A Facile Design of Solution-Phase Based VS2 Multifunctional Electrode for Green Energy Harvesting and Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of VS2 Microflower Structured Powder
2.2. VS2-Based Electrode Fabrication
2.3. Electrochemical Measurements
3. Results and Discussion
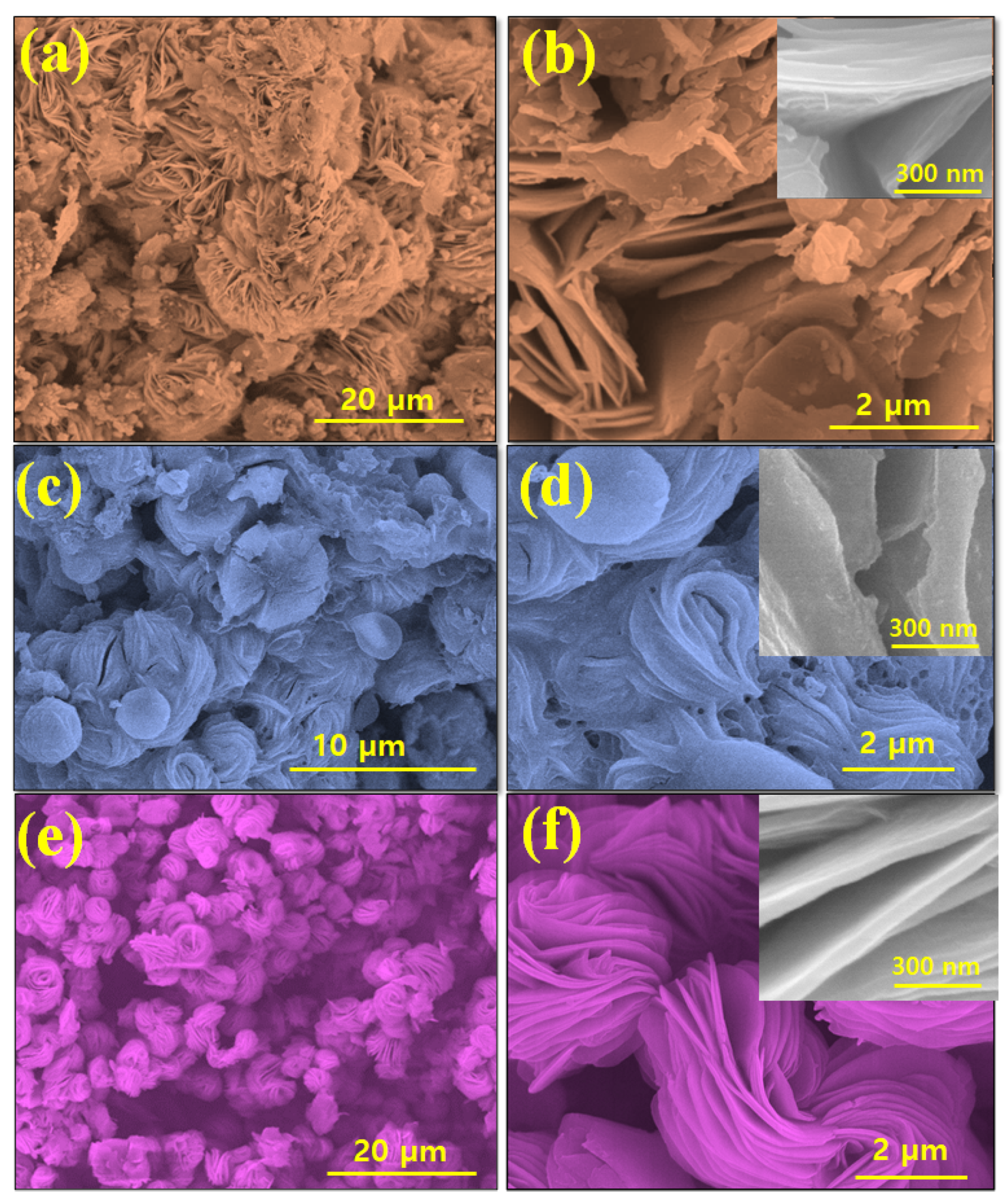
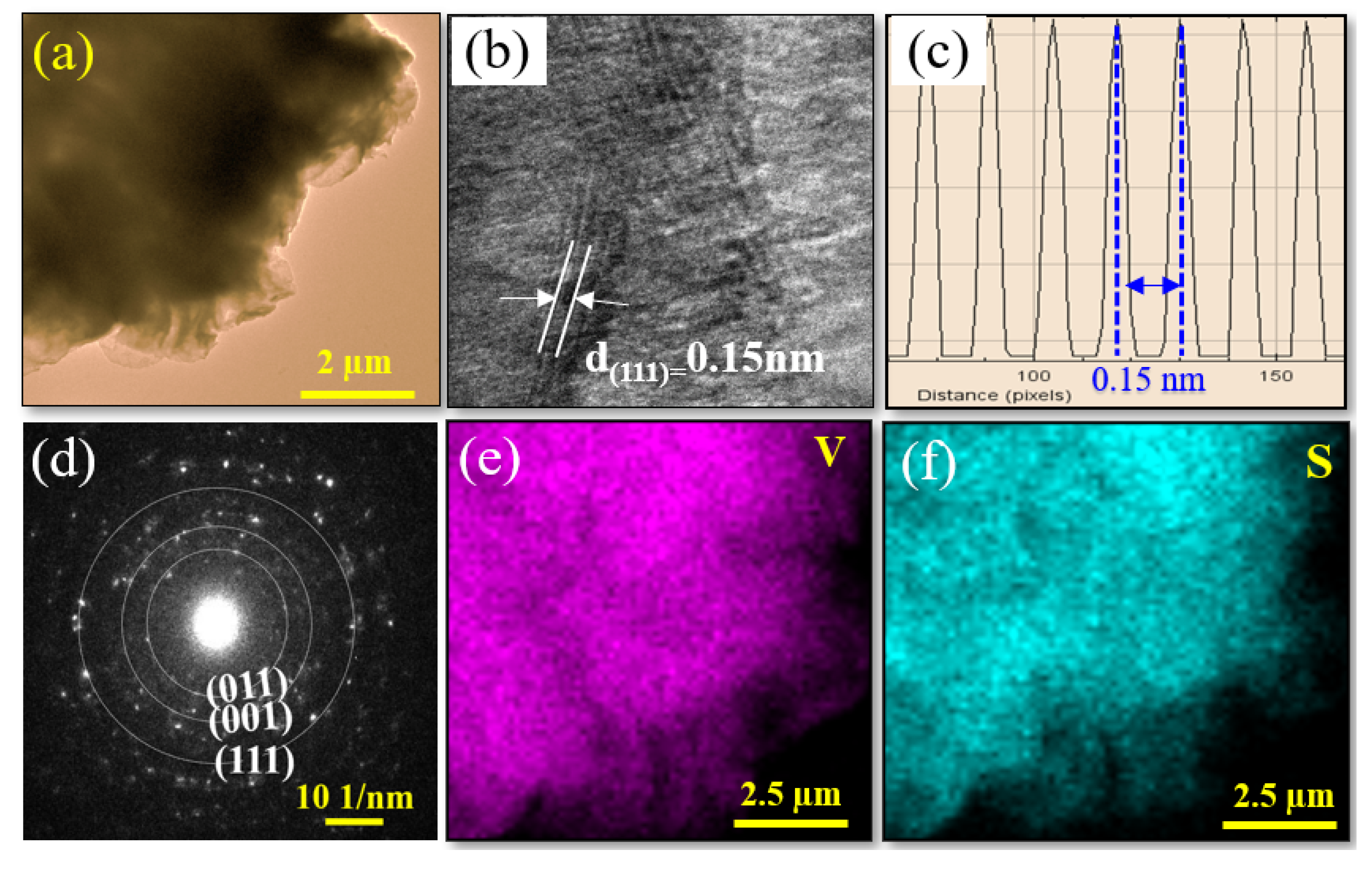
3.1. Topographic, Crystal Structure and Chemical Composition Charaterization of VS2
