Aqueous Organic Zinc-Ion Hybrid Supercapacitors Prepared by 3D Vertically Aligned Graphene-Polydopamine Composite Electrode
Abstract
:1. Introduction
2. Experimental
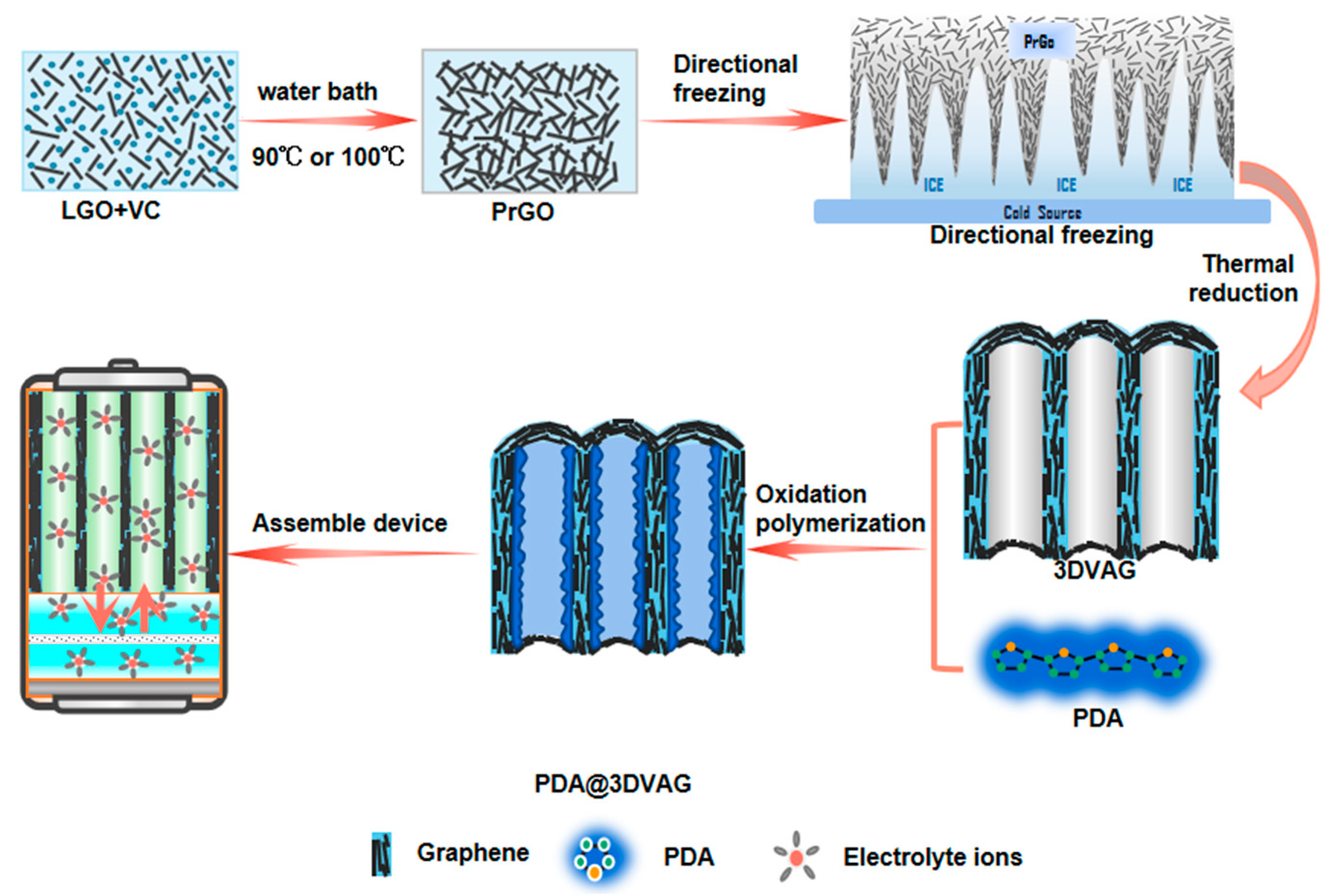
2.1. Preparation of 3DVAG
2.2. Preparation of PDA@3DVAG
2.3. Materials and Electrochemical Characterization
3. Results and Discussion
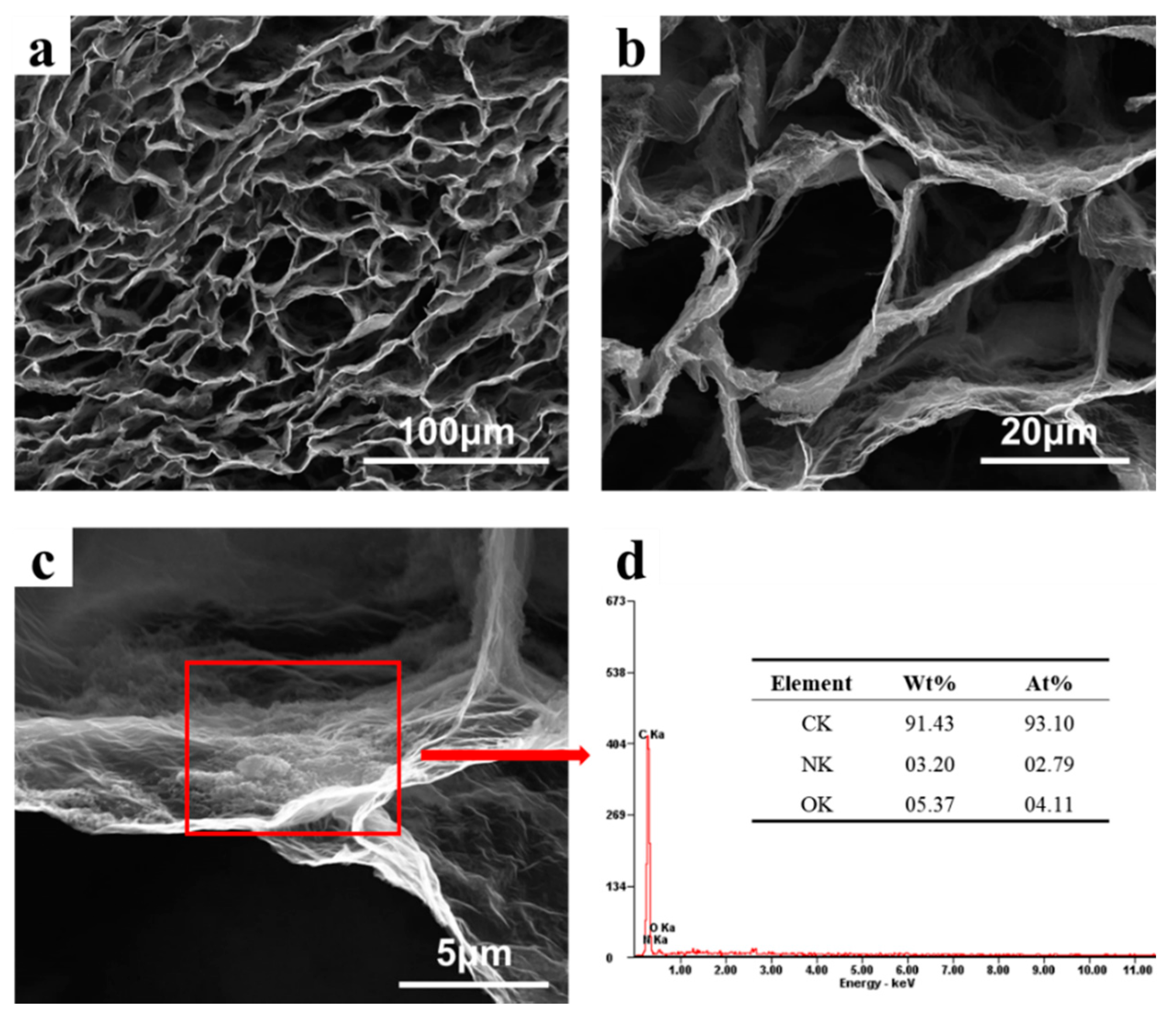
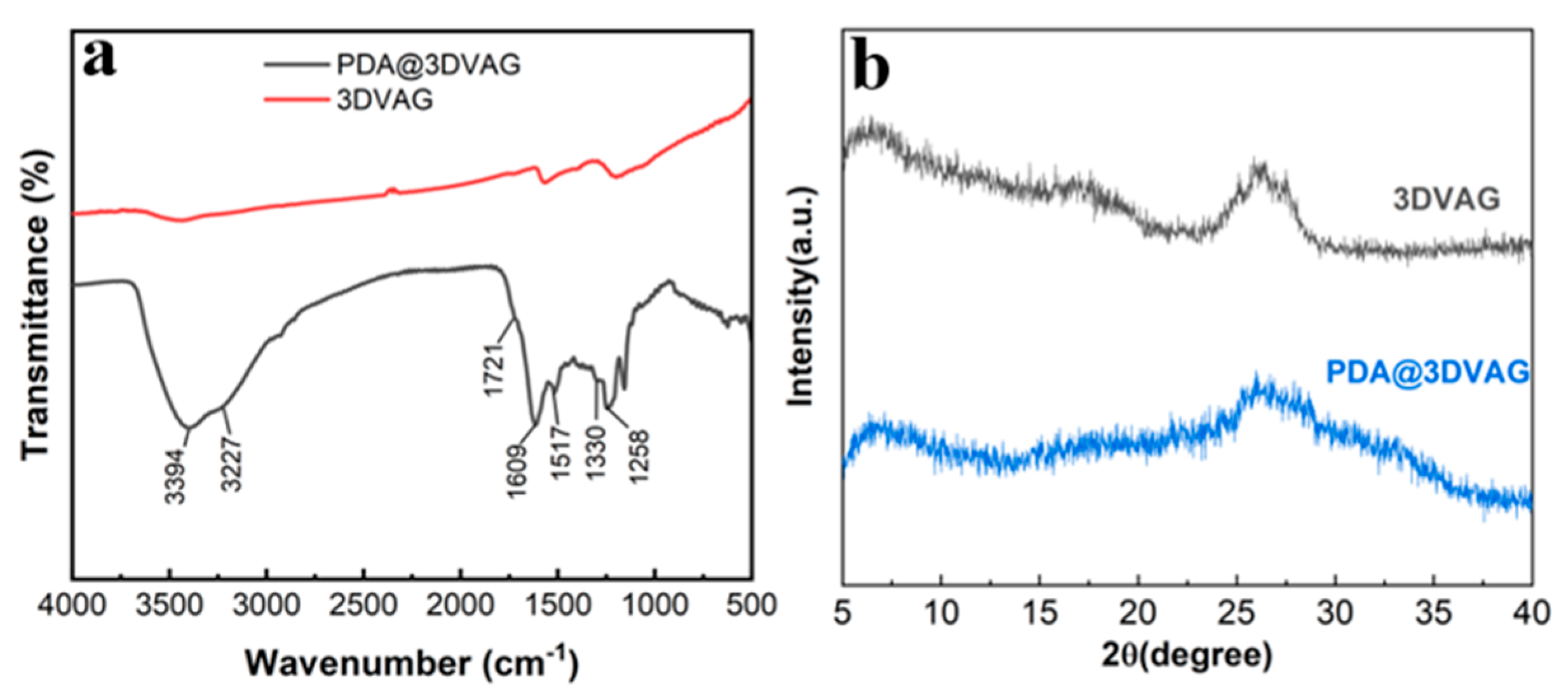
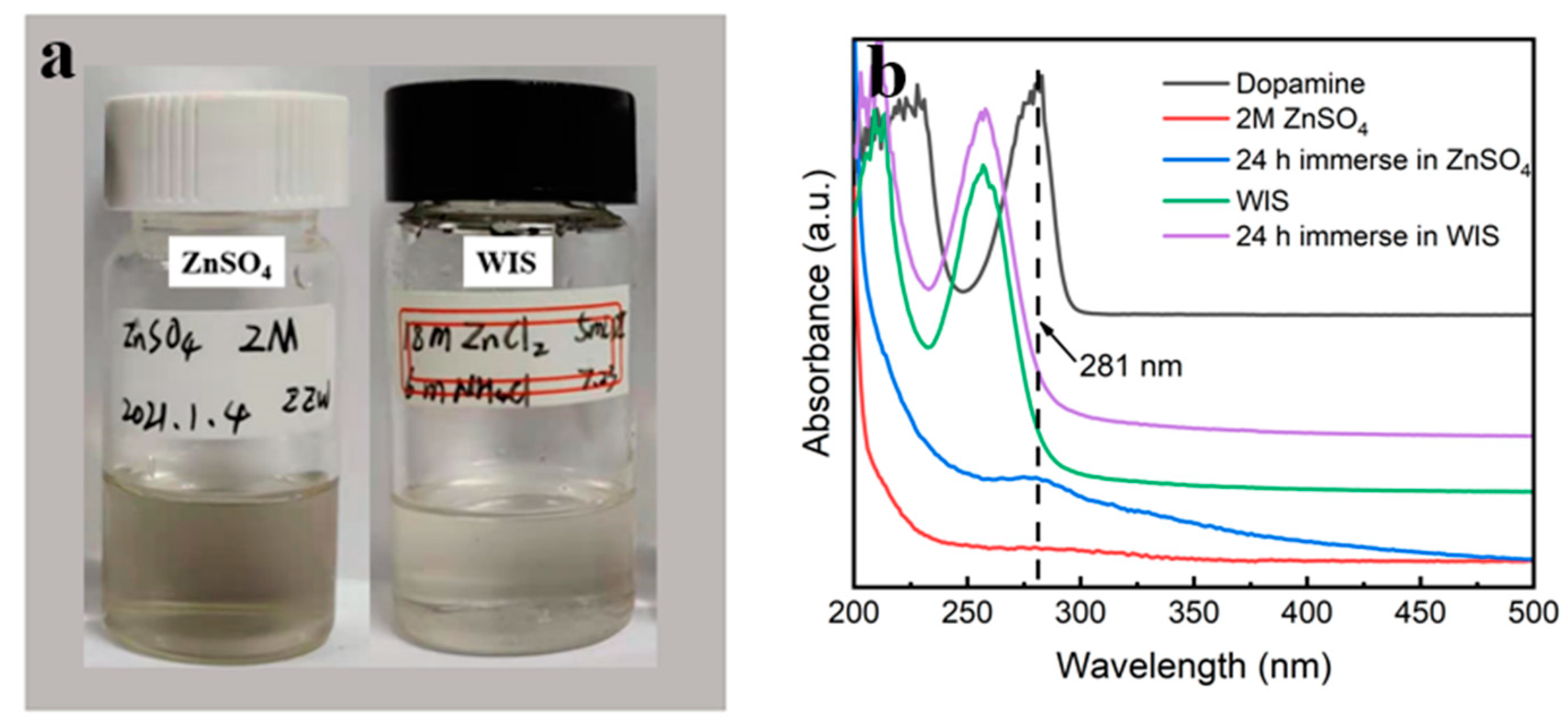
3.1. Morphology and Structure of PDA@3DVAG
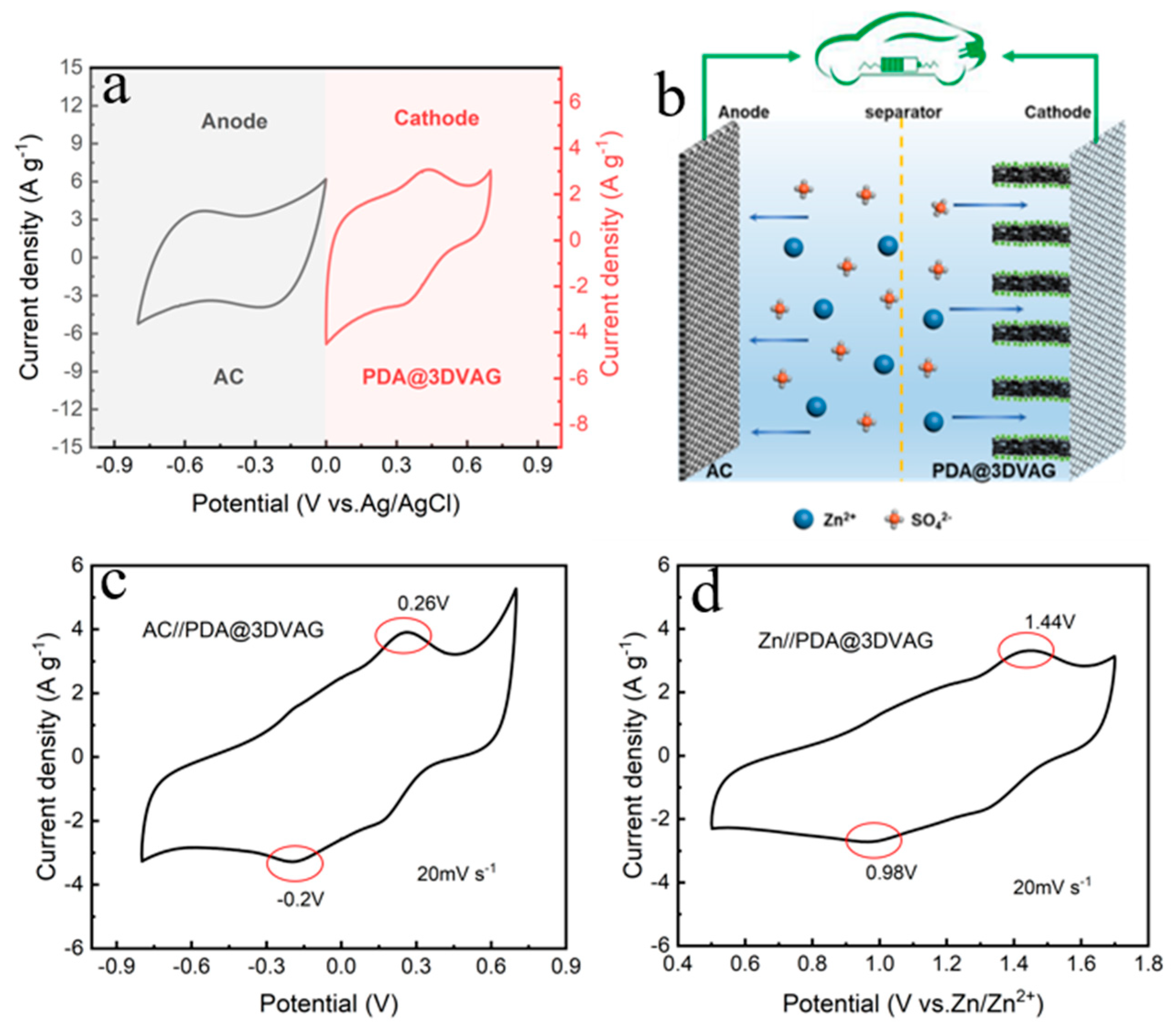
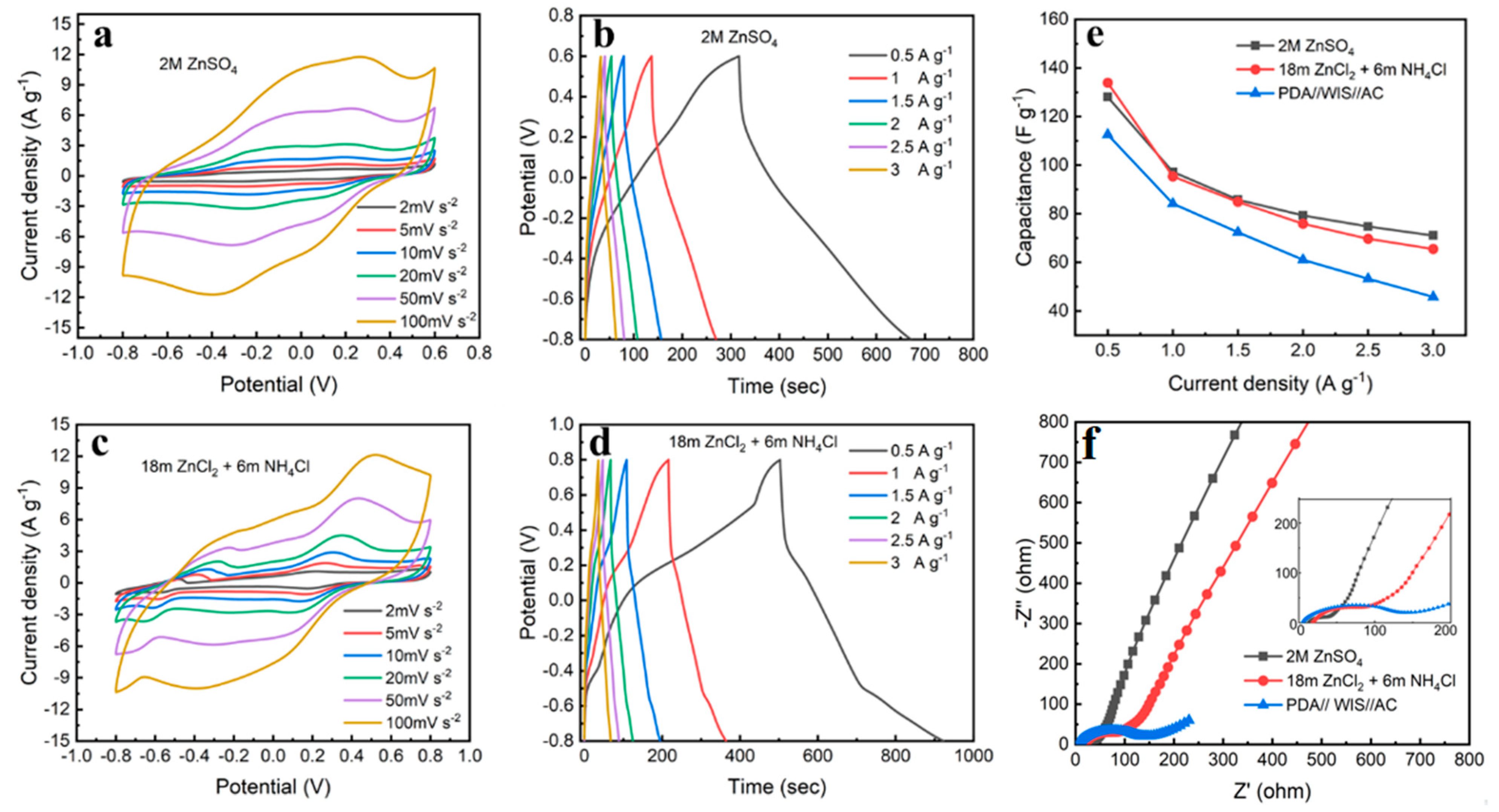
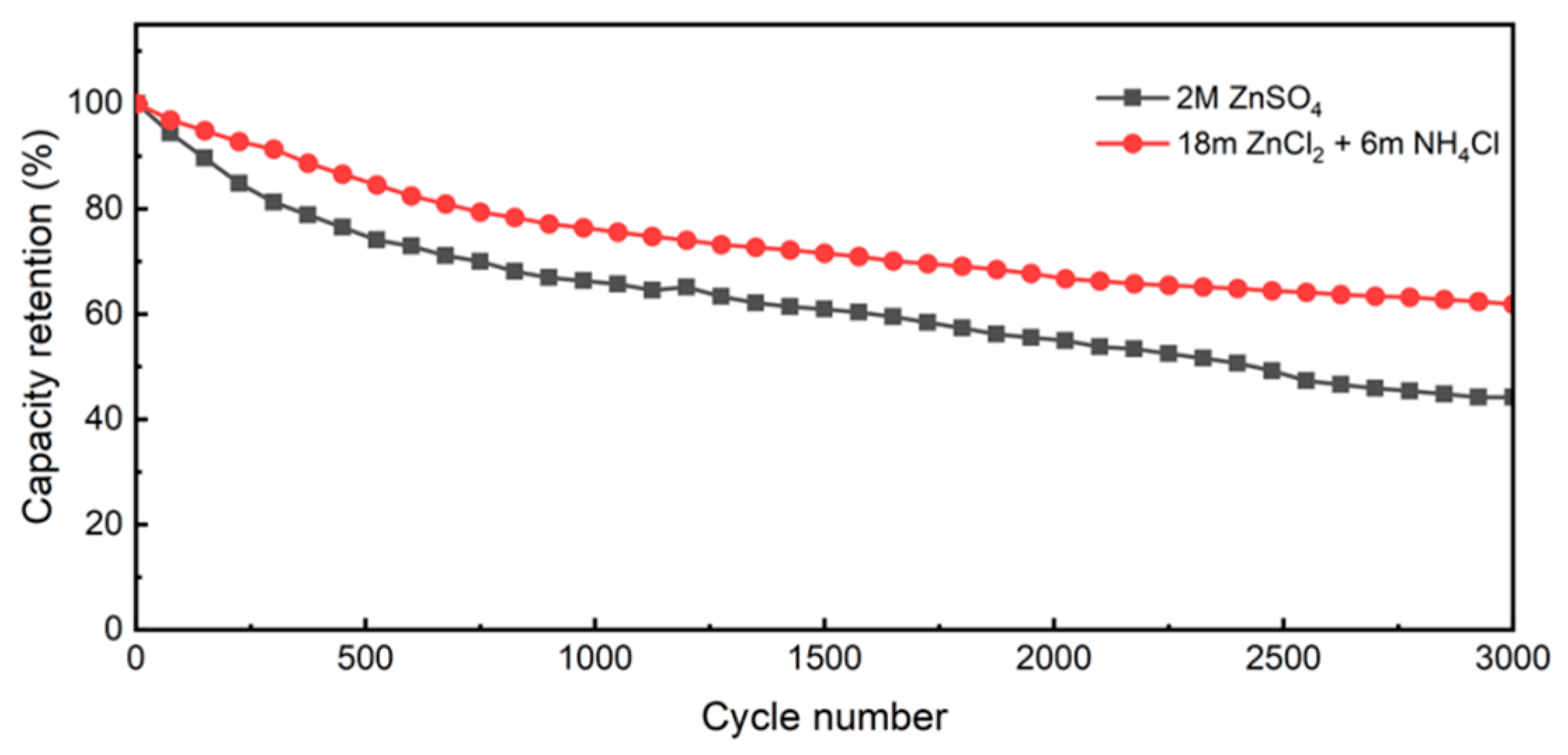
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaeian, M.; Abbas, Q.; Ogwu, A.; Hall, P.; Goldin, M.; Mirzaeian, M.; Jirandehi, H.F. Electrode and electrolyte materials for electrochemical capacitors. Int. J. Hydrogen Energy 2017, 42, 25565–25587. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, J.; Zhang, T.; Song, X.; Chen, W.; Chen, L. 2D Redox-Active Covalent Organic Frameworks for Supercapacitors: Design, Synthesis, and Challenges. Small 2021, 17, e2005073. