Particles Emission from an Industrial Spray Coating Process Using Nano-Materials
Abstract
:1. Introduction
2. Materials and Methods
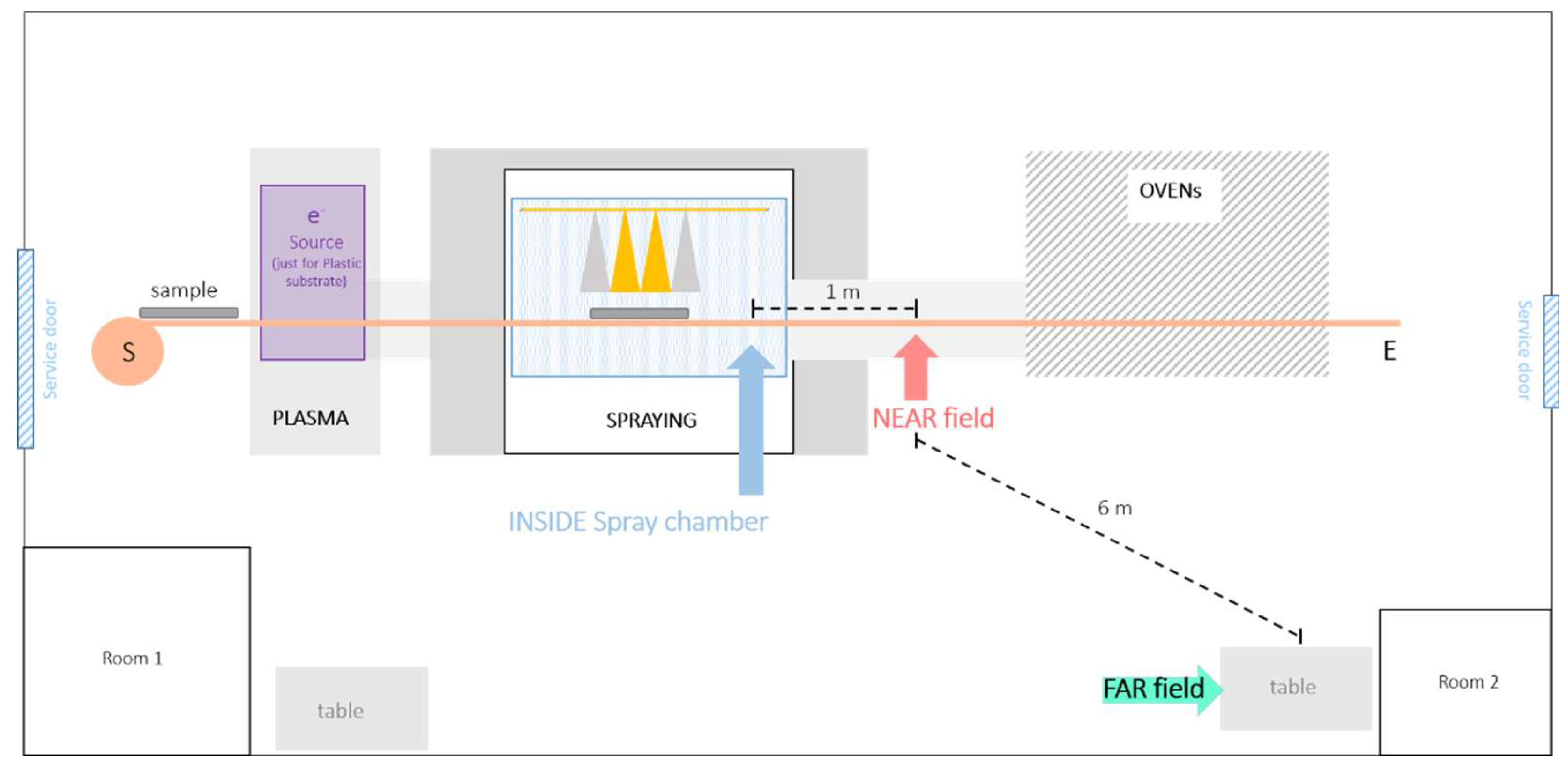
2.1. Materials and Coating Process
2.2. Methods
- Particle mobility size distributions were obtained by Scanning Mobility Particle Sizer (SMPS), composed by a differential mobility analyzer (L-DMA mod. Grimm mod. 5400, Grimm Aerosol, Ainring, Germany), a condensation particle counter (CPC, Grimm mod. 5403, Grimm Aerosol, Ainring, Germany), and an X-ray soft charges neutralizer (TSI mod. 3088; Shoreview, MN, USA) instead of the original one based on 241Am (Grimm Mod. 5522). Nicosia et al. [24], applied a TSI soft X-Ray neutralizer to the Grimm L-DMA column obtaining a transfer function to correct the data. The SMPS scan time was ca 4.5 min with a 1.5 min retrace time. Mobility size was measured in the range from 10 nm to 1 μm.
