The Oxygen Vacancy Defect of ZnO/NiO Nanomaterials Improves Photocatalytic Performance and Ammonia Sensing Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
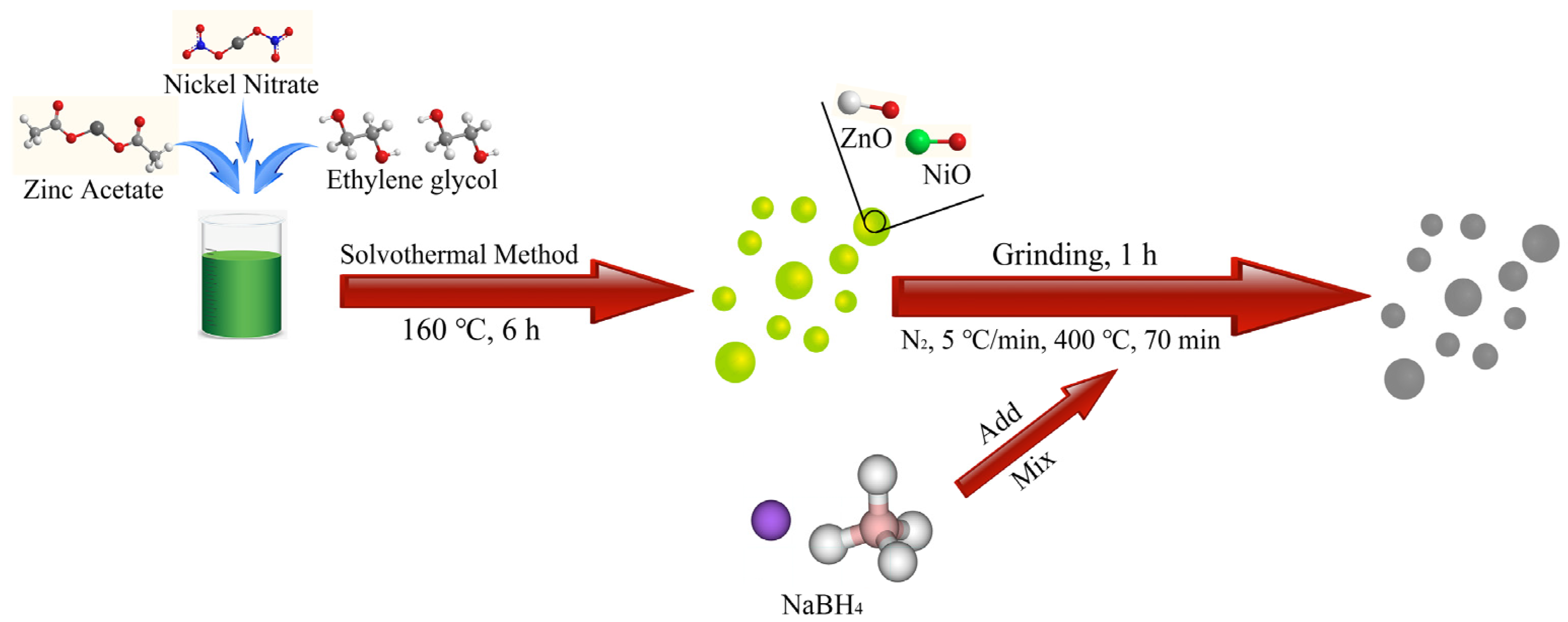
2.2. Synthesis of Spherical ZnO/NiO Nanoparticles with Oxygen Vacancy