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Q.; Hou, Y.; Shen, Z.; Fan, L.; Zhou, S.; Lu, Y.; Archer, L.A. Dynamic interphase–mediated assembly for deep cycling metal batteries. Sci. Adv. 2021, 7, 3752. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Chevrier, V.L. Correction to Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2015, 115, 2043. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nat. Cell Biol. 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.-S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem. Rev. 2014, 114, 11619–11635. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, H.; Zhang, H.; Liu, A.; Liu, Y.; Zhang, S. Highly bonded T-Nb2O5/rGO nanohybrids for 4 V quasi-solid state asymmetric supercapacitors with improved electrochemical performance. Nano Res. 2018, 11, 4673–4685. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode Materials, Electrolytes, and Challenges in Nonaqueous Lithium-Ion Capacitors. Adv. Mater. 2018, 30, e1705670. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Ma, M.; Zhong, Z.; Lin, X.; Chen, M. Interfacial growth of free-standing PANI films: Toward high-performance all-polymer supercapacitors. Chem. Sci. 2021, 12, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cheng, Z.; Feng, J.; Xie, Z.; Liu, Y.; Wang, Y. Ultrahigh volumetric performance of a free-standing compact N-doped holey graphene/PANI slice for supercapacitors. J. Mater. Chem. A 2017, 5, 16689–16701. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, J.; Sun, X.; Cheng, Z.; Liu, Y.; Wang, Y. High Density of Free-Standing Holey Graphene/PPy Films for Superior Volumetric Capacitance of Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 21763–21772. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Hu, Y.; Chen, Z.; Zhong, L. Cellulose carbon aerogel/PPy composites for high-performance supercapacitor. Carbohydr. Polym. 2019, 215, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Diao, Y.; Lu, Y.; Yang, H.; Zhou, Q.; Chrulski, K.; D’Arcy, J.M. Energy storing bricks for stationary PEDOT supercapacitors. Nat. Commun. 2020, 11, 3882. [Google Scholar] [CrossRef]
