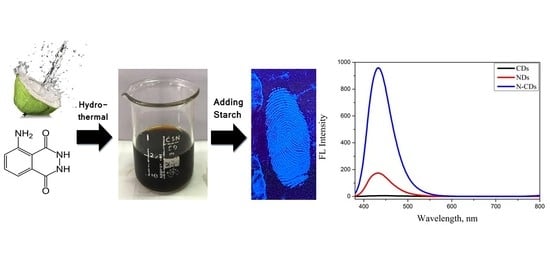
Highly Sensitive Fingerprint Detection under UV Light on Non-Porous Surface Using Starch-Powder Based Luminol-Doped Carbon Dots (N-CDs) from Tender Coconut Water as a Green Carbon Source
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Characteristics of the N-CDs by XPS and FTIR
2.2. Morphology Image Analysis of N-CDs by FIB-SEM and HR-TEM
2.3. Elemental Analysis of the N-CDs by EDX
2.4. Optical Properties of CDs and N-CDs
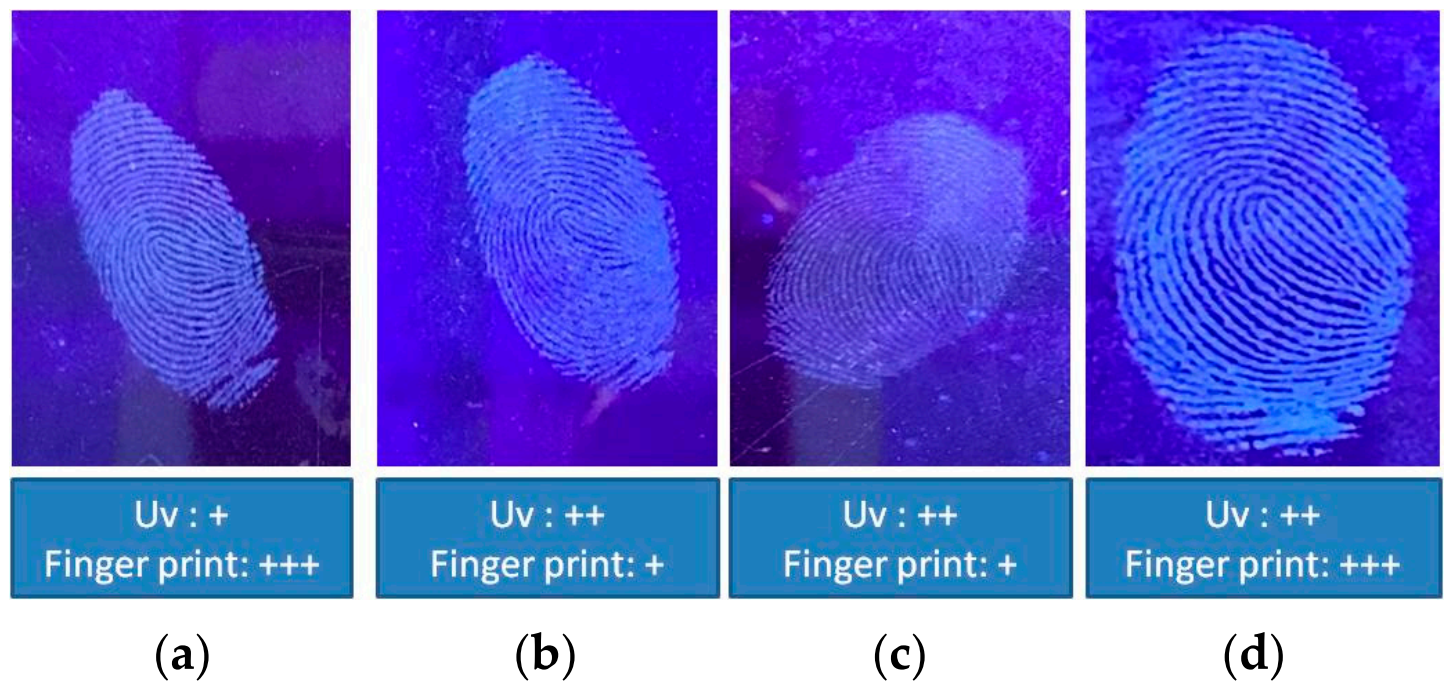
2.5. Application of the N-CDs for Latent Fingerprint Detection
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instruments
3.3. Synthesis of the Luminol Doped Carbon Dots (N-CDs)
3.4. Fluorescent N-CDs Powder Preparation
3.5. Method Detection of a Latent Fingerprint
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sari, S.A.; Qalbiah, U.; Putri, I.C. Comparison between Latent Fingerprint Identification using Black Powder and Cyanoacrylate Glue. Asian J. Chem. 2018, 30, 2615–2620. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Kaushal, P. Human Identification and Fingerprints: A Review. J. Biomet. Biostat. 2011, 2, 123. [Google Scholar] [CrossRef] [Green Version]
- Vokey, J.R.; Tangen, J.M.; Cole, S.A. On the preliminary psychophysics of fingerprint identification. Q. J. Exp. Psychol. 2009, 62, 1023–1040. [Google Scholar] [CrossRef]
- Tang, M.; Ren, G.; Zhu, B.; Yu, L.; Liu, X.; Chai, F.; Wu, H.; Wang, C. Facile synthesis of orange emissive carbon dots and their application for mercury ion detection and fast fingerprint development. Anal. Methods 2019, 11, 2072–2081. [Google Scholar] [CrossRef]
- Nayak, V.C.; Rastogi, P.; Kanchan, T.; Lobo, S.W.; Yoganarasimha, K.; Nayak, S.; Rao, N.G.; Kumar, G.P.; Shetty, B.S.K.; Menezes, R.G. Sex differences from fingerprint ridge density in the Indian population. J. Forensic Leg. Med. 2010, 17, 84–86. [Google Scholar] [CrossRef]
- Jayan, S.S.; Jayan, J.S.; Sneha, B.; Abha, K. Facile synthesis of carbon dots using tender coconut water for the fluorescence detection of heavy metal ions. Mater. Today Proc. 2021, 43, 3821–3825. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, M.; Moumen, A.; Duina, G.; Comini, E. 1D Titanium Dioxide: Achievements in Chemical Sensing. Material 2020, 13, 2974. [Google Scholar] [CrossRef]