- Particle optical size distributions were obtained by an optical particles counter (OPC Grimm mod. 1107 D, Grimm Aerosol, Ainring, Germany) in the 0.3–30 μm size range (in 32 channels) with a time resolution of 6 sec.
- Aerosol mass concentration was detected using an aerosol photometer (DustTrack mod. 8530, TSI Inc., Shoreview, MN, USA).
- Particle optical size distributions were obtained by optical particles counter (OPC Grimm mod. 1107 A, Grimm Aerosol, Ainring, Germany) in the 0.3–30 μm size range (in 32 channels) with a time resolution of 6 sec.
- LDSA concentrations (µm2/cm3) measured by a second diffusion charger (Naneos Partector, Switzerland) in the size range from 10 to 400 nm.
- Two low-cost optical particles counters SPS30 (Sensirion, Staefa, Switzerland) positioned at the left (SPS30_L) and at the right side of the spray nozzles (SPS30_R), respectively. SPS30 can measure number concentration (in the range 0–3000 p/cm3) of particles with diameter > 0.3 µm, in four dimensional classes: 0.5–1 µm; 1.0–2.5 µm; 2.5–4 µm; 4–10 µm.
- Aerosol mass concentration was detected by means of an aerosol photometer (DustTrack mod. 8520, TSI Inc., Shoreview, MN, USA).
- The UFP number concentration and the lung deposited surface area (LDSA) was obtained with a DiSCmini (Testo, Lenzkirch, Germany). Maximum detectable particle concentrations depends on particle size and averaging time. Typical value is 1·106 p/cm3.
3. Results and Discussion
3.1. Inside the Spray Chamber