2.3. Characterization Instrument
3. Results
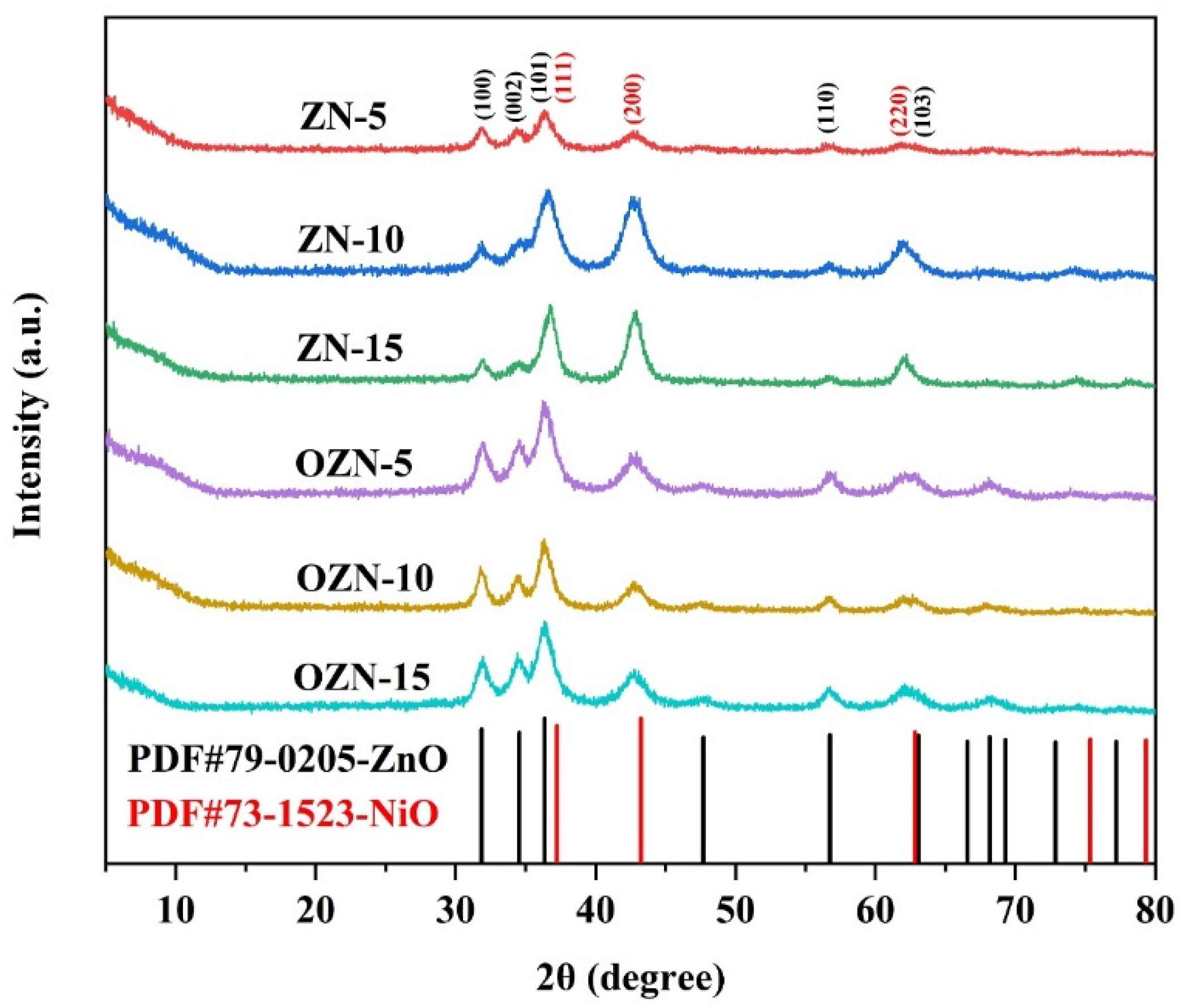
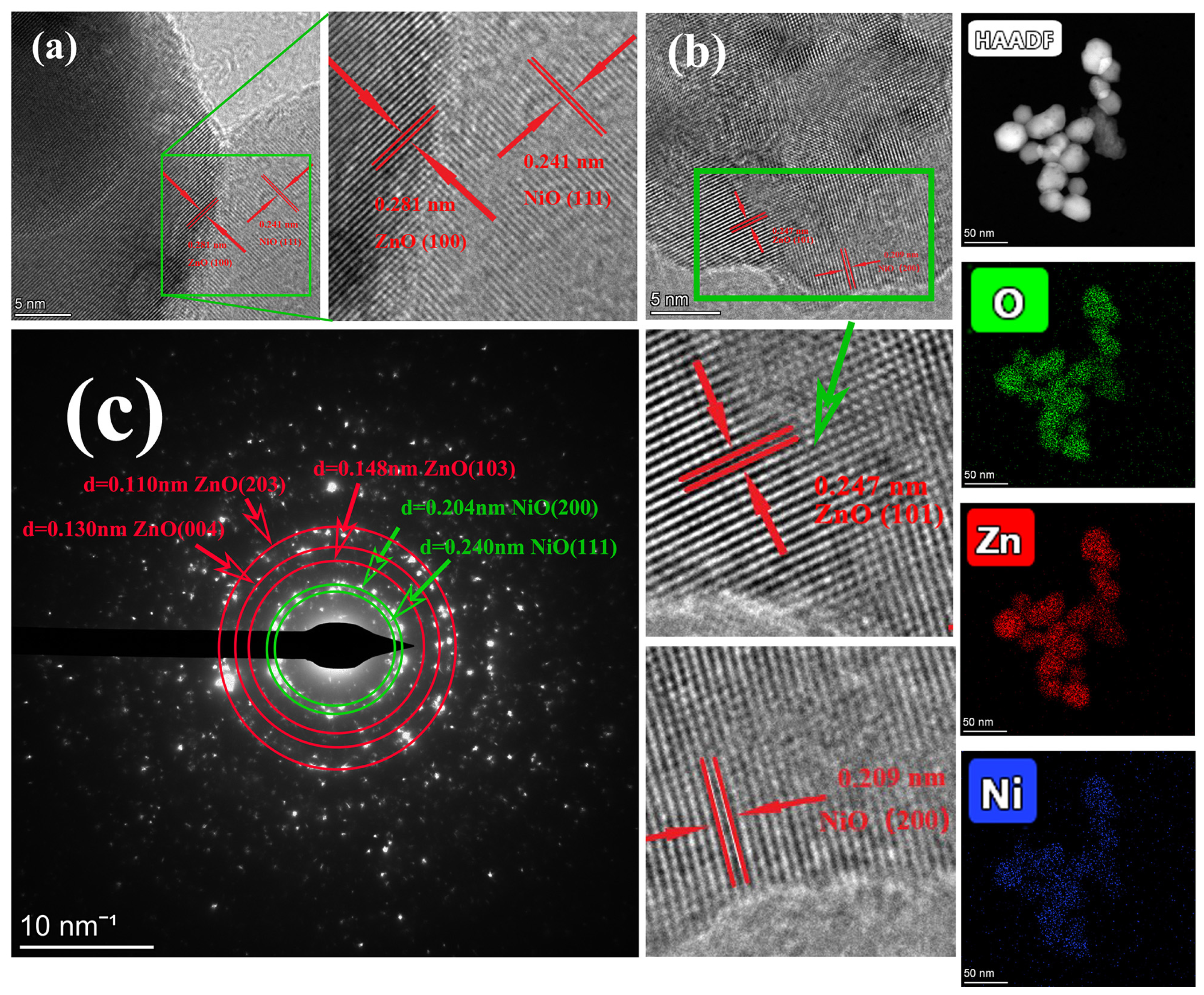