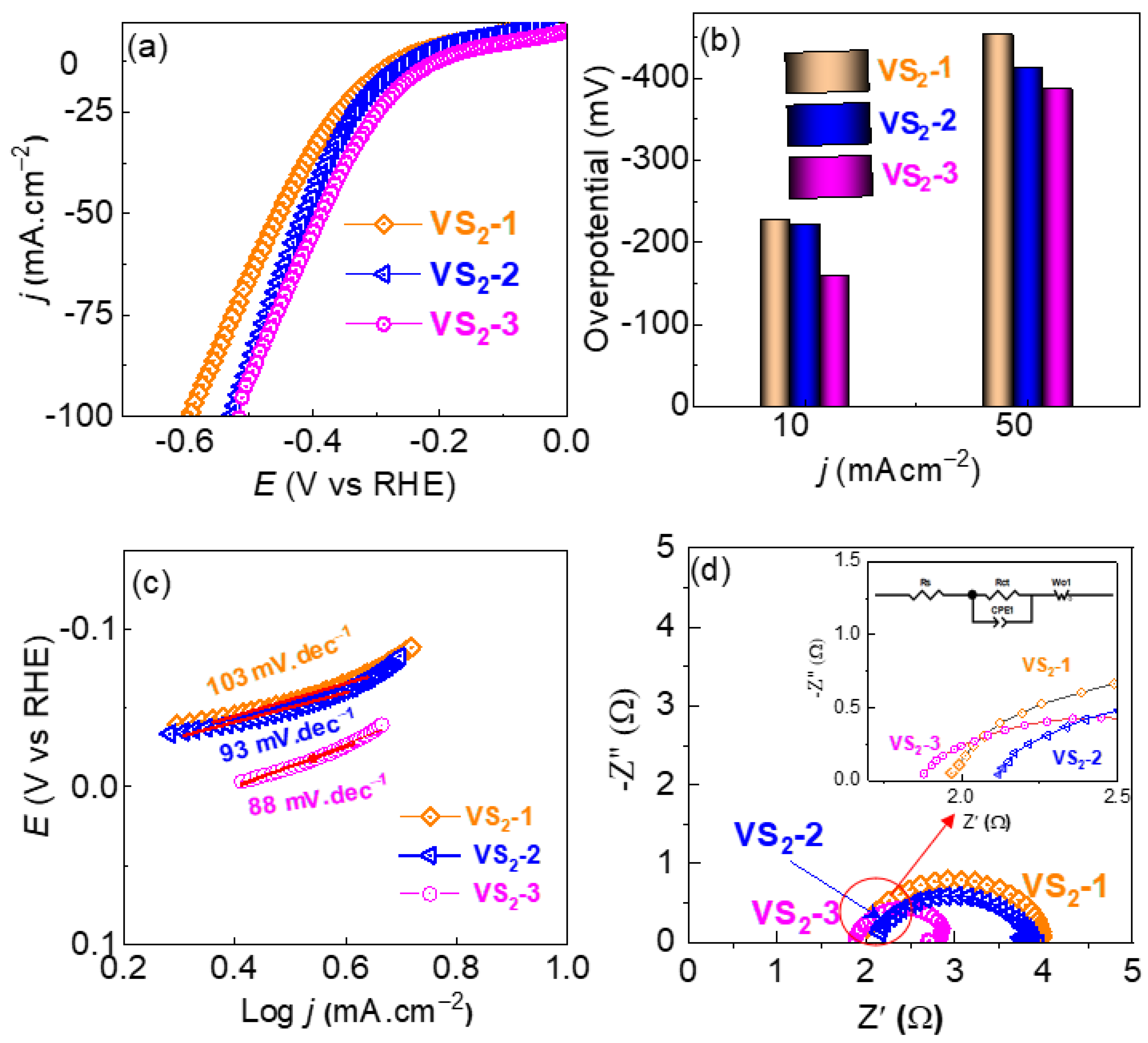
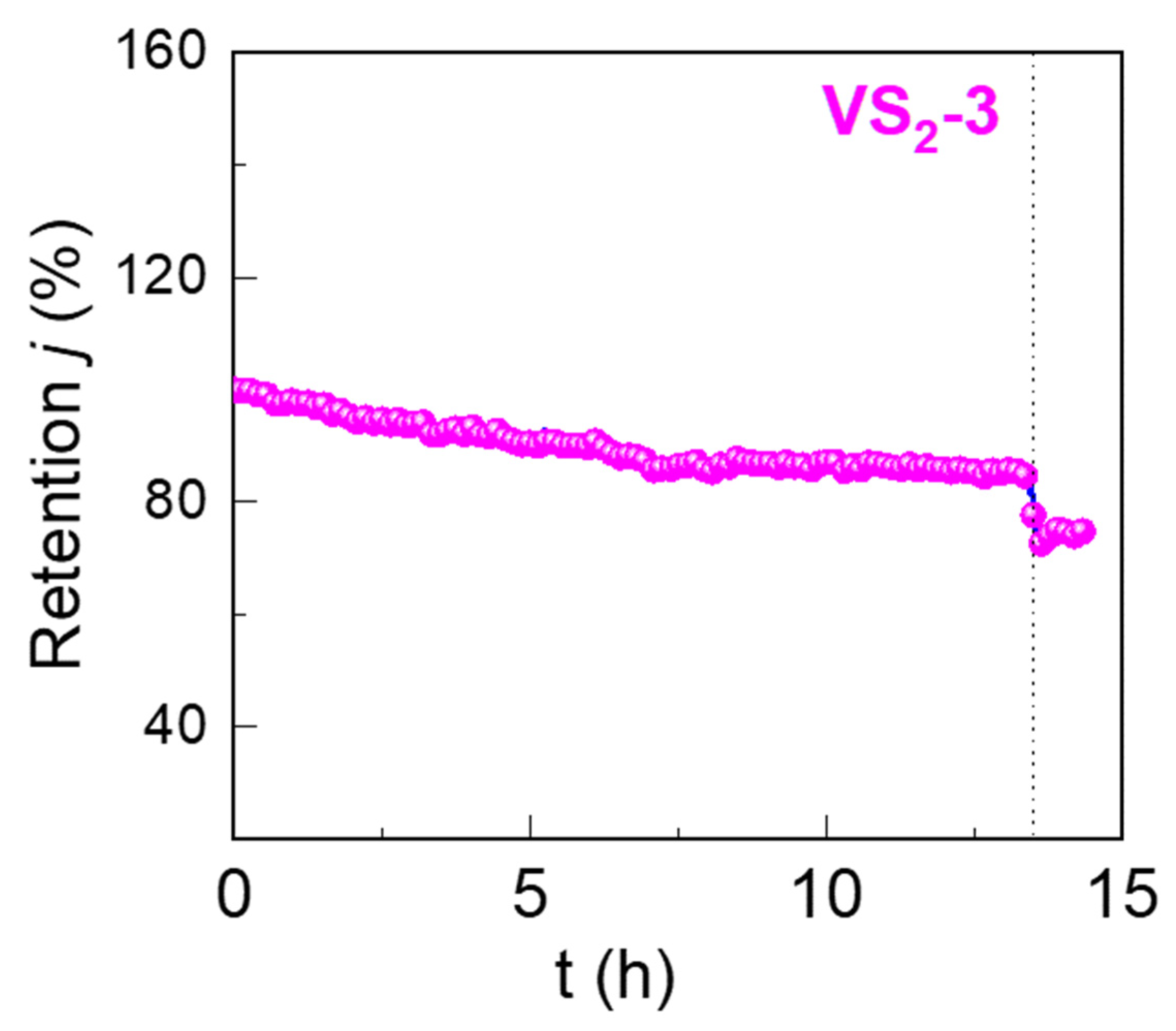
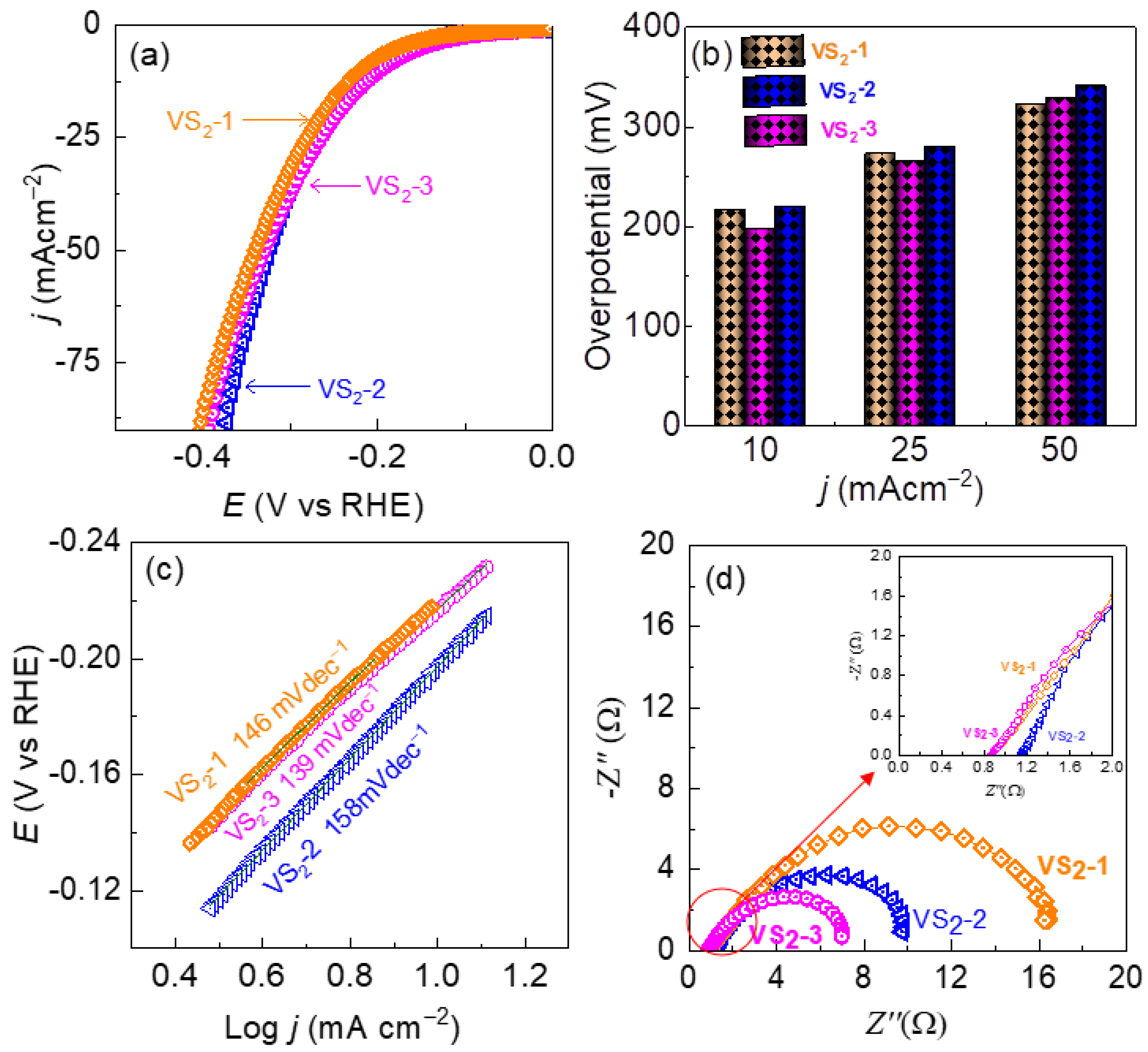
3.2. HER Performance of VS2 Electrodes