- Tisawat, N.; Samart, C.; Jaiyong, P.; Bryce, R.A.; Nueangnoraj, K.; Chanlek, N.; Kongparakul, S. Enhancement performance of carbon electrode for supercapacitors by quinone derivatives loading via solvent-free method. Appl. Surf. Sci. 2019, 491, 784–791. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Cheng, J.; Guan, Q.; Wang, B. Bioinspired Interface Design of Sewable, Weavable, and Washable Fiber Zinc Batteries for Wearable Power Textiles. Adv. Funct. Mater. 2020, 30, 2004430. [Google Scholar] [CrossRef]
- Liu, T.; Kim, K.C.; Lee, B.; Chen, Z.; Noda, S.; Jang, S.S.; Lee, S.W. Self-polymerized dopamine as an organic cathode for Li- and Na-ion batteries. Energy Environ. Sci. 2017, 10, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Huang, W.; Luo, Z.; Liu, L.; Lu, Y.; Li, Y.; Li, L.; Hu, J.; Ma, H.; Chen, J. High-capacity aqueous zinc batteries using sustainable quinone electrodes. Sci. Adv. 2018, 4, eaao1761. [Google Scholar] [CrossRef] [Green Version]
- Kundu, D.; Oberholzer, P.; Glaros, C.; Bouzid, A.; Tervoort, E.; Pasquarello, A.; Niederberger, M. Organic Cathode for Aqueous Zn-Ion Batteries: Taming a Unique Phase Evolution toward Stable Electrochemical Cycling. Chem. Mater. 2018, 30, 3874–3881. [Google Scholar] [CrossRef]
- Ye, J.; Simon, P.; Zhu, Y. Designing ionic channels in novel carbons for electrochemical energy storage. Natl. Sci. Rev. 2020, 7, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Liu, H.; Liu, P. Polymer grafted on carbon nanotubes as a flexible cathode for aqueous zinc ion batteries. Chem. Commun. 2019, 55, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xie, Z.; Fang, Y.; Zhu, K.; Gao, Y.; Wang, G.; Yan, J.; Cheng, K.; Ye, K.; Cao, D. Polydopamine-Modified Reduced Graphene Oxides as a Capable Electrode for High-Performance Supercapacitor. Chemistryselect 2019, 4, 2711–2715. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Ding, B.; Wang, J.; Malgras, V.; Jiang, J.; Li, Z.; Chen, S.; Dou, H.; Alshehri, S.M.; et al. Lithium-ion capacitor based on nanoarchitectured polydopamine/graphene composite anode and porous graphene cathode. Carbon 2020, 167, 627–633. [Google Scholar] [CrossRef]