- Fàbrega, C.; Casals, O.; Hernández-Ramírez, F.; Prades, J.D. A review on efficient self-heating in nanowire sensors: Prospects for very-low power devices. Sens. Actuators B Chem. 2018, 256, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Xian, M.; Shuang, S.; Dong, C. Facile synthesis of orange fluorescence 1 carbon dots with excitation independent emissionfor pH sensing and cellular imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys.-X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Choi, C.A.; Mazrad, Z.A.; Lee, G.; In, I.; Lee, K.D.; Park, S.Y. Boronate-based fluorescent carbon dot for rapid and selectively bacterial sensing by luminescence off/on system. J. Pharm. Biomed. Anal. 2018, 159, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.; Chen, X.; Zhang, Y.; Colvin, V.L.; Rice, Q.; Seo, J.; Feng, S.; Wang, S.; Yu, W.W. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Q.; Fu, M.; Fan, X.; Lu, H.; Wang, H.; Zhang, Y.; Wang, H. Carbon quantum dots encapsulated in super small platinum nanocrystals core-shell architecture/nitrogen doped graphene hybrid nanocomposite for electrochemical biosensing of DNA damagebiomarker-8’-hydroxy-2’-deoxyguanosine. Anal. Chim. Acta 2018, 1047, 9–20. [Google Scholar] [CrossRef]
- Macairan, J.-R.; Jaunky, D.B.; Naccache, R.; Piekny, A. Intracellular ratiometric temperature sensing using fluorescent carbon dots. Nanoscale Adv. 2019, 1, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Huang, J.-L.; Wang, Y.-T.; Zheng, A.-Q.; Shu, Y.; Wang, J.-H. β-cyclodextrin decorated carbon dots serve as nanocarrier for targeted drug delivery and controlled release. ChemNanoMat 2019, 5, 479–487. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; Ong, H.; Zhao, Y. Dual-Responsive Carbon Dots for Tumor Extracellular Microenvironment Triggered Targeting and Enhanced Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 18732–18740. [Google Scholar] [CrossRef]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Ma, J.J.; Chen, J.R.; Feng, H. DNA nanosensor based on biocompatible graphene quantum dots and carbon nanotubes. Biosens. Bioelectron. 2014, 60, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, M.-X.; Yu, Y.-L.; Wang, J.-H. Room-temperature synthesis of fluorescent carbon-based nanoparticles and their application in multidimensional sensing. Sens. Actuators B Chem. 2019, 288, 749–756. [Google Scholar] [CrossRef]
- Diao, H.; Li, T.; Zhang, R.; Kang, Y.; Liu, W.; Cui, Y.; Wei, S.; Wang, N.; Li, L.; Wang, H.; et al. Facile and green synthesis of fluorescent carbon dots with tunable emission for sensors and cells imaging. Spectrochim. Acta—Part A Mol. 2018, 200, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar]
- Zhong, D.; Zhuo, Y.; Feng, Y.; Yang, X. Employing carbon dots modified with vancomycin for assaying gram-positive bacteria like Staphylococcus Aureus. Biosens. Bioelectron. 2015, 74, 546–553. [Google Scholar] [CrossRef]
- Wang, H.; Mu, Q.; Wang, K.; Revia, R.A.; Yen, C.; Gu, X.; Tian, B.; Liu, J.; Zhang, M. Nitrogen and boron dual-doped graphene quantum dots for near-infrared second window imaging and photothermal therapy. Appl. Mater. Today 2019, 14, 108–117. [Google Scholar] [CrossRef]
