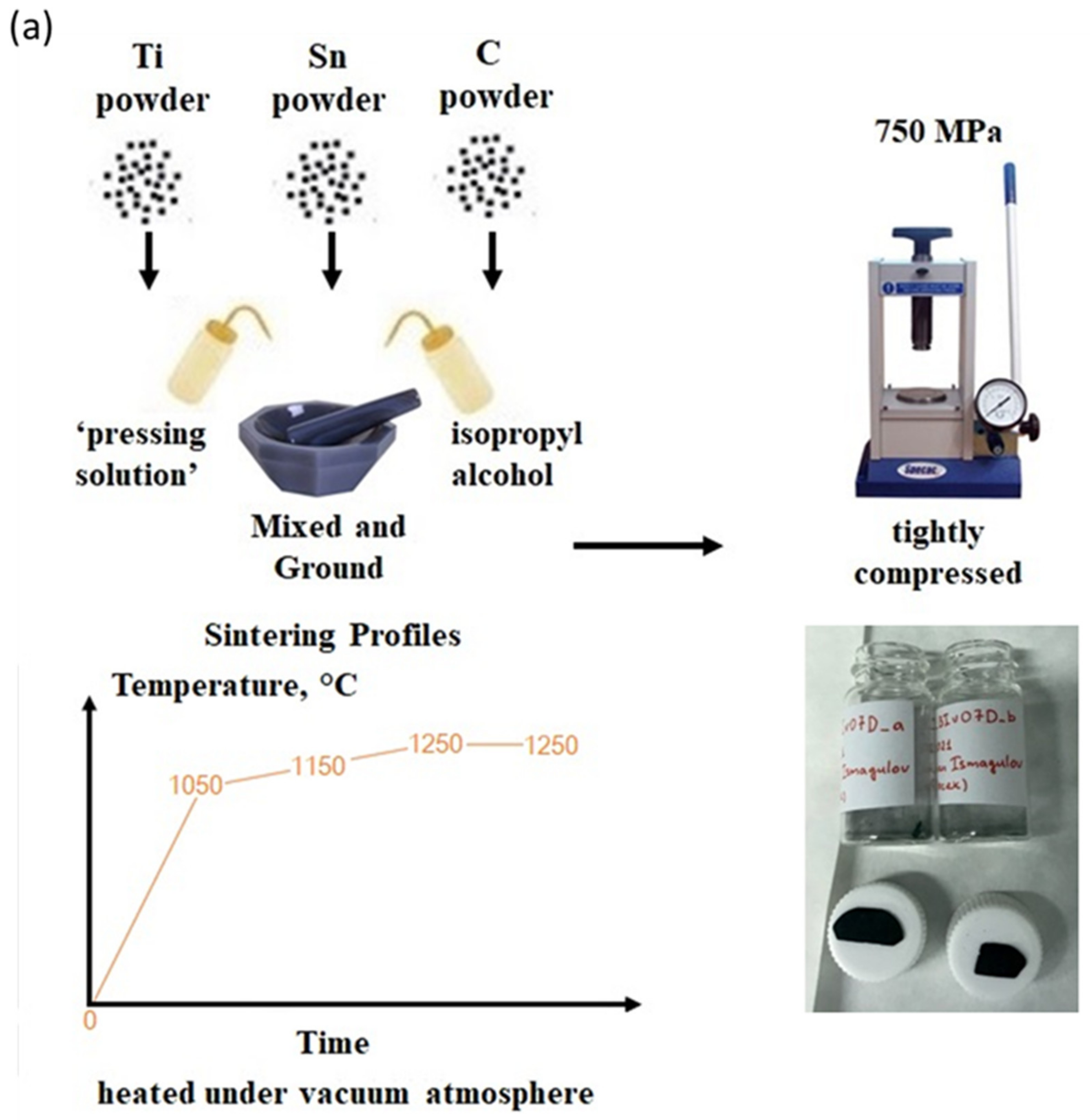
2.1. Synthesis of Ti2SnC M2AX PPSs
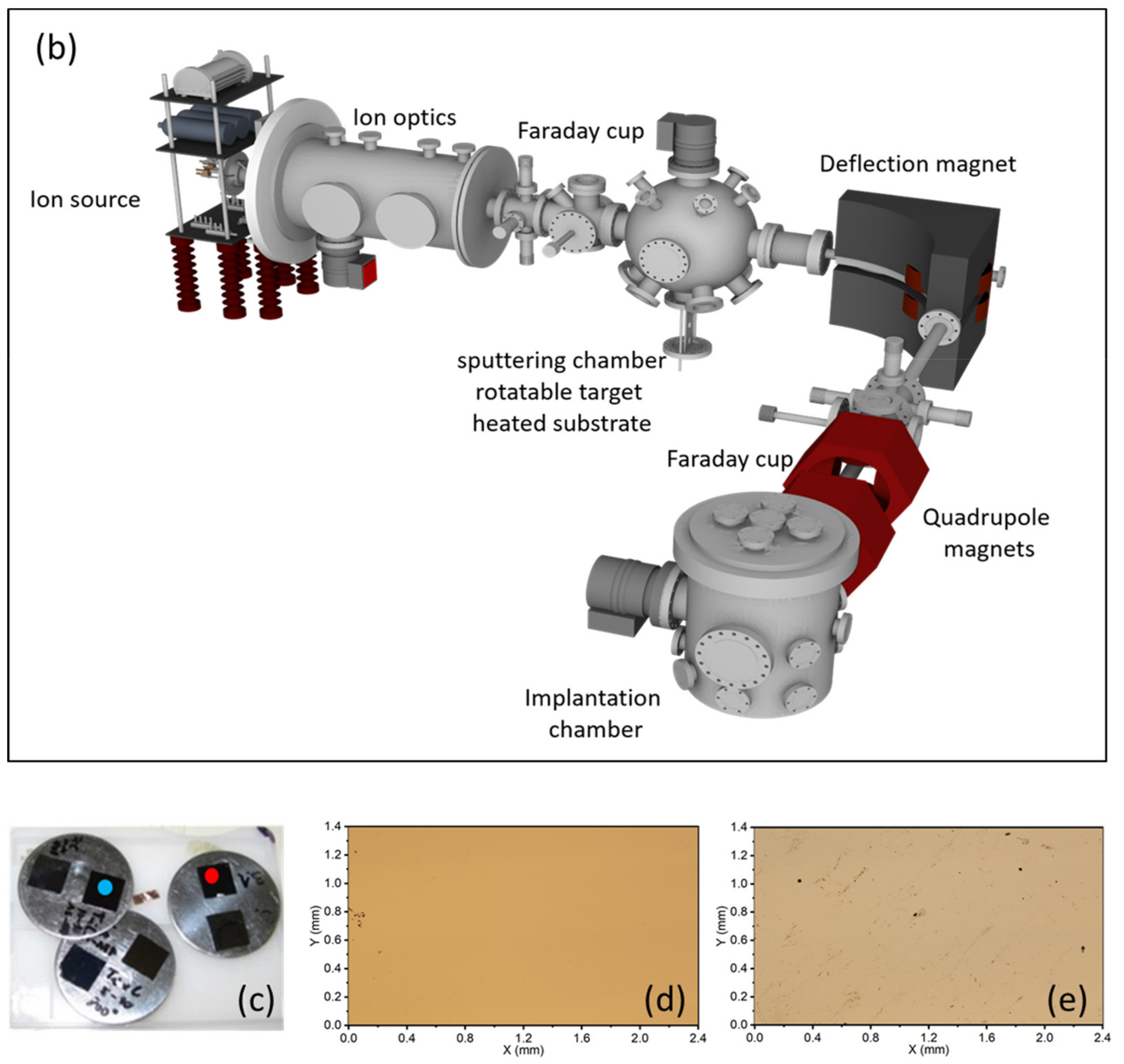
2.2. Synthesis of Ti2SnC TNCFs
2.3. Ion Beam Irradiation
2.4. Methods of Characterization
3. Results and Discussion
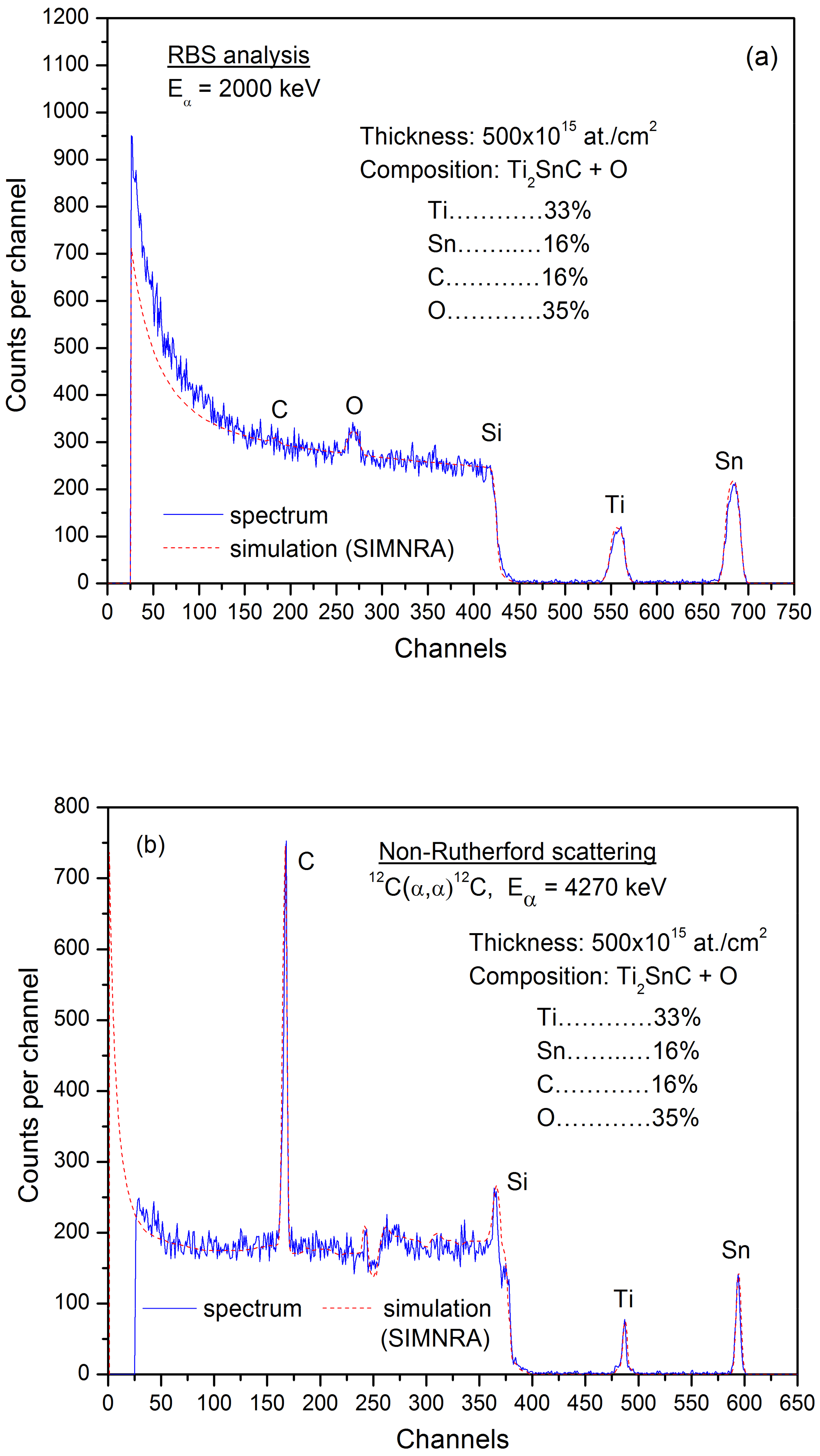
3.1. Elemental Detection by Nuclear Analytical Methods
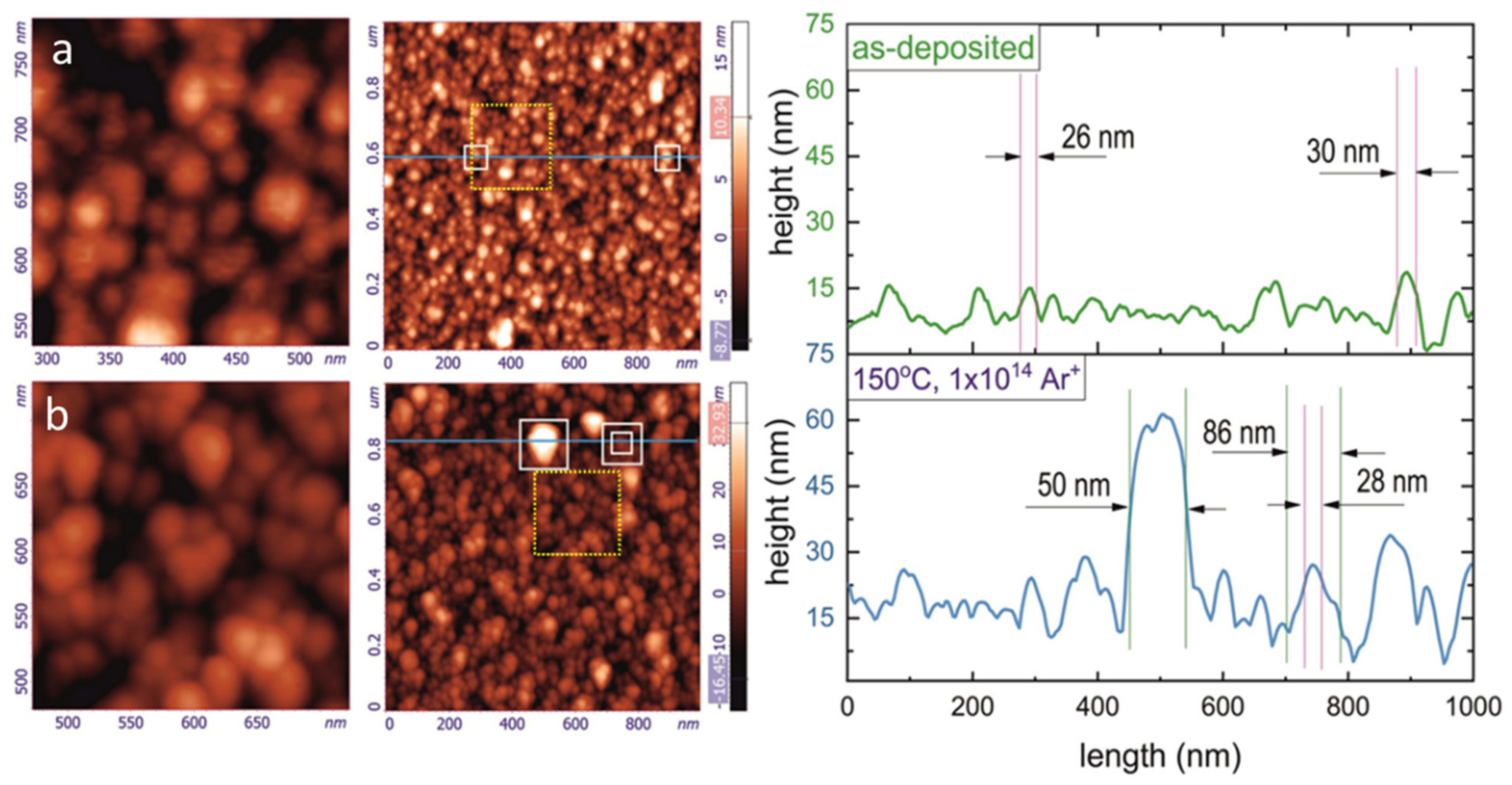
3.2. Morphology of Ti2SnC_AD, Ti2SnC_Ar+TNCFs, and Ti2SnC PPS Imaged by AFM and SEM