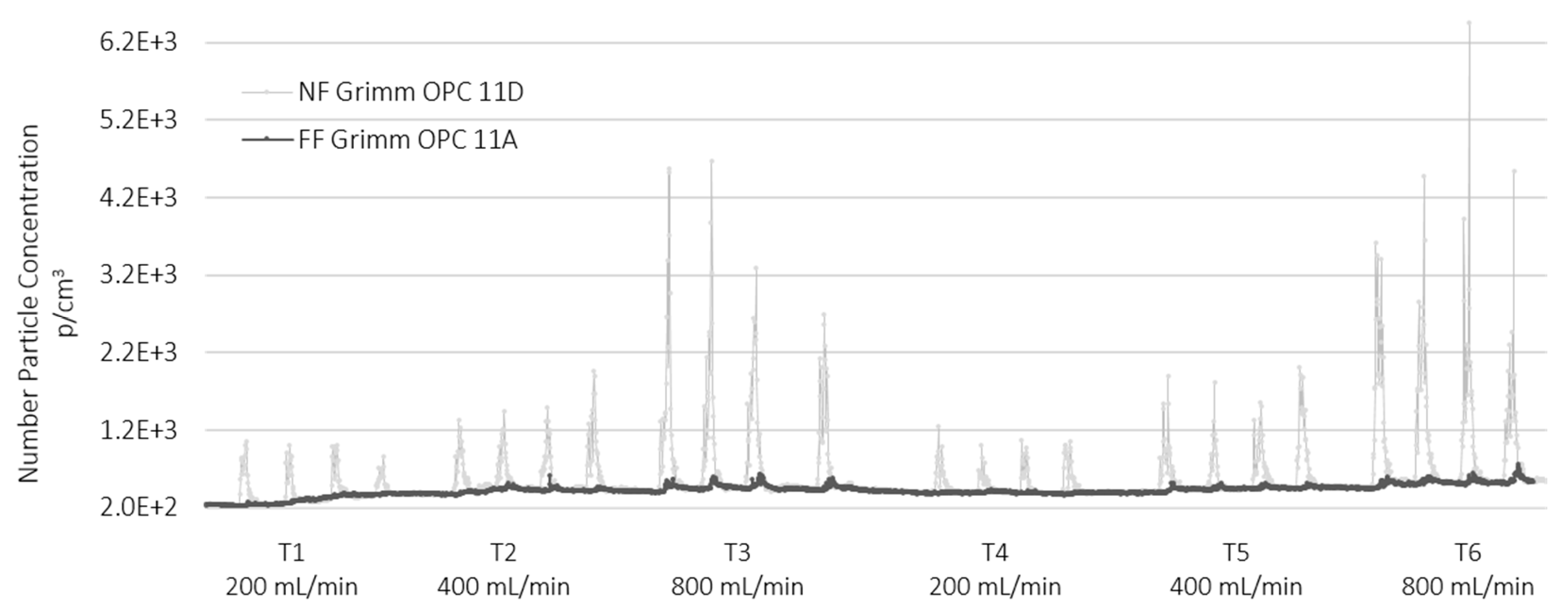
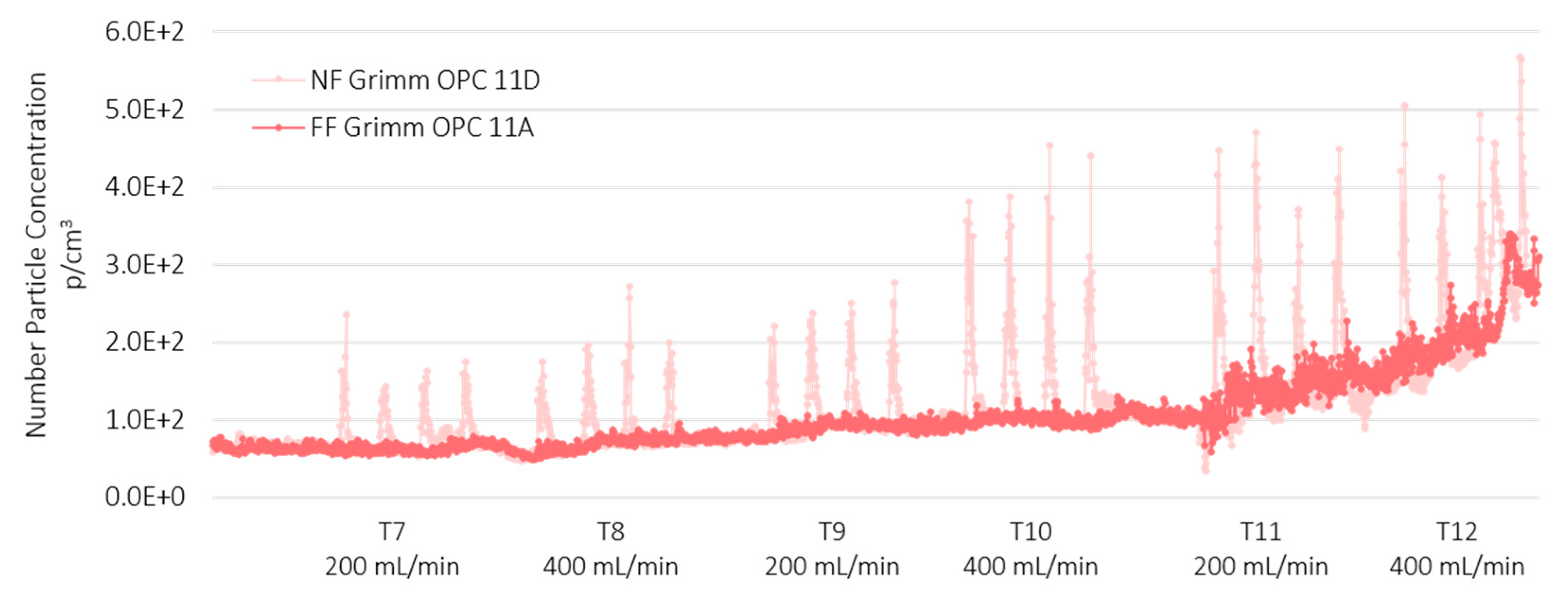
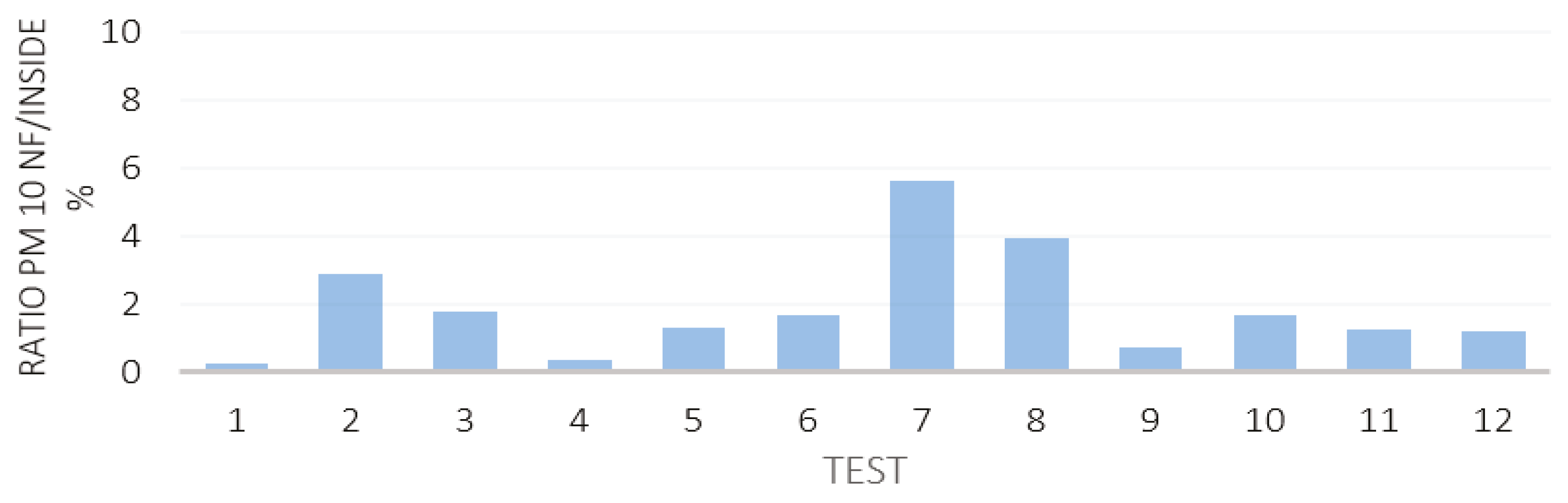
3.2. Near Field and Far Field
3.3. Comparison of Substrates and Suspensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhlbusch, T.A.J.; Wijnhoven, S.W.P.; Haase, A. Nanomaterial exposures for worker, consumer and the general public. NanoImpact 2018, 10, 11–25. [Google Scholar] [CrossRef]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health Effects—Pros and Cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef] [Green Version]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2000, 74, 1–8. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Pekkanen, J.; Peters, A.; Hoek, G.; Tiittanen, P.; Brunekreef, B.; de Hartog, J.; Heinrich, J.; Ibald-Mulli, A.; Kreyling, W.G.; Lanki, T. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: The Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation 2002, 106, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A., III; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Nørgaard, A.W.; Jensen, K.A.; Janfelt, C.; Lauritsen, F.R.; Clausen, P.A.; Wolkoff, P. Release of VOCs and particles during use of nanofilm spray products. Environ. Sci. Technol. 2009, 43, 7824–7830. [Google Scholar] [CrossRef]