- Yuksel, R.; Buyukcakir, O.; Panda, P.K.; Lee, S.H.; Jiang, Y.; Singh, D.; Hansen, S.; Adelung, R.; Mishra, Y.K.; Ahuja, R.; et al. Necklace-like Nitrogen-Doped Tubular Carbon 3D Frameworks for Electrochemical Energy Storage. Adv. Funct. Mater. 2020, 30, 1909725. [Google Scholar] [CrossRef]
- Ma, X.; Wang, J.; Wang, X.; Zhao, L.; Xu, C. Aqueous V2O5/activated carbon zinc-ion hybrid capacitors with high energy density and excellent cycling stability. J. Mater. Sci. Mater. Electron. 2019, 30, 5478–5486. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Zeng, W.; Wang, M.; Ruan, L.; Ma, Y. A New Free-Standing Aqueous Zinc-Ion Capacitor Based on MnO2–CNTs Cathode and MXene Anode. Nano-Micro Lett. 2019, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Peng, S.; Bin Wu, H.; Yu, L.; Madhavi, S.; Lou, X.W. A Flexible Quasi-Solid-State Asymmetric Electrochemical Capacitor Based on Hierarchical Porous V2O5Nanosheets on Carbon Nanofibers. Adv. Energy Mater. 2015, 5, 1500753. [Google Scholar] [CrossRef]