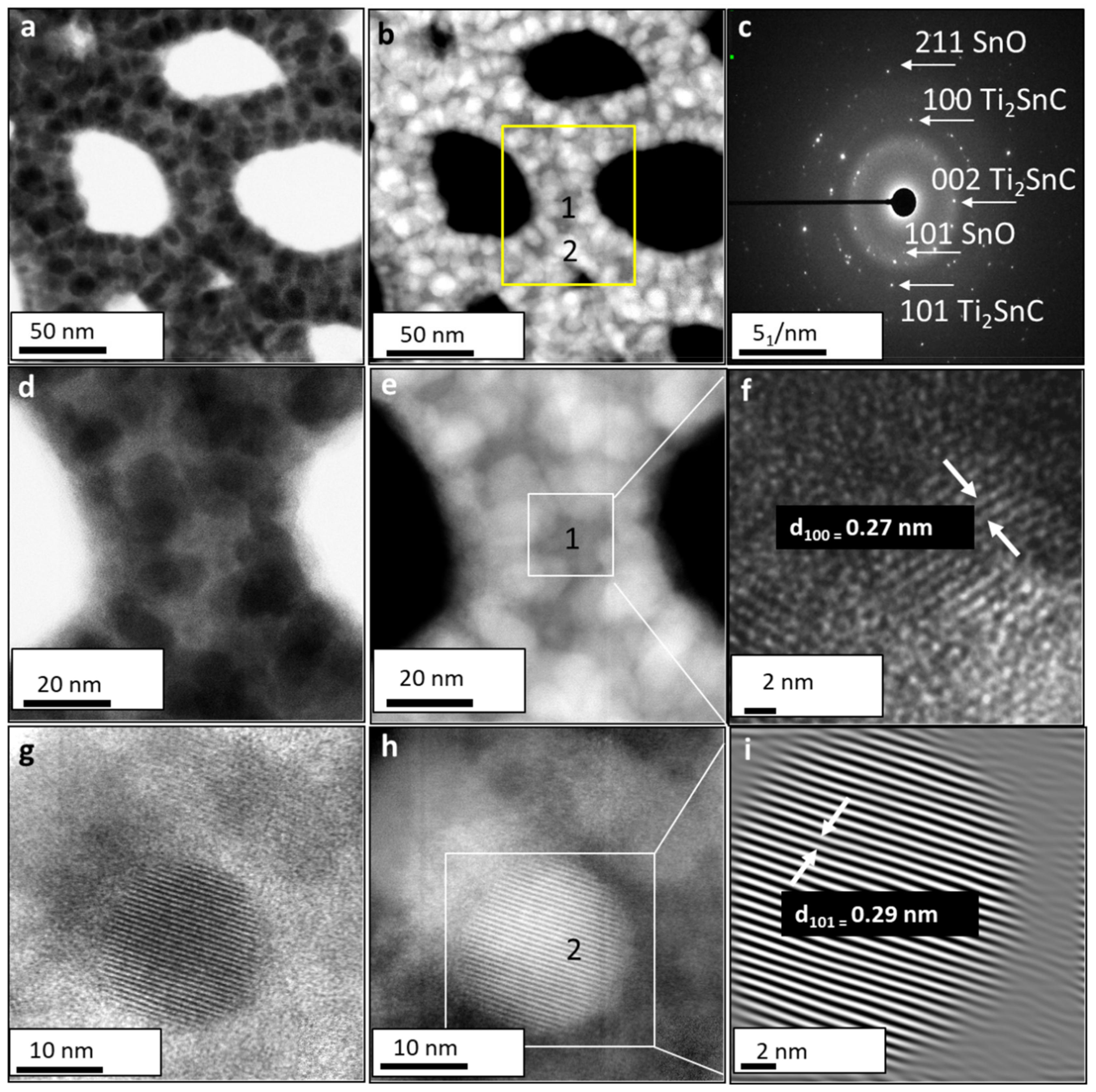
3.3. Structural Analysis of the Ti2SnC_AGTNCF with Atomic-Resolution STEM
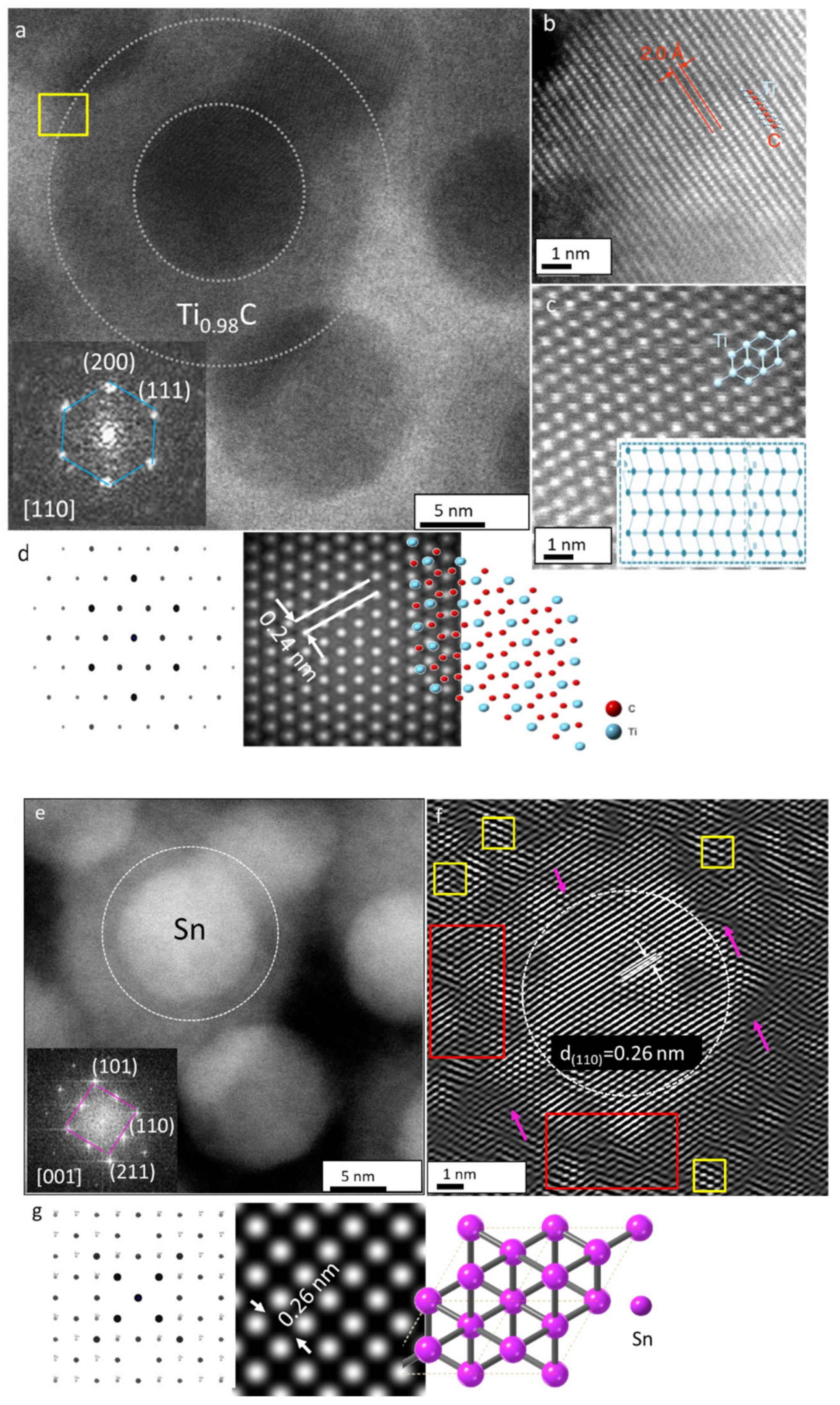
3.4. Structural Analysis of the Ti2SnC_Ar+TNCF with Atomic-Resolution STEM
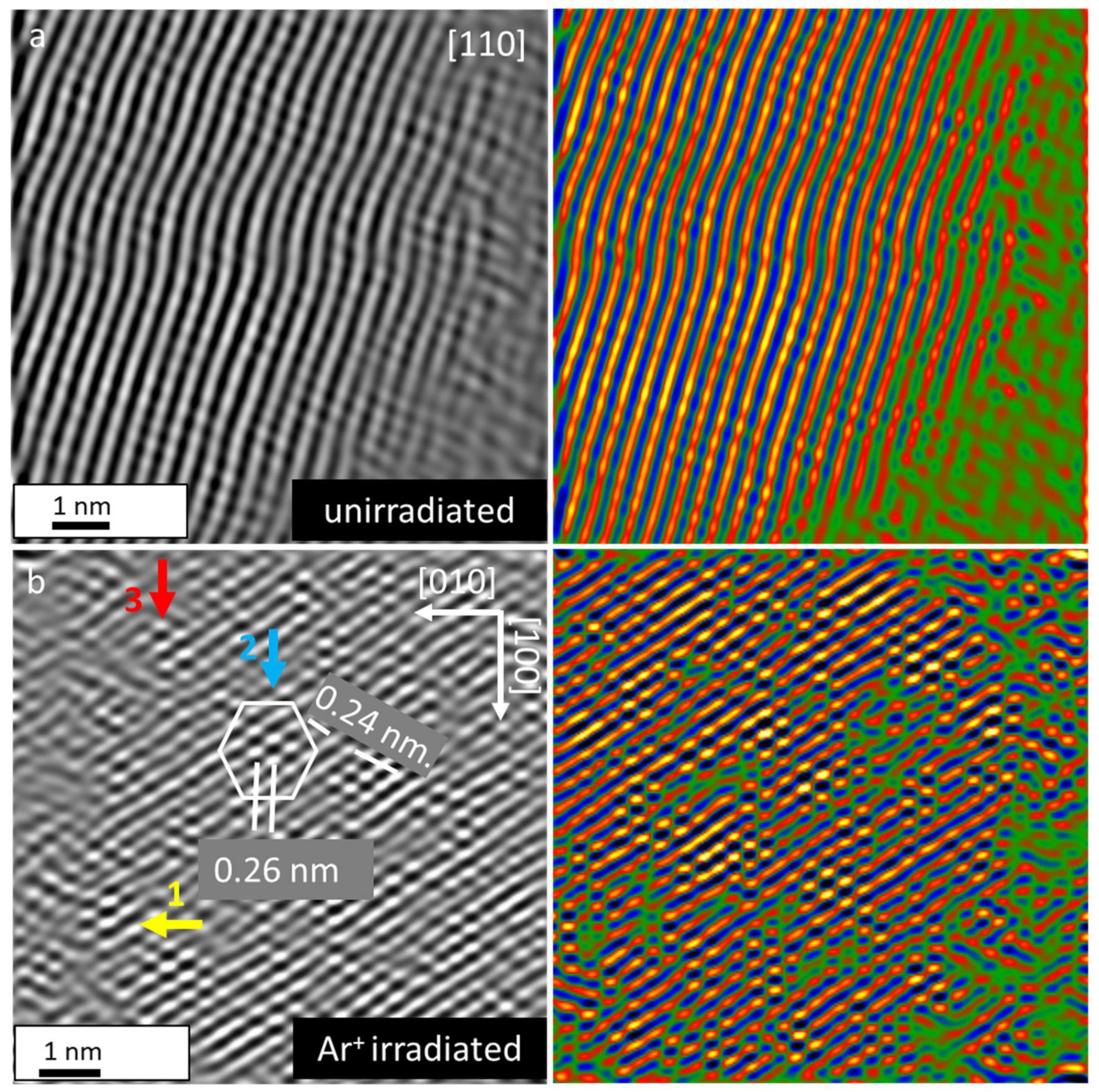
3.5. Mechanism of Irradiation-Induced Structural Transformation in Ti2SnC TNCFs
3.6. Structural Analysis of the Ti2SnC_PPS with HRTEM/SAED
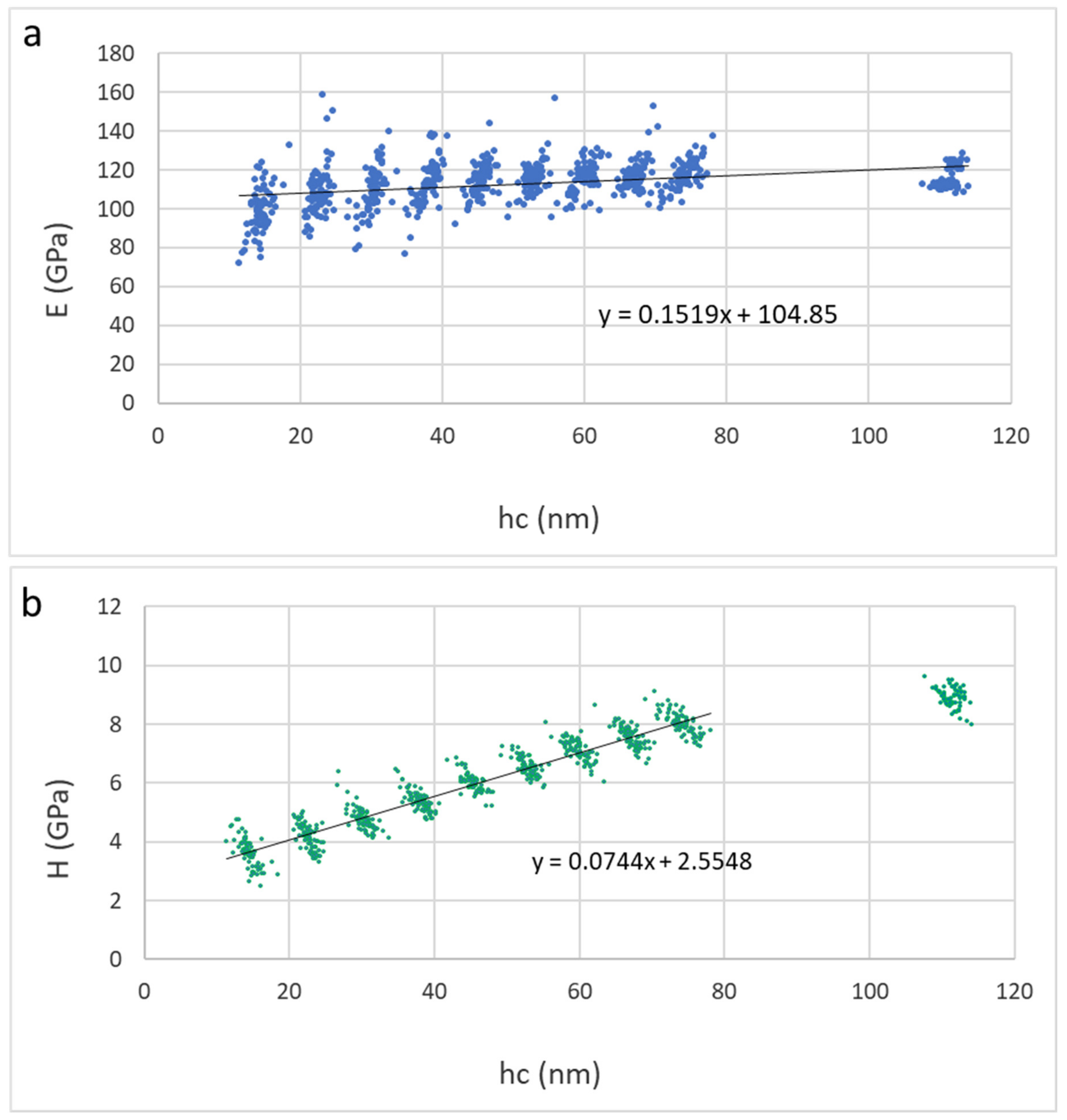