- Göhler, D.; Stintz, M. Granulometric characterization of airborne particulate release during spray application of nanoparticle-doped coatings. J. Nanoparticle Res. 2014, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Girotto, C.; Rand, B.P.; Steudel, S.; Genoe, J.; Heremans, P. Nanoparticle-based, spray-coated silver top contacts for efficient polymer solar cells. Org. Electron. 2009, 10, 735–740. [Google Scholar] [CrossRef]
- Agrawal, A.M.; Pandey, P. Scale up of pan coating process using quality by design principles. J. Pharm. Sci. 2015, 104, 3589–3611. [Google Scholar] [CrossRef]
- Savolainen, K.; Pylkkänen, L.; Norppa, H.; Falck, G.; Lindberg, H.; Tuomi, T.; Vippola, M.; Alenius, H.; Hämeri, K.; Koivisto, J. Nanotechnologies, engineered nanomaterials and occupational health and safety–A review. Saf. Sci. 2010, 48, 957–963. [Google Scholar] [CrossRef]
- Ding, Y.; Kuhlbusch, T.A.J.; Van Tongeren, M.; Jiménez, A.S.; Tuinman, I.; Chen, R.; Alvarez, I.L.; Mikolajczyk, U.; Nickel, C.; Meyer, J.; et al. Airborne engineered nanomaterials in the workplace—a review of release and worker exposure during nanomaterial production and handling processes. J. Hazard. Mater. 2017, 322, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmatonidis, A.; Ribalta, C.; Sanfélix, V.; Bezantakos, S.; Biskos, G.; Vulpoi, A.; Simion, S.; Monfort, E.; Viana, M. Workplace exposure to nanoparticles during thermal spraying of ceramic coatings. Ann. Work Expo. Health 2019, 63, 91–106. [Google Scholar] [CrossRef]
- Viana, M.; Fonseca, A.S.; Querol, X.; López-Lilao, A.; Carpio, P.; Salmatonidis, A.; Monfort, E. Workplace exposure and release of ultrafine particles during atmospheric plasma spraying in the ceramic industry. Sci. Total Environ. 2017, 599, 2065–2073. [Google Scholar] [CrossRef]