- Chen, S.; Wei, L.; Chen, X.-W.; Wang, J.-H. Suspension Array of Ionic Liquid or Ionic Liquid−Quantum Dots Conjugates for the Discrimination of Proteins and Bacteria. Anal. Chem. 2015, 87, 10902–10909. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Demchenko, A.P. Nanoparticles and nanocomposites for fluorescence sensing and imaging. Methods Appl. Fluoresc. 2013, 1, 022001. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Li, B.; Fan, X.; Wang, W.; Liu, P.; Xu, S.; Luo, X. Nitrogen doped carbon dots: Mechanism investigation and their application for label free CA125 analysis. J. Mater. Chem. B 2019, 10, 1039. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Zheng, B.; Yan, L.; Dai, J.; Zhuang, Z.; Du, J.; Guo, Y.; Xiao, D. N-doped carbon dots: Green and efficient synthesis on large-scale and their application in fluorescent pH sensing. New J. Chem. 2017, 41, 10607–10612. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-Synthesized Polysaccharide-Derived Carbon Dots as Therapeutic Cargoes and Toughening Agents for Elastomeric Gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef]
- Luo, H.; Dimitrov, S.; Daboczi, M.; Kim, J.-S.; Guo, Q.; Fang, Y.; Stoeckel, M.-A.; Samorì, P.; Fenwick, O.; Sobrido, A.B.J.; et al. Nitrogen-Doped Carbon Dots/TiO2 Nanoparticle Composites for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2020, 3, 3371–3381. [Google Scholar] [CrossRef]
- Milenkovic, I.; Algarra, M.; Alcoholado, C.; Cifuentes, M.; Lázaro-Martínez, J.M.; Rodríguez-Castellón, E.; Mutavdžić, D.; Radotić, K.; Bandosz, T.J. Fingerprint imaging using N-doped carbon dots. Carbon 2019, 144, 791–797. [Google Scholar] [CrossRef]
- Das, R.B. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Ansi, V.A.; Sreelakshmi, P.; Poovathinthodiyil, R.; Renuka, N.K. Table sugar derived carbon dot—A promising green reducing agent. Mater. Res. Bull. 2021, 139, 111284. [Google Scholar]
- Hashemi, N.; Mousazadeh, M.H. Green synthesis of photoluminescent carbon dots derived from red beetroot as a selective probe for Pd2+ detection. J. Photochem. Photobiol. A Chem. 2021, 421, 113534. [Google Scholar] [CrossRef]
- Hu, Z.; Jiao, X.-Y.; Xu, L. The N,S co-doped carbon dots with excellent luminescent properties from green tea leaf residue and its sensing of gefitinib. Microchem. J. 2020, 154, 104588. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xu, X.; Hao, H.; Liu, R.; Zhang, D.; Gao, F.; Lu, Q. Green synthesis of fluorescent carbon quantum dots and carbon spheres from pericarp. Sci. China Chem. 2015, 58, 863–870. [Google Scholar] [CrossRef]