3.7. Nanomechanical Properties Ti2SnC_AG, Ti2SnC_Ar+TNCFs and Ti2SnC_PPS
4. Conclusions
- In conclusion, the microstructure and mechanical properties of Ti2SnC TNCFs, synthesized by an unconventional low-energy ion facility (LEIF) based on Ar+ ion beam sputtering of the Ti, Sn, and C targets have been investigated. Combining high-resolution HAADF-STEM analysis with simulations of SAED patterns, observed that Ti2SnC_AGTNCFs coexist with SnO due to oxidation of Sn during the preparation process. A significant microstructural instability was observed after irradiation of the Ti2SnC_AGTNCFs with Ar+ ion beam having an energy of 30 keV and fluence of 1.1015 cm−2. The results from simulated SEAD patterns are compatible with experimental HAADF-STEM analysis and have suggested the existence of a heterostructure composed of binary Ti0.98C carbide and metallic β-Sn, which could be attributed to the irradiation-induced instability of the ultrathin Ti2SnC film. In addition, Ar+ ion-beam irradiation-induced dislocation and point defects can provide channels for very fast Sn mass transport. The analysis by nanoindentation showed that the irradiated Ti2SnC TNCFs and irradiated Ti2SnC_PPS exhibited promising Young’s modulus and hardness even for the locally disordered structure in Ti2SnC TNCFs. This fact opens the possibility of exploiting the β-Sn/Ti0.98C structure as a composite where a harsh radiation environment could have occurred.