- West, G.H.; Cooper, M.R.; Burrelli, L.G.; Dresser, D.; Lippy, B.E. Exposure to airborne nano-titanium dioxide during airless spray painting and sanding. J. Occup. Environ. Hyg. 2019, 16, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.J.; Kling, K.I.; Hänninen, O.; Jayjock, M.; Löndahl, J.; Wierzbicka, A.; Fonseca, A.S.; Uhrbrand, K.; Boor, B.E.; Jiménez, A.S.; et al. Source specific exposure and risk assessment for indoor aerosols. Sci. Total Environ. 2019, 668, 13–24. [Google Scholar] [CrossRef]
- Ortelli, S.; Belosi, F.; Bengalli, R.; Ravegnani, F.; Baldisserri, C.; Perucca, M.; Azoia, N.; Blosi, M.; Mantecca, P.; Costa, A.L. Influence of spray-coating process parameters on the release of TiO2 particles for the production of antibacterial textile. NanoImpact 2020, 19, 100245. [Google Scholar] [CrossRef]
- Asbach, C.; Alexander, C.; Clavaguera, S.; Dahmann, D.; Dozol, H.; Faure, B.; Fierz, M.; Fontana, L.; Iavicoli, I.; Kaminski, H.; et al. Review of measurement techniques and methods for assessing personal exposure to airborne nanomaterials in workplaces. Sci. Total Environ. 2017, 603–604, 793–806. [Google Scholar] [CrossRef]
- Belut, E.; Sánchez Jiménez, A.; Meyer-Plath, A.; Koivisto, A.J.; Koponen, I.K.; Jensen, A.C.Ø.; MacCalman, L.; Tuinman, I.; Fransman, W.; Domat, M.; et al. Indoor dispersion of airborne nano and fine particles: Main factors affecting spatial and temporal distribution in the frame of exposure modeling. Indoor Air 2019, 29, 803–816. [Google Scholar] [CrossRef]