3.1. Crystal Structure and Morphology of ZnO/NiO Nanoparticles
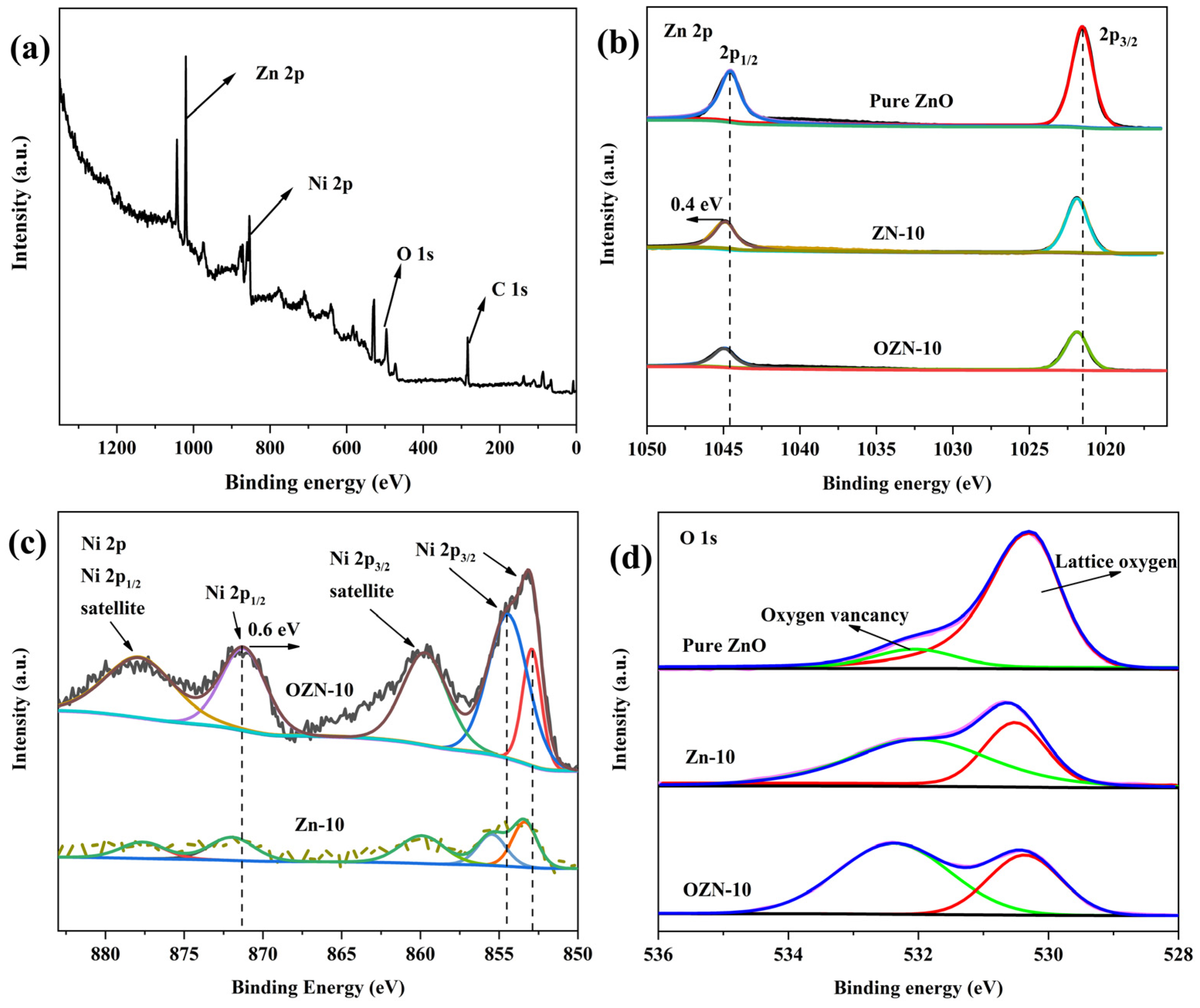
3.2. Valence Analysis