- Alam, A.-M.; Park, B.-Y.; Ghouri, Z.K.; Park, M.; Kim, H.-Y. Synthesis of Carbon Quantum Dot from Cabbage with Down- and Up-Conversion Photoluminescence Properties: Excellent Imaging Agent for Biomedical Application. Green Chem. 2015, 17, 3791–3797. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Ye, S.-L.; Huang, J.-J.; Luo, L.; Fu, H.-J.; Sun, Y.M.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L. Preparation of Carbon Dots and Their Application in Food Analysis as Signal Probe. Chin. J. Anal. Chem. 2017, 45, 1517–1581. [Google Scholar] [CrossRef]
- Roshni, V.; Ottoor, D. Synthesis of carbon nanoparticles using one step green approach and their application as mercuric ion sensor. J. Lumin. 2015, 161, 117–122. [Google Scholar] [CrossRef]
- Du, F.; Zeng, F.; Ming, Y.; Wu, S. Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim. Acta 2013, 180, 453–460. [Google Scholar] [CrossRef]
- Yong, J.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Li, J.; Cheng, Y.; Wan, F.; Jia, R.; Wang, Y. Compound repair effect of carbon dots and Fe2+ on iron deficiency in Cucumis melon L. Plant Physiol. Biochem. 2019, 142, 137–142. [Google Scholar] [CrossRef]
- Chandrajith, V.; Marapana, R. Physicochemical characters of bark exudates of Lannea coromandelica and its application as a natural fruit coating. J. Pharmacogn. Phytochem. 2018, 7, 1798–1802. [Google Scholar]
- Djunaidi, M.C. Gold imprinted adsorption based on eugenol. IOP Publ. 2020, 1524, 012077. [Google Scholar] [CrossRef]
- Naik, G.G.; Alam, M.B.; Pandey, V.; Dubey, P.K.; Parmar, A.; Sahu, A. Pink Fluorescent Carbon Dots Derived from the Phytomedicine for Breast Cancer Cell Imaging. ChemistrySelect 2020, 5, 6594–6960. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Liu, Z.; Deng, B.; Yu, M.; Li, L.; Jiang, H.; Li, J. Preparation of the antifouling microfiltration membranes from poly(N,N-dimethylacrylamide). J. Mater. Chem. 2011, 21, 11908. [Google Scholar] [CrossRef]
- Guan, W.; Gu, W.; Ye, L.; Guo, C.; Su, S.; Xu, P.; Ye, L. Microwave-assisted polyol synthesis of carbon nitride dots from folic acid for cell imaging. Int. J. Nanomed. 2014, 9, 5071–5078. [Google Scholar]
- Lu, S.; Guo, S.; Xu, P.; Li, X.; Zhao, Y.; Gu, W.; Xue, M. Hydrothermal synthesis of nitrogen-doped carbon dots with real-time live-cell imaging and blood–brain barrier penetration capabilities. Int. J. Nanomed. 2016, 11, 6235–6336. [Google Scholar] [CrossRef] [Green Version]












| Coconut Type | Young | Mature |
|---|---|---|
| Average Weight (g) | 565 | 393 |
| Age of Coconut | 6 months | 12 months |
| sugar | (g/100 g) | |
| Total | 5.23 | 3.42 |
| Sucrose | 0.06 | 0.51 |
| Glucose | 2.61 | 1.48 |
| Fructose | 2.55 | 1.43 |