- Arun, N.; Jain, A.; Aravindan, V.; Jayaraman, S.; Ling, W.C.; Srinivasan, M.; Madhavi, S. Nanostructured spinel LiNi 0.5 Mn 1.5 O 4 as new insertion anode for advanced Li-ion capacitors with high power capability. Nano Energy 2015, 12, 69–75. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Zhao, Y.; Liu, Z.; Chang, Z.; Fu, L.; Zhu, Y.; Wu, Y.; Zhao, D. A Quasi-Solid-State Li-Ion Capacitor Based on Porous TiO2Hollow Microspheres Wrapped with Graphene Nanosheets. Small 2016, 12, 6207–6213. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, F.; Chen, J.; Yuan, Z.; Liu, C.; Zhang, X.; Xu, M.; Wei, L.; Chen, Y. A graphene-covalent organic framework hybrid for high-performance supercapacitors. Energy Storage Mater. 2020, 32, 448–457. [Google Scholar] [CrossRef]


| Electrode Material | Electrolyte | Voltage (V) | Energy Density (Wh kg−1) | Power Density (W kg−1) | Reference |
|---|---|---|---|---|---|
| PDA@3DVAG//AC | WIS | 0.8 | 46.14 | 393.75 | This work |
| NTC | 1M H2SO4 | 0.8 | 4.5 | 40 | [26] |
| V2O5//AC | 2M ZnSO4 | 2 | 34.6 | 1300 | [27] |
| MnO2–CNTs//MXene | 2M ZnSO4 | 1.9 | 29.7 | 2480 | [28] |