in Acidic and Alkaline Electrolyte
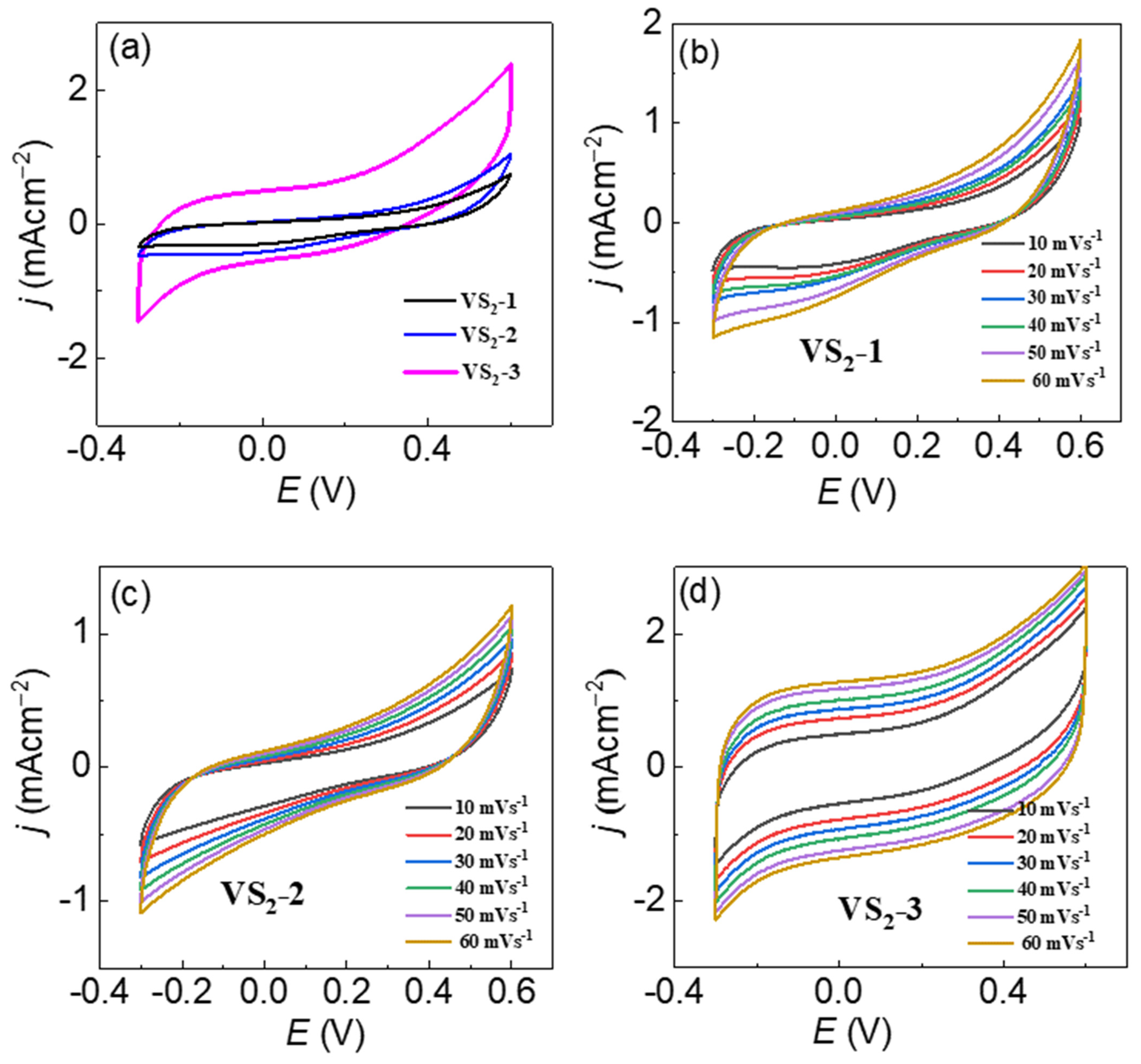
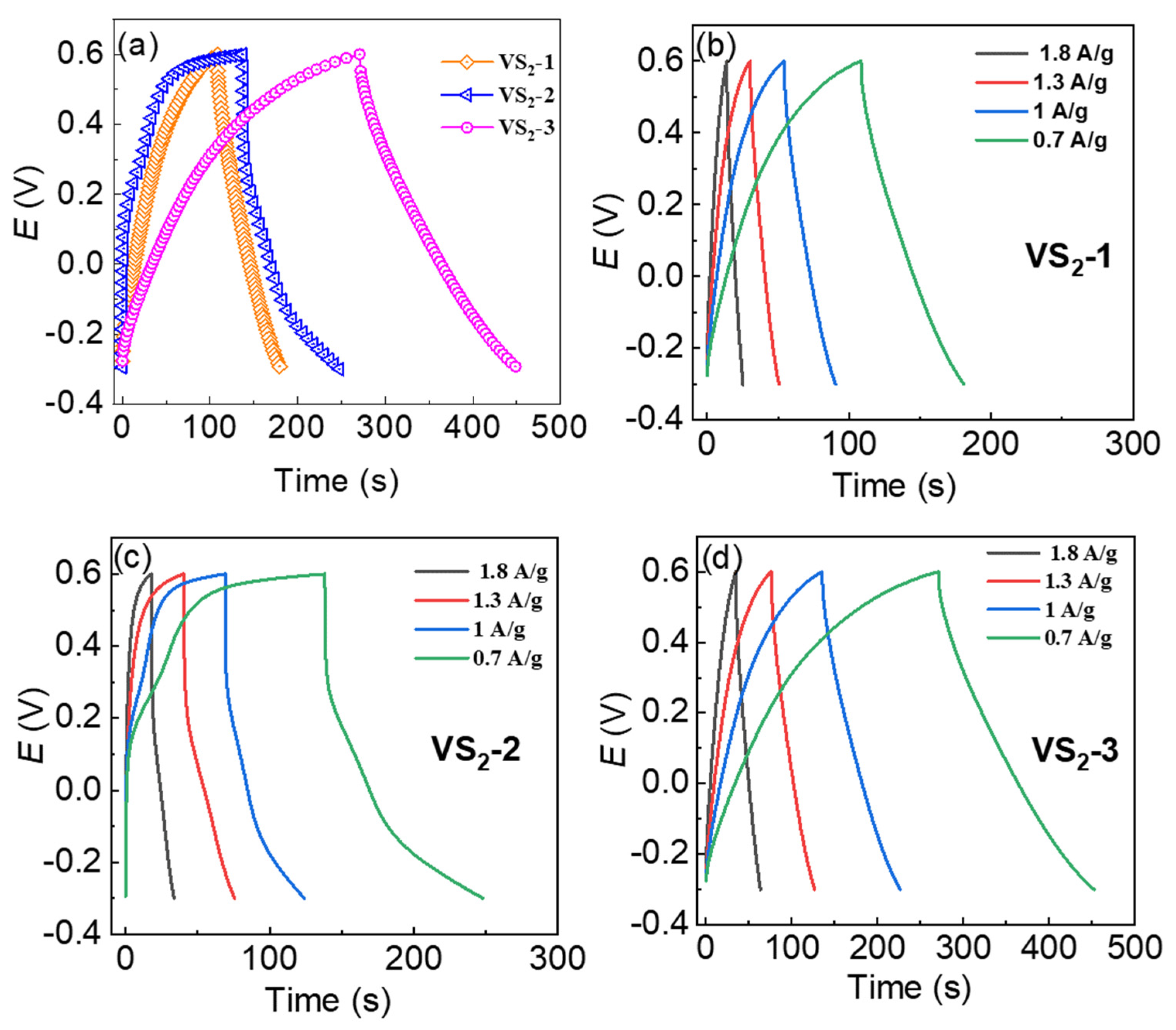
3.3. Supercapacitor Performance of VS2 in Symmetric Device-Configuration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; IPCC-Sr15: Geneva, Switzerland, 2018; Volume 2, pp. 17–20.