of Chemical Composition and Elements
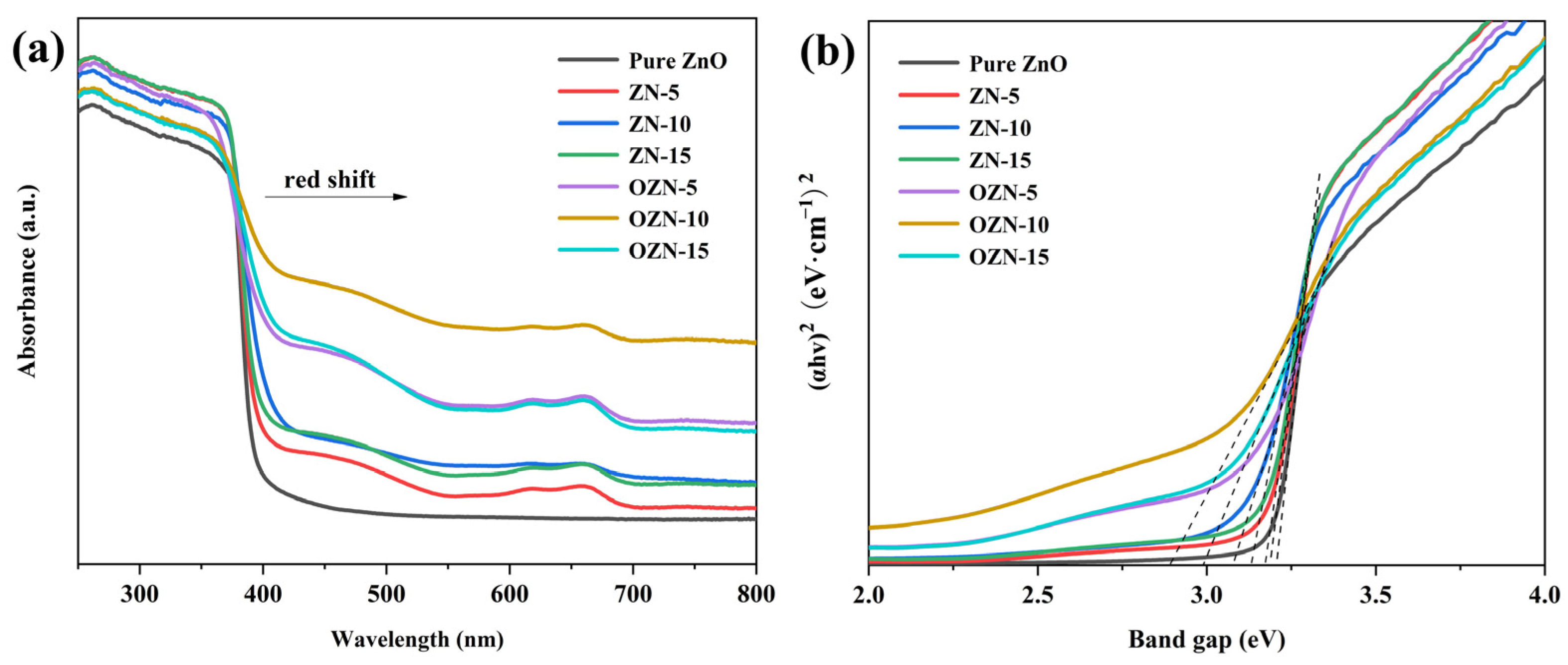
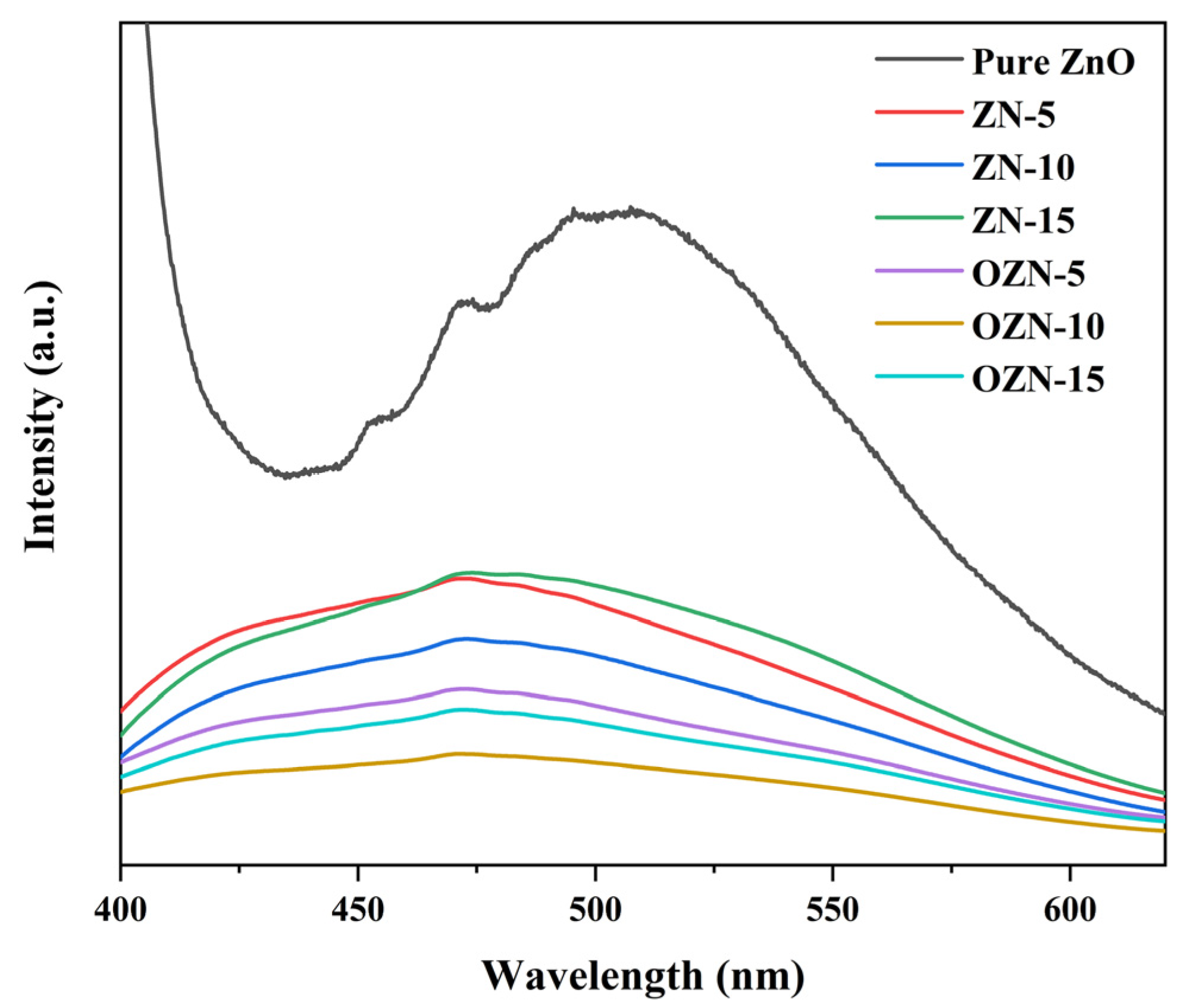