- HRTEM/SAED observations and XRD analyses of the Ti2SnC_PPS documented 70.4% of a single Ti2SnC phase. The calculated value for NPs size by Scherrer’s formula was estimated to be 120 nm. The presence of 9% of Sn avoided the formation of stable intermetallic impurities (Ti3Sn, Ti6Sn5, Ti2Sn, and Ti5Sn3) in Ti2SnC_PPS. The Ti2SnC_PPS irradiated yield the lowest hardness (H) when compared with Ti2SnC_PPS and unirradiated and irradiated Ti2SnC TNCFs. Probably, the low degree of nano crystallinity and tendency to agglomeration upon irradiation can contribute to the surface hardness of polycrystalline bulk materials.
- The approach here presented may be extendable to other M2AX nanostructured materials, and can keep attention for material science applications ranging from protective nanocoating films, ion-beam irradiation resistant parts for nuclear applications and nanoceramics, to their utilization as a precursor for MX phases.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsoum, M.W. The Mn+1AXn Phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Hogberg, H.; Hultman, L. The Mn+1AXn Phases: Materials science and thin-film processing. Thin. Solid Film. 2010, 518, 1851–1878. [Google Scholar] [CrossRef] [Green Version]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Kohlenstoffhaltige ternäre verbindungen (H-phase). Mon. Chem. Verwandte Teile And. Wiss. 1963, 94, 672–676. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. The Max Phases: Unique new carbide and nitride materials: Ternary ceramics turn out to be surprisingly soft and machinable, yet also heat-tolerant, strong and lightweight. Am. Sci. 2001, 89, 334–343. [Google Scholar] [CrossRef]
- Tallman, D.J. On the Potential of Max Phases for Nuclear Applications. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, June 2015. [Google Scholar]
- Whittle, K.R.; Blackford, M.G.; Aughterson, R.D.; Moricca, S.; Lumpkin, G.R.; Riley, D.P.; Zaluzec, N.J. Radiation tolerance of Mn+1AXn Phases, Ti3ALC2 and Ti3SiC2. Acta Mater. 2010, 58, 4362–4368. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Qi, Q.; O’Connor, D.J.; King, B.V.; Kisi, E.H.; Qing, X.B.; Wang, B.Y. Surface damage of Ti3SiC2 By Mev Iodine bombardment. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2013, 307, 536–540. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhou, Y.C.; Wang, J.Y. Tribological behavior of Ti2SnC particulate reinforced copper matrix composites. Mater. Sci. Eng. 2006, 422, 266–271. [Google Scholar] [CrossRef]
- Dong, H.Y.; Yan, C.K.; Chen, S.Q.; Zhou, Y.C. Solid–Liquid reaction synthesis and thermal stability of Ti2SnC powders. J. Mater. Chem. 2001, 11, 1402–1407. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P. The microstructural evolution of Ti2SnC from Sn–Ti–C system by Self-propagating High-temperature Synthesis (SHS). Int. J. Refract. Met. Hard Mater. 2011, 29, 751–754. [Google Scholar] [CrossRef]
- Li, S.; Bei, G.-P.; Zhai, H.-X.; Zhou, Y. Bimodal microstructure and reaction mechanism of Ti2SnC synthesized by a high—temperature reaction using Ti/Sn/C and Ti/Sn/TiC powder compacts. J. Am. Ceram. Soc. 2006, 89, 3617–3623. [Google Scholar] [CrossRef]
- Yeh, C.L.; Kuo, C.W. Effects of TiC addition on formation of Ti2SnC by self-propagating combustion of Ti–Sn–C–TiC powder compacts. J. Alloy. Compd. 2010, 502, 461–465. [Google Scholar] [CrossRef]
- Li, S.; Bei, G.-P.; Zhai, H.-X.; Zhou, Y. Synthesis of Ti2SnC from Ti/Sn/TiC powder mixtures by pressureless sintering technique. Mater. Lett. 2006, 60, 3530–3532. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzales-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Rathel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Wang, X.; Zhang, J. Synthesis of Ti2SnC under optimized experimental parameters of pressureless spark plasma sintering assisted by al addition. Adv. Mater. Sci. Eng. 2018, 2018, 9861894. [Google Scholar] [CrossRef] [Green Version]
- Canam Project. Available online: Http://Www.Ujf.Cas.Cz/En/Research-Development/Large-Research-Infrastructures-And-Centres/Canam/About-The-Project/ (accessed on 12 December 2021).