- Fonseca, A.S.; Viana, M.; Querol, X.; Moreno, N.; De Francisco, I.; Estepa, C.; De La Fuente, G.F. Ultrafine and nanoparticle formation and emission mechanisms during laser processing of ceramic materials. J. Aerosol Sci. 2015, 88, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Nymark, P.; Bakker, M.; Dekkers, S.; Franken, R.; Fransman, W.; García-Bilbao, A.; Greco, D.; Gulumian, M.; Hadrup, N.; Halappanavar, S. Toward rigorous materials production: New approach methodologies have extensive potential to improve current safety assessment practices. Small 2020, 16, 1904749. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.; Blosi, M. Process for the preparation of nanoparticles of noble metals in hydrogel and nanoparticles thus obtained. WO2016125070A1, 7 January 2020. [Google Scholar]
- Nicosia, A.; Manodori, L.; Trentini, A.; Ricciardelli, I.; Bacco, D.; Poluzzi, V.; Di Matteo, L.; Belosi, F. Field study of a soft X-ray aerosol neutralizer combined with electrostatic classifiers for nanoparticle size distribution measurements. Particuology 2018, 37, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Lung-deposited surface area concentration measurements in selected occupational and non-occupational environments. J. Aerosol Sci. 2016, 96, 24–37. [Google Scholar] [CrossRef]