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.P.; Gallachóir, B.P.Ó. The role of hydrogen in low carbon energy futures—A review of existing perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shifa, T.A.; Zhan, X.; Huang, Y.; Liu, K.; Cheng, Z.; Jiang, C.; He, J. Recent advances in transition-metal dichalcogenide based nanomaterials for water splitting. Nanoscale 2015, 7, 19764–19788. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Wang, H. Strategies for Developing Transition Metal Phosphides in Electrochemical Water Splitting. Front. Chem. 2021, 9, 700020. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liao, G.; Liu, S. Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. NPJ 2D Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Patil, S.A.; Cho, S.; Jo, Y.; Kim, H.; Im, H. Cu-Fe-NH2 based metal-organic framework nanosheets: Via drop-casting for highly efficient oxygen evolution catalysts durable at ultrahigh currents. J. Mater. Chem. A 2020, 8, 24408–24418. [Google Scholar] [CrossRef]
- Patil, S.A.; Cho, S.; Jo, Y.; Shrestha, N.K.; Kim, H.; Im, H. Bimetallic Ni-Co@hexacyano nano-frameworks anchored on carbon nanotubes for highly efficient overall water splitting and urea decontamination. Chem. Eng. J. 2021, 426, 130773. [Google Scholar] [CrossRef]
- Rabani, I.; Hussain, S.; Vikraman, D.; Seo, Y.S.; Jung, J.; Jana, A.; Shrestha, N.K.; Jalalah, M.; Noh, Y.Y.; Patil, S.A. 1D-CoSe2 nanoarray: A designed structure for efficient hydrogen evolution and symmetric supercapacitor characteristics. Dalt. Trans. 2020, 49, 14191–14200. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Landers, A.T.; Fields, M.; Torelli, D.A.; Xiao, J.; Hellstern, T.R.; Francis, S.A.; Tsai, C.; Kibsgaard, J.; Lewis, N.S.; Chan, K.; et al. The Predominance of Hydrogen Evolution on Transition Metal Sulfides and Phosphides under CO2 Reduction Conditions: An Experimental and Theoretical Study. ACS Energy Lett. 2018, 3, 1450–1457. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Xu, L.; Guo, X.T.; Lv, T.T.; Pang, H. Vanadium sulfide-based materials: Synthesis, energy storage and conversion. J. Mater. Chem. A 2020, 8, 20781–20802. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, M.; Huang, W.; Liao, W.; Bian, H.; Chen, D.; Wang, L.; Tang, J.; Liu, C. Synergism on Electronic Structures and Active Edges of Metallic Vanadium Disulfide Nanosheets via Co Doping for Efficient Hydrogen Evolution Reaction in Seawater. ChemCatChem 2021, 13, 2138–2144. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Yu, K.; Feng, Y.; Zhu, Z. 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting. Nanoscale 2020, 12, 6176–6187. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Barzegar, F.; Dangbegnon, J.K.; Bello, A.; Madito, M.J.; Momodu, D.; Manyala, N. Asymmetric supercapacitor based on VS2 nanosheets and activated carbon materials. RSC Adv. 2016, 6, 38990–39000. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic few-layered VS2 ultrathin nanosheets: High two-dimensional conductivity for in-plane supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef]
- Meyer, E.; Bede, A.; Mutukwa, D.; Taziwa, R.; Zingwe, N. Optimization, and analysis of carbon supported VS2 nanocomposites as potential electrodes in supercapacitors. J. Energy Storage 2020, 27, 101074. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Wang, J.; Luo, N.; Wu, J.; Huang, S.; Yu, L.; Wei, M. Hierarchical spheres constructed by ultrathin VS2 nanosheets for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 3691–3696. [Google Scholar] [CrossRef]