| V2O5–ECF//ECF | 6M LiCl | 2 | 22.3 | 1500 | [29] |
| LiNi0.5Mn1.5O4//AC | 1M LiPF6 | 1.75 | 19 | 103 | [30] |
| TiO2@EEG//EEG | 1M LiPF6 | 1.5 | 10 | 2000 | [31] |
| rGO/COF//rGO | 1M H2SO4 | 1 | 10.3 | 50 | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, R.; Zhang, Z.; Zhang, H.; Tang, Z.; Xue, Y.; Yang, G. Aqueous Organic Zinc-Ion Hybrid Supercapacitors Prepared by 3D Vertically Aligned Graphene-Polydopamine Composite Electrode. Nanomaterials 2022, 12, 386. https://doi.org/10.3390/nano12030386
Cui R, Zhang Z, Zhang H, Tang Z, Xue Y, Yang G. Aqueous Organic Zinc-Ion Hybrid Supercapacitors Prepared by 3D Vertically Aligned Graphene-Polydopamine Composite Electrode. Nanomaterials. 2022; 12(3):386. https://doi.org/10.3390/nano12030386
Chicago/Turabian StyleCui, Ruowei, Zhenwang Zhang, Huijuan Zhang, Zhihong Tang, Yuhua Xue, and Guangzhi Yang. 2022. "Aqueous Organic Zinc-Ion Hybrid Supercapacitors Prepared by 3D Vertically Aligned Graphene-Polydopamine Composite Electrode" Nanomaterials 12, no. 3: 386. https://doi.org/10.3390/nano12030386