- Cosnier, F.; Seidel, C.; Valentino, S.; Schmid, O.; Bau, S.; Vogel, U.; Devoy, J.; Gaté, L. Retained particle surface area dose drives inflammation in rat lungs following acute, subacute, and subchronic inhalation of nanomaterials. Part. Fibre Toxicol. 2021, 18, 29. [Google Scholar] [CrossRef]
- Hama, S.M.L.; Ma, N.; Cordell, R.L.; Kos, G.P.A.; Wiedensohler, A.; Monks, P.S. Lung deposited surface area in Leicester urban background site/UK: Sources and contribution of new particle formation. Atmos. Environ. 2017, 151, 94–107. [Google Scholar] [CrossRef] [Green Version]
- Schulte, P.A.; Murashov, V.; Zumwalde, R.; Kuempel, E.D.; Geraci, C.L. Occupational exposure limits for nanomaterials: State of the art. J. Nanoparticle Res. 2010, 12, 1971–1987. [Google Scholar] [CrossRef]
- Hatto, P. International standards for risk management in nanotechnology. Nat. Nanotechnol. 2009, 4, 205. [Google Scholar] [CrossRef]
- Dankovic, D.A.; Kuempel, E.D. Occupational exposure to titanium dioxide. NIOSH Curr. Intell. Bull. 2011, 63. [Google Scholar]
- Kuempel, E.D.; Roberts, J.R.; Roth, G.; Zumwalde, R.D.; Nathan, D.; Hubbs, A.F.; Trout, D.; Holdsworth, G. Health effects of occupational exposure to silver nanomaterials. Curr. Intell. Bull. 2021, 70, 1–332. [Google Scholar]




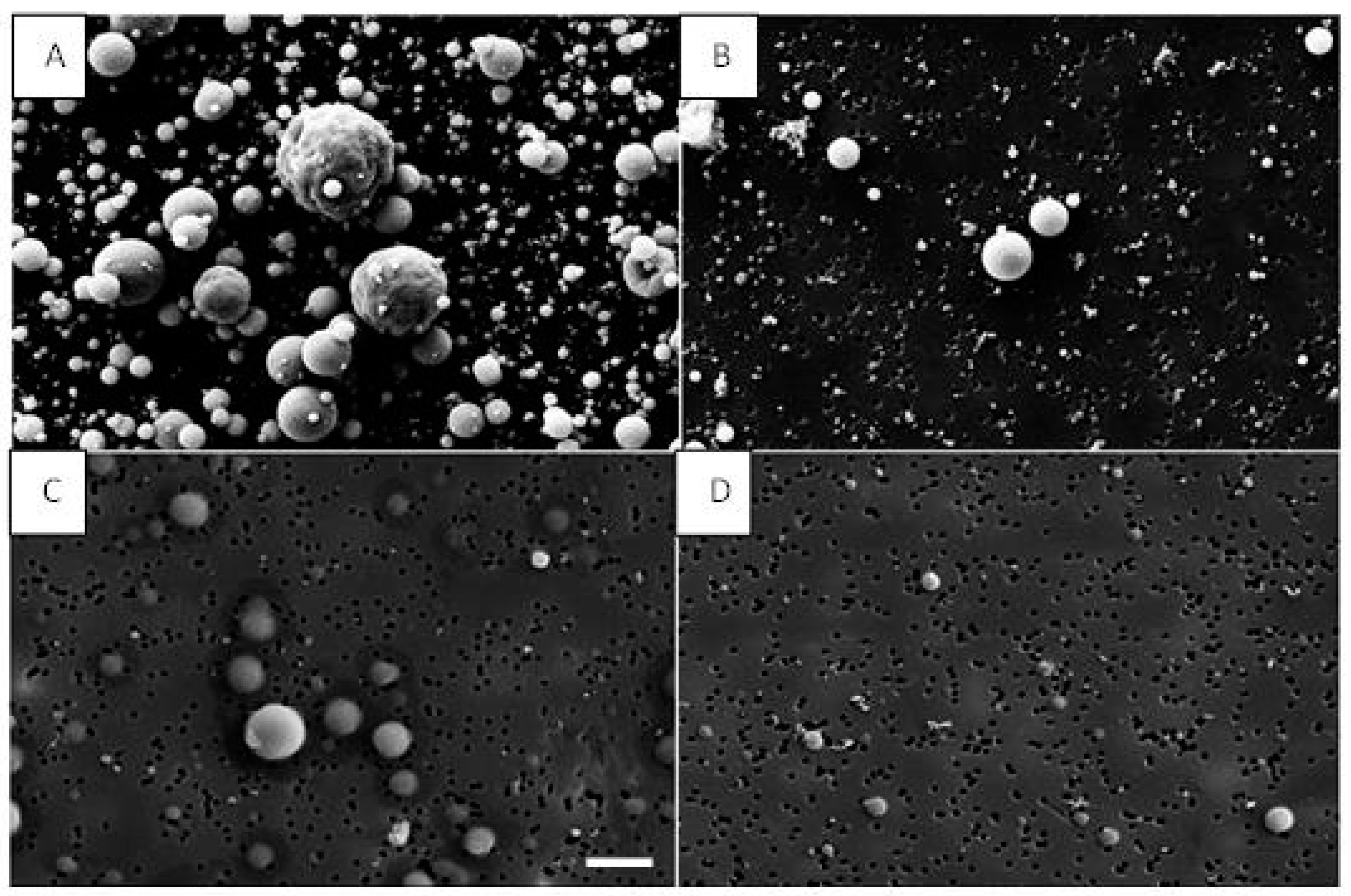
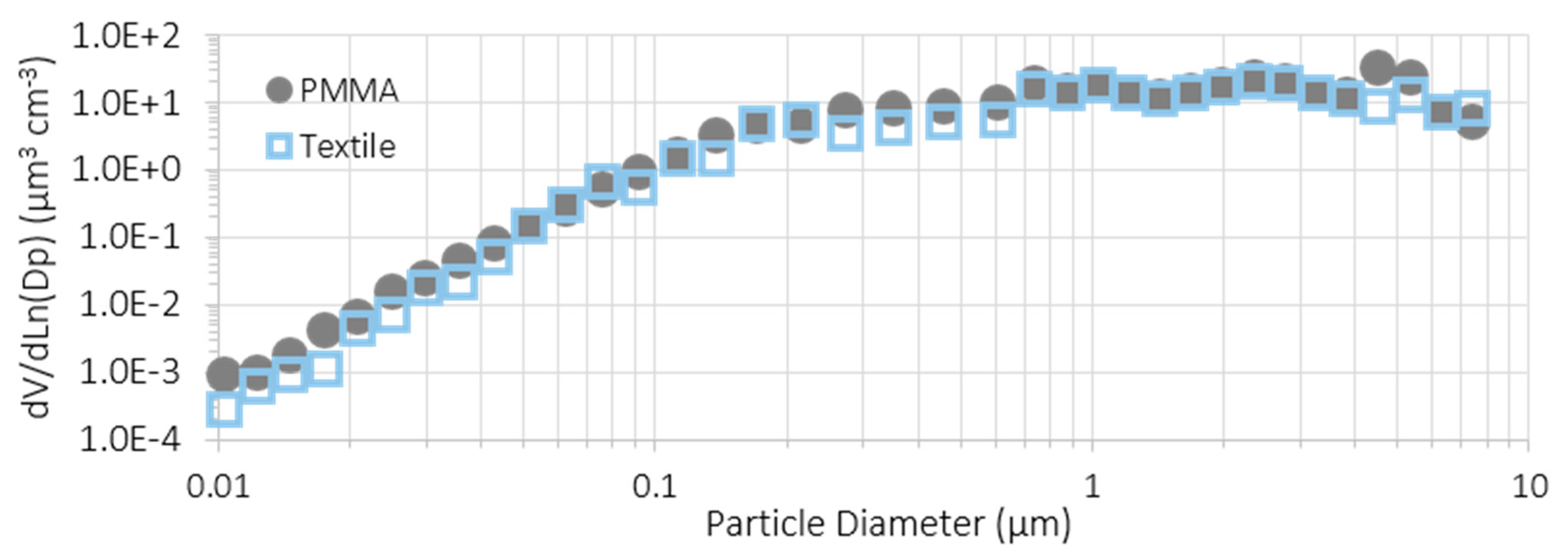
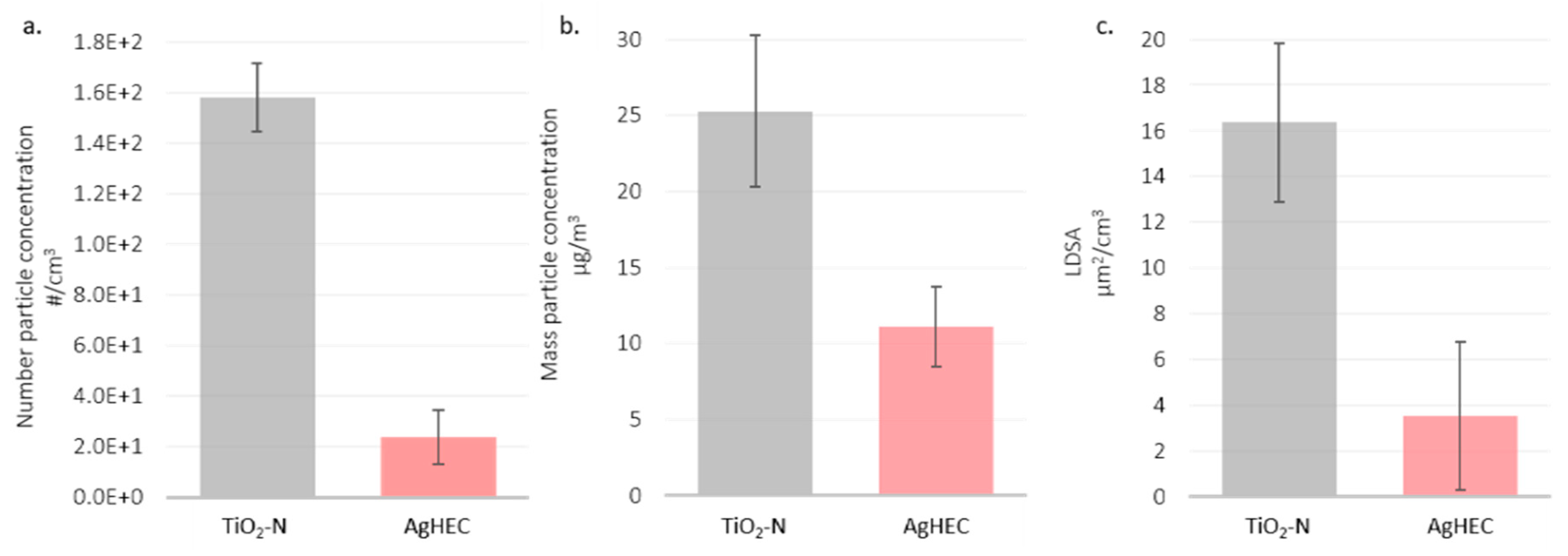
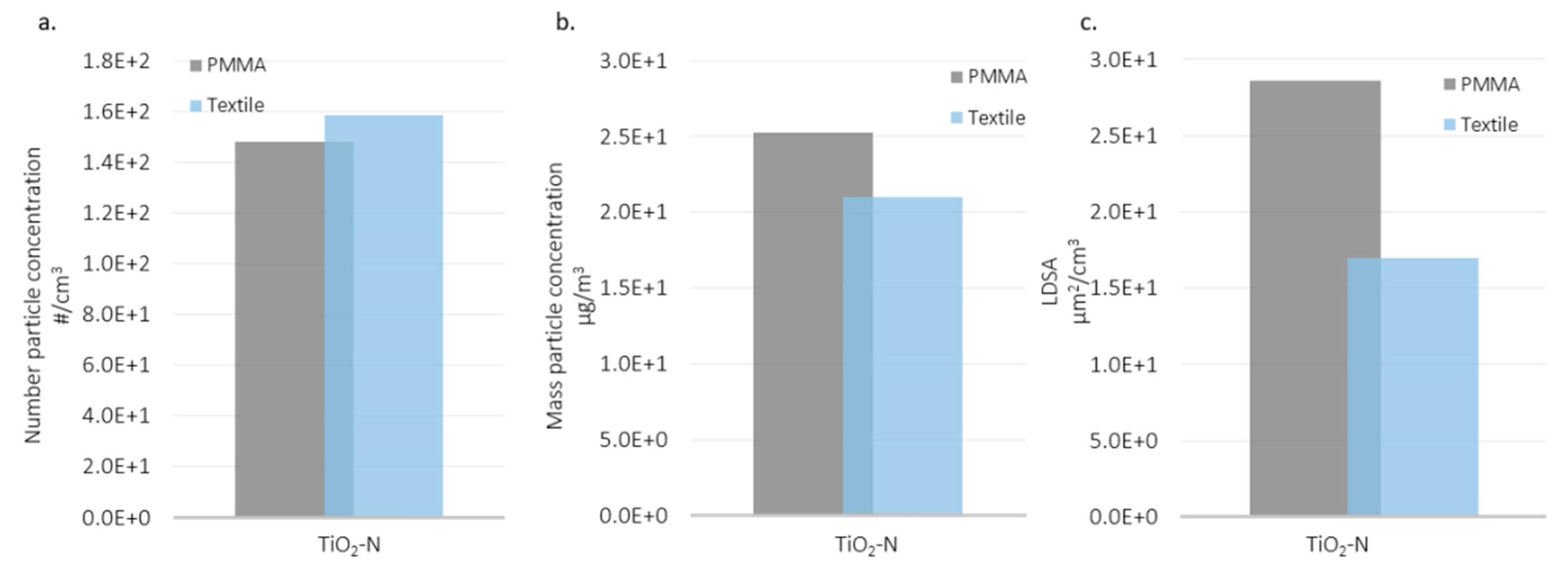
| Material | Inside Spray Chamber (µg/m3) | NF (µg/m3) | Ratio (%) |