- Bakardjieva, S.; Ceccio, G.; Vacik, J.; Calcagno, L.; Cannavo, A.; Horak, P.; Lavrentiev, V.; Nemecek, J.; Michalcova, A.; Klie, R. Surface morphology and mechanical properties changes induced In Ti3InC2 (M3AX2) thin nanocrystalline films by irradiation of 100 Kev Ne+ ions. Surf. Coat. Technol. 2021, 426, 127775. [Google Scholar] [CrossRef]
- Gates-Rector, S.D.; Blanton, T.N. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Lábár, J.L. Consistent Index. A (Set) SAED Pattern(S) ProcessDiffraction Program. Ultramicroscopy 2005, 103, 237–249. [Google Scholar]
- Klie, R.F.; Zheng, J.C.; Zhu, Y.; Varela, M.; Wu, J.; Leighton, C. Direct measurement of the low-temperature spin-state transition in LaCoO3. Phys. Rev. Lett. 2007, 99, 047203. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Wang, J.Y.; Zhou, Y.C. Low-temperature instability of Ti2SnC: A combined transmission electron microscopy, differential scanning calorimetry, and X-ray diffraction investigations. J. Mater. Res. 2009, 24, 39–49. [Google Scholar] [CrossRef]
- Cannavò, A.; Vacik, J.; Bakardjieva, S.; Kupcik, J.; Lavrentiev, V.; Ceccio, G.; Horak, P.; Nemecek, J.; Calcagno, L. Effect of medium energy He+, Ne+ And Ar+ ion irradiation on the Hf-In-C thin film composites. Thin. Solid Films 2021, 743, 139025. [Google Scholar] [CrossRef]
- Dias, J.S.; Batista, F.R.M.; Bacani, R.; Triboni, E.R. Structural characterization of SnO nanoparticles synthesized by the hydrothermal and microwave routes. Sci. Rep. 2020, 10, 9446. [Google Scholar] [CrossRef]
- Vázquez-López, A.; Maestre, D.; Ramirez-Castellanos, J.; Cremades, A. In situ local oxidation of SnO induced by laser irradiation: A stability study. Nanomaterials 2021, 11, 976. [Google Scholar] [CrossRef]
- Jaśkaniec, S.; Kavanagh, S.R.; Coelho, J.; Ryan, S.; Hobbs, C.; Walsh, A.; Scanlon, D.O.; Nicolosi, V. Solvent engineered synthesis of layered SnO for high-performance anodes. npj 2D Mater. Appl. 2021, 5, 27. [Google Scholar] [CrossRef]
- Campo, C.M.; Rodríguez, J.E.; Ramírez, A.E. Thermal behaviour of romarchite phase SnO in different atmospheres: A hypothesis about the phase transformation. Heliyon 2016, 2, E00112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, M.S.; Egerton, R.F.; Midgley, P.A. Differentiation of Tin Oxides using electron energy-loss spectroscopy. Phys. Rev. B 2004, 69, 233304. [Google Scholar] [CrossRef]
- Adelmann, C.; Brault, J.; Rouviere, J.-R.; Mariette, H.; Mula, G.; Daudin, B. Atomic-layer epitaxy of GaN quantum wells and quantum dots on (0001) AlN. J. Appl. Phys. 2002, 91, 5498–5500. [Google Scholar] [CrossRef]
- Voyles, P.M.; Muller, D.A.; Grazul, J.L.; Citrin, P.H.; Gossmann, H.J. Atomic-scale imaging of individual dopant atoms and clusters in highly N-Type bulk Si. Nature 2002, 416, 826–829. [Google Scholar] [CrossRef]
- Rafferty, B.; Nellist, D.; Pennycook, J. On the origin of transverse incoherence in z-contrast stem. Microscopy 2001, 50, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.K.; Wiffen, F.W.; Bentley, J.; Stiegler, J.O. Irradiation induced precipitation in tungsten based, W-Re alloys. Metall. Trans. 1983, 14, 655–666. [Google Scholar] [CrossRef]
- Nordlund, K.; Averback, R.S. Point defect movement and annealing in collision cascades. Phys. Rev. 1997, 56, 2421. [Google Scholar] [CrossRef] [Green Version]
- Haberland, H. A model for the processes happening in a rare-gas cluster after ionization. Surf. Sci. 1985, 156, 305–312. [Google Scholar] [CrossRef]
- Ding, A.; Futrell, J.H.; Cassidy, R.A.; Cordis, L.; Hesslich, J. Mass spectrometric and photoionisation investigations of the structure of heterogeneous clusters. Surf. Sci. 1985, 156, 282–291. [Google Scholar] [CrossRef]
- Begemann, W.; Dreihöfer, S.; Meiwes-Broer, K.H.; Lutz, H.O. Sputtered metal cluster ions: Unimolecular decomposition and collision induced fragmentation. Z. Phys. D At. Mol. Clust. 1986, 3, 183–188. [Google Scholar] [CrossRef]
- Fondell, M.; Gorgoi, M.; Boman, M.; Lindblad, A. An HAXPES study of sn, SnS, SnO and SnO2. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Matsumoto, K. Fragmentation of Sputtered Neutrals by SNMS. J. Mass Spectrom. Soc. Jpn. 1991, 39, 93–96. [Google Scholar] [CrossRef]
- Chapon, C.; Gillet, M.F.; Henry, C.R. (Eds.) Small Particles and Inorganic Clusters: Proceedings of the Fourth International Meeting on Small Particles and Inorganic Clusters University Aix-Marseille III Aix-en-Provence; Springer Science & Business Media: Marseille, France, 2012. [Google Scholar]
- Bras, P.; Sterner, J.; Platzer-Björkman, C. Investigation of blister formation in sputtered Cu2ZnSnS4 absorbers for thin film solar cells. J. Vac. Sci. Technol. A: Vac. Surf. Film. 2015, 33, 061201. [Google Scholar] [CrossRef]
- Hardy, H.K.; Silcock, J.M. The Phase Sections at 500 and 350 °C of Al Rich Al-Cu-Li Alloys. J. Inst. Met. 1955, 84, 423–428. [Google Scholar]
- Parish, C.M.; Field, K.G.; Certain, A.G.; Wharry, J.P. Application of STEM Characterization for Investigating Radiation Effects in BCC Fe-Based. J. Mater. Res. 2015, 30, 1275–1289. [Google Scholar] [CrossRef]
- Schäublin, R. Effect of helium on irradiation-induced hardening of iron: A simulation point of view. J. Nucl. Mater. 2007, 362, 152–160. [Google Scholar] [CrossRef]
- Riviere, J.P. Radiation Induced Point Defects and Diffusion. In Application of Particle and Laser Beams in Materials Technology; Misaelides, P., Ed.; NATO ASI Series (Applied Sciences); Springer: Dordrecht, The Netherlands, 1995; Volume 283. [Google Scholar] [CrossRef]
- Lecterc, S.J.; Li, X.; Lescoat, M.L.; Fortuna, F.; Gentils, A. Microstructure of Au-ion irradiated 316L and FeNiCr austenitic stainless steels. J. Nucl. Mater. 2016, 480, 436–446. [Google Scholar]