- Shinde, P.V.; Gavali, D.S.; Thapa, R.; Singh, M.K.; Rout, C.S. Ternary VS2/ZnS/CdS hybrids as efficient electrocatalyst for hydrogen evolution reaction: Experimental and theoretical insights. AIP Adv. 2021, 11, 105010. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Wang, Z.; Zhu, J.; Wen, Z.; Zhao, X.; Zhang, X.; Xu, J.; Lu, Z. Synergistic Interlayer and Defect Engineering in VS2 Nanosheets toward Efficient Electrocatalytic Hydrogen Evolution Reaction. Small 2018, 14, 1703098. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tang, Z.; Pan, Z.; Shi, W.; Wang, Y.; Tian, Z.Q.; Shen, P.K. Template-free growth of spherical vanadium disulfide nanoflowers as efficient anodes for sodium/potassium ion batteries. Mater. Des. 2020, 192, 108780. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Xia, Y. A Solution-Phase, Precursor Route to Polycrystalline SnO2 Nanowires That Can Be Used for Gas Sensing under Ambient Conditions. J. Am. Chem. Soc. 2003, 125, 16176–16177. [Google Scholar] [CrossRef] [PubMed]
- Talebi-Esfandarani, M.; Savadogo, O. Effect of ethylene glycol on morphology, crystallinity, and electrochemical properties of LiFePO4/C in lithium-ion batteries. J. Appl. Electrochem. 2015, 45, 245–251. [Google Scholar] [CrossRef]
- Saravanan, K.; Balaya, P.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Morphology controlled synthesis of LiFePO4/C nanoplates for Li-ion batteries. Energy Environ. Sci. 2010, 3, 457–464. [Google Scholar] [CrossRef]
- Sun, R.; Wei, Q.; Sheng, J.; Shi, C.; An, Q.; Liu, S.; Mai, L. Novel layer-by-layer stacked VS2 nanosheets with intercalation pseudocapacitance for high-rate sodium ion charge storage. Nano Energy 2017, 35, 396–404. [Google Scholar] [CrossRef]
- Liang, H.; Shi, H.; Zhang, D.; Ming, F.; Wang, R.; Zhuo, J.; Wang, Z. Solution Growth of Vertical VS2Nanoplate Arrays for Electrocatalytic Hydrogen Evolution. Chem. Mater. 2016, 28, 5587–5591. [Google Scholar] [CrossRef]
- Sharma, A.; Mane, P.; Chakraborty, B.; Rout, C.S. 1T-VS2/MXene Hybrid as a Superior Electrode Material for Asymmetric Supercapacitors: Experimental and Theoretical Investigations. ACS Appl. Energy Mater. 2021, 4, 14198–14209. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, D.; Das, S.; Kumar, A.; Patil, S.; Nanda, K.K.; Sarma, D.D.; Shukla, A. Expanding Interlayer Spacing in MoS2 for Realizing an Advanced Supercapacitor. ACS Energy Lett. 2019, 4, 1602–1609. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, S.A.; Rabani, I.; Hussain, S.; Seo, Y.-S.; Jung, J.; Shrestha, N.K.; Im, H.; Kim, H. A Facile Design of Solution-Phase Based VS2 Multifunctional Electrode for Green Energy Harvesting and Storage. Nanomaterials 2022, 12, 339. https://doi.org/10.3390/nano12030339
Patil SA, Rabani I, Hussain S, Seo Y-S, Jung J, Shrestha NK, Im H, Kim H. A Facile Design of Solution-Phase Based VS2 Multifunctional Electrode for Green Energy Harvesting and Storage. Nanomaterials. 2022; 12(3):339. https://doi.org/10.3390/nano12030339
Chicago/Turabian StylePatil, Supriya A., Iqra Rabani, Sajjad Hussain, Young-Soo Seo, Jongwan Jung, Nabeen K. Shrestha, Hyunsik Im, and Hyungsang Kim. 2022. "A Facile Design of Solution-Phase Based VS2 Multifunctional Electrode for Green Energy Harvesting and Storage" Nanomaterials 12, no. 3: 339. https://doi.org/10.3390/nano12030339
APA StylePatil, S. A., Rabani, I., Hussain, S., Seo, Y.-S., Jung, J., Shrestha, N. K., Im, H., & Kim, H. (2022). A Facile Design of Solution-Phase Based VS2 Multifunctional Electrode for Green Energy Harvesting and Storage. Nanomaterials, 12(3), 339. https://doi.org/10.3390/nano12030339