| Inorganic Ions | (g/100 g) | |
| Calcium, Ca | 27.35 | 31.64 |
| Potassium, K | 203.7 | 257.52 |
| Phosphorus, P | 4.66 | 12.77 |
| Sodium, Na | 1.75 | 16.1 |
| Precursors | Reaction Condition | Fluorescence | Size | Application | Reference |
|---|---|---|---|---|---|
| Sodium alginate | 1100 W for 8 min | Blue | 5.6 nm | Toughening agent | [33] |
| Watermelon peel | 220 °C for 2 h | Blue | 2 nm | Optical imaging | [39] |
| Table sugar | 120 °C for 3 min | Blue | 3–4 nm | Green reducing agent | [40] |
| Red beetroot | 180 °C for 10 h | - | 5–7 nm | Pd2+ detection | [41] |
| Green tea leaf | 350 °C for 2 h | Blue | 2 nm | Optical imaging | [42] |
| Orange juice | 120 °C for 150 min | yellow | 1.5–4.5 nm | Biolabeling and optoelectronics | [43] |
| Aloe | 180 °C for 11 h | Blue | 5 nm | Detection of tetrazine in food samples | [44] |
| Orange pericarp | 100 °C for 5 h | Blue | 2.9 nm | Nano biotechnology | [45] |
| Cabbage | 140 °C for 5 h | Blue | 2–6 nm | Bioimaging | [46] |
| Coconut water | 180 °C for 4 h | Blue | 12.9 nm | Detection fingerprint | Our work |
| Material Type | Visible Lamp | UV-Light |
|---|---|---|
| Glass |  |  |
| Stapler |  |  |
| Coin |  |  |
| Coaster |  |  |
| Material | Day | 60 °C | −4 °C | Room Temp |
|---|---|---|---|---|
| Glass | 7 | DM | D | DM |
| 14 | D | D | DM | |
| 21 | D | D | DM | |
| 28 | D | D | DM | |
| Iron | 7 | DM | DM | DM |
| 14 | DM | DM | DM | |
| 21 | DM | D | DM | |
| 28 | D | D | DM | |
| Plastic | 7 | D | D | DM |
| 14 | D | D | DM | |
| 21 | D | D | D | |
| 28 | D | D | D | |
| D | Detected | |||
| DM | Detected Minutiae | |||
| ND | Not Detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugroho, D.; Keawprom, C.; Chanthai, S.; Oh, W.-C.; Benchawattananon, R. Highly Sensitive Fingerprint Detection under UV Light on Non-Porous Surface Using Starch-Powder Based Luminol-Doped Carbon Dots (N-CDs) from Tender Coconut Water as a Green Carbon Source. Nanomaterials 2022, 12, 400. https://doi.org/10.3390/nano12030400
Nugroho D, Keawprom C, Chanthai S, Oh W-C, Benchawattananon R. Highly Sensitive Fingerprint Detection under UV Light on Non-Porous Surface Using Starch-Powder Based Luminol-Doped Carbon Dots (N-CDs) from Tender Coconut Water as a Green Carbon Source. Nanomaterials. 2022; 12(3):400. https://doi.org/10.3390/nano12030400
Chicago/Turabian StyleNugroho, David, Chayanee Keawprom, Saksit Chanthai, Won-Chun Oh, and Rachadaporn Benchawattananon. 2022. "Highly Sensitive Fingerprint Detection under UV Light on Non-Porous Surface Using Starch-Powder Based Luminol-Doped Carbon Dots (N-CDs) from Tender Coconut Water as a Green Carbon Source" Nanomaterials 12, no. 3: 400. https://doi.org/10.3390/nano12030400