- Lapauw, T.; Tunca, B.; Potashnikov, D.; Pesach, A.; Ozeri, O.; Vleugels, J.; Lambrinou, K. The double solid solution (Zr, Nb)2(Al, Sn)C MAX phase: A steric stability approach. Sci. Rep. 2018, 8, 12801. [Google Scholar] [CrossRef] [PubMed]
- Titus, M.S.; Rhein, R.K.; Wells, P.B.; Dodge, P.C.; Viswanathan, G.B.; Mills, M.J.; Van derVan, A.; Pollock, T.M. Solute segregation and deviation from bulk thermodynamics at nanoscale crystalline defects. Sci. Adv. 2016, 2, e1601796. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Assali, S.; Oussama Moutanabbir, O. Atomic Pathways of Solute Segregation in the Vicinity of Nanoscale Defects. Nano Lett. 2021, 23, 9882–9888. [Google Scholar] [CrossRef] [PubMed]
- Rathika, R.; Kovendhan, M.; Paul Joseph, D.; Vijayarangamuthu, K.; Sendil Kamur, A.; Venkateswaran, C.; Asokan, K.; Johnson Jeyakumar, S. 200 MeV Ag15+ ion beam irradiation induced modifications in spray deposited MoO3 thin films by fluence variation. Nucl. Eng. Technol. 2019, 51, 1983–1990. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhou, Y.C.; Liao, T.; Zhang, J.; Lin, Z.J. A first principles investigation of the phase stability of Ti2AlC with Al vacancies. Scr. Mater. 2008, 58, 227–231. [Google Scholar] [CrossRef]
- Herring, C.; Galt, J.K. Elastic and plastic properties of very small metal specimens. Phys. Rev. 1952, 85, 1060. [Google Scholar] [CrossRef]
- Sears, G.W. A mechanism of whisker growth. Acta Metall. 1955, 3, 367. [Google Scholar] [CrossRef]
- Tu, K.N.; Thompson, R.D. Kinetics of interfacial reaction in bimetallic Cu-Sn thin films. Acta Metall. 1982, 30, 947–952. [Google Scholar] [CrossRef]
- Zenou, V.Y.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc-doped TiO2 anatase nanoparticles using Scherrer equation and Debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Yaroschuk, G.; Tyagi, S. Fabrication and characterization of M2SnC (M= Ti, Zr, Hf and Nb). Scr. Mater. 1997, 37, 1583–1591. [Google Scholar] [CrossRef]
- Yin, F.; Tedenac, J.C.; Gascoin, F. Thermodynamic modelling of the Ti–Sn system and calculation of the Co–Ti–Sn system. Comput. Coupling Phase Diagr. Thermochem. 2007, 31, 370–379. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, W.J. The effect of addition of Sn to copper on hot compressive deformation mechanisms, microstructural evolution and processing maps. J. Mater. Res. Technol. 2020, 9, 749–761. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Lapauw, T.; Vanmeensel, K.; Lambrinou, K.; Vleugels, J. Rapid synthesis and elastic properties of fine-grained Ti2SnC produced by spark plasma sintering. J. Alloy. Compd. 2015, 631, 72–76. [Google Scholar] [CrossRef]
- Nie, J.F. Applications of atomic-resolution HAADF-STEM and EDS-STEM characterization of light alloys. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Riso, Denmark, 2017; p. 012005. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.F.; Aaronson, H.I.; Muddle, B.C. Proceedings of an International Conference on Solid-Solid Phase Transform; Koiwa, M., Otsuka, K., Miyazaki, T., Eds.; The Japan Institute of Metals: Tokyo, Japan, 1999; p. 157. [Google Scholar]
- Nie, J.F.; Muddle, B.C. The lattice correspondence and diffusional-displacive phase transformations. Mater. Forum 1999, 23, 23–40. [Google Scholar]
- Bakardjieva, S.; Horak, P.; Vacik, J.; Cannavo, A.; Lavrentiev, V.; Torrisi, A.; Michalcova, A.; Klie, R.; Rui, X.; Calcagno, L. Effect of Ar+ irradiation of Ti3InC2 at different ion beam fluences. Surf. Coat. Technol. 2020, 394, 125834. [Google Scholar] [CrossRef]
- Högberg, H.; Emmerlich, J.; Eklund, P.; Wilhelmsson, O.; Palmquist, J.P.; Jansson, U.; Hultman, L. Growth and property characterization of epitaxial MAX-phase thin films from the Tin+1 (Si, Ge, Sn) Cn systems. In Advances in Science and Technology; Trans Tech Publications Ltd.: Acireale, Italy, 2006; Volume 45, pp. 2648–2655. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Ghosh, S.; Gutierrez, G.; Rajput, P.; Grover, V.; Sattonnay, G.; Avasthi, D.K. Grain size effect on the radiation damage tolerance of cubic zirconia against simultaneous low and high energy heavy ions: Nano triumphs bulk. Sci Rep. 2021, 11, 10886. [Google Scholar] [CrossRef]
- Liew, P.J.; Yap, C.Y.; Wang, J. Surface modification and functionalization by electrical discharge coating: A comprehensive review. Int. J. Extrem. Manuf. 2020, 2, 012004. [Google Scholar] [CrossRef]





| Sample | E(GPa) | H(GPa) |
|---|---|---|
| Ti2SnC_AGTNCF (film) | 87.7 | 2.48 |
| Ti2SnC_Ar+TNCF (film) | 97.6 | 2.44 |
| Ti2SnC_PPS pristine (bulk) | 104.9 | 2.56 |
| Ti2SnC_PPS irradiated (bulk) | 100.8 | 2.08 |
| Si substrate, ref. [17] | 166.6 | 15.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakardjieva, S.; Plocek, J.; Ismagulov, B.; Kupčík, J.; Vacík, J.; Ceccio, G.; Lavrentiev, V.; Němeček, J.; Michna, Š.; Klie, R. The Key Role of Tin (Sn) in Microstructure and Mechanical Properties of Ti2SnC (M2AX) Thin Nanocrystalline Films and Powdered Polycrystalline Samples. Nanomaterials 2022, 12, 307. https://doi.org/10.3390/nano12030307
Bakardjieva S, Plocek J, Ismagulov B, Kupčík J, Vacík J, Ceccio G, Lavrentiev V, Němeček J, Michna Š, Klie R. The Key Role of Tin (Sn) in Microstructure and Mechanical Properties of Ti2SnC (M2AX) Thin Nanocrystalline Films and Powdered Polycrystalline Samples. Nanomaterials. 2022; 12(3):307. https://doi.org/10.3390/nano12030307
Chicago/Turabian StyleBakardjieva, Snejana, Jiří Plocek, Bauyrzhan Ismagulov, Jaroslav Kupčík, Jiří Vacík, Giovanni Ceccio, Vasily Lavrentiev, Jiří Němeček, Štefan Michna, and Robert Klie. 2022. "The Key Role of Tin (Sn) in Microstructure and Mechanical Properties of Ti2SnC (M2AX) Thin Nanocrystalline Films and Powdered Polycrystalline Samples" Nanomaterials 12, no. 3: 307. https://doi.org/10.3390/nano12030307