|---|---|---|---|
| TiO2-N a | 1198 ± 2 | 93 ± 6 | 7.7 |
| Ti b | 491 ± 4 | 24.7 ± 0.6 | 5.1 |
| AgHEC a | 172 ± 5 | 37 ± 6 | 21.5 |
| Ag b | 13.2 ± 0.3 | 0.35 ± 0.03 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Secco, B.; Trabucco, S.; Ravegnani, F.; Koivisto, A.J.; Zanoni, I.; Blosi, M.; Ortelli, S.; Altin, M.; Bartolini, G.; Costa, A.L.; et al. Particles Emission from an Industrial Spray Coating Process Using Nano-Materials. Nanomaterials 2022, 12, 313. https://doi.org/10.3390/nano12030313
Del Secco B, Trabucco S, Ravegnani F, Koivisto AJ, Zanoni I, Blosi M, Ortelli S, Altin M, Bartolini G, Costa AL, et al. Particles Emission from an Industrial Spray Coating Process Using Nano-Materials. Nanomaterials. 2022; 12(3):313. https://doi.org/10.3390/nano12030313
Chicago/Turabian StyleDel Secco, Benedetta, Sara Trabucco, Fabrizio Ravegnani, Antti Joonas Koivisto, Ilaria Zanoni, Magda Blosi, Simona Ortelli, Marko Altin, Gianni Bartolini, Anna Luisa Costa, and et al. 2022. "Particles Emission from an Industrial Spray Coating Process Using Nano-Materials" Nanomaterials 12, no. 3: 313. https://doi.org/10.3390/nano12030313
APA StyleDel Secco, B., Trabucco, S., Ravegnani, F., Koivisto, A. J., Zanoni, I., Blosi, M., Ortelli, S., Altin, M., Bartolini, G., Costa, A. L., & Belosi, F. (2022). Particles Emission from an Industrial Spray Coating Process Using Nano-Materials. Nanomaterials, 12(3), 313. https://doi.org/10.3390/nano12030313