3.3. Photophysical Property of Samples
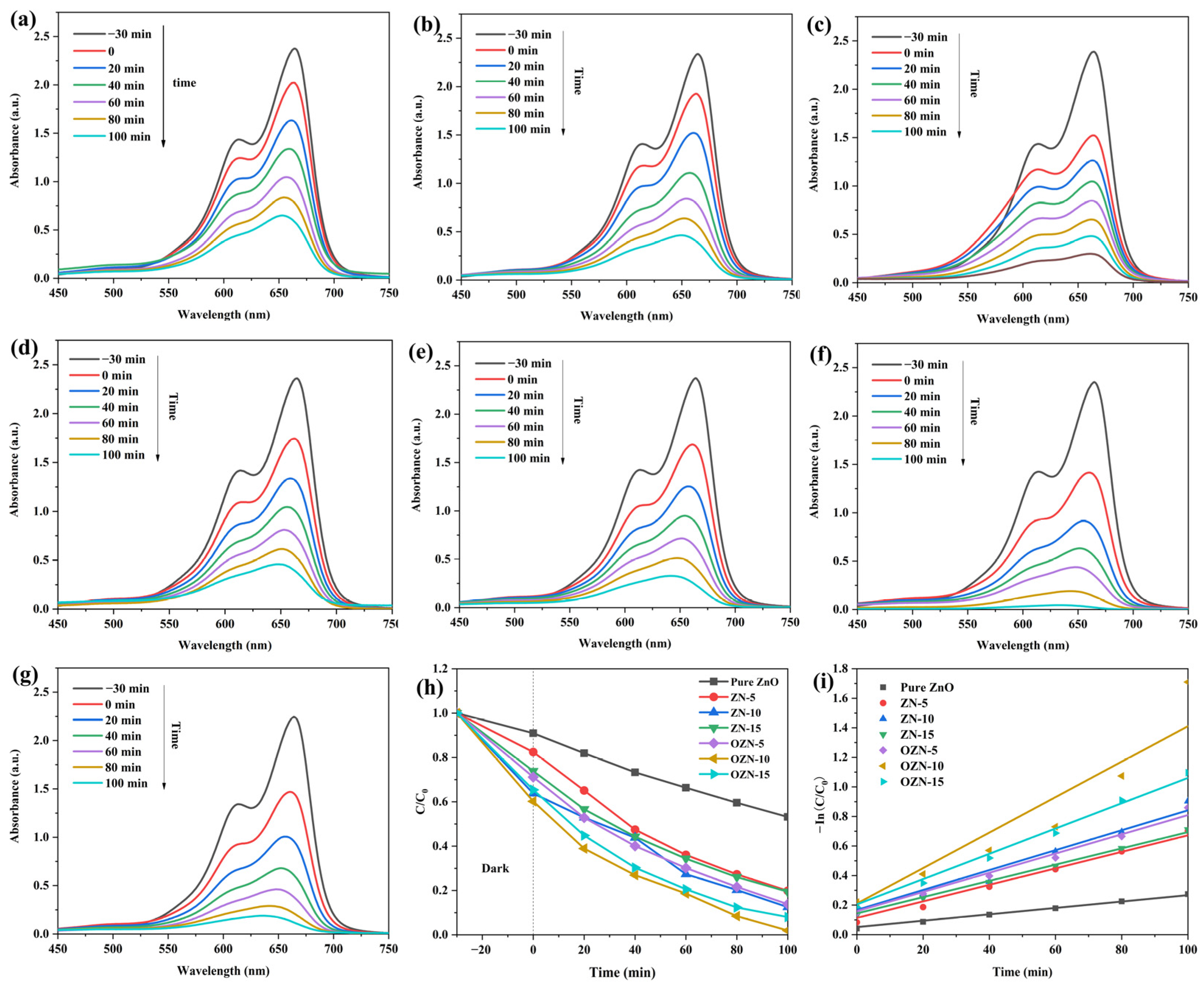
3.4. Analysis of the Photocatalytic Properties of ZN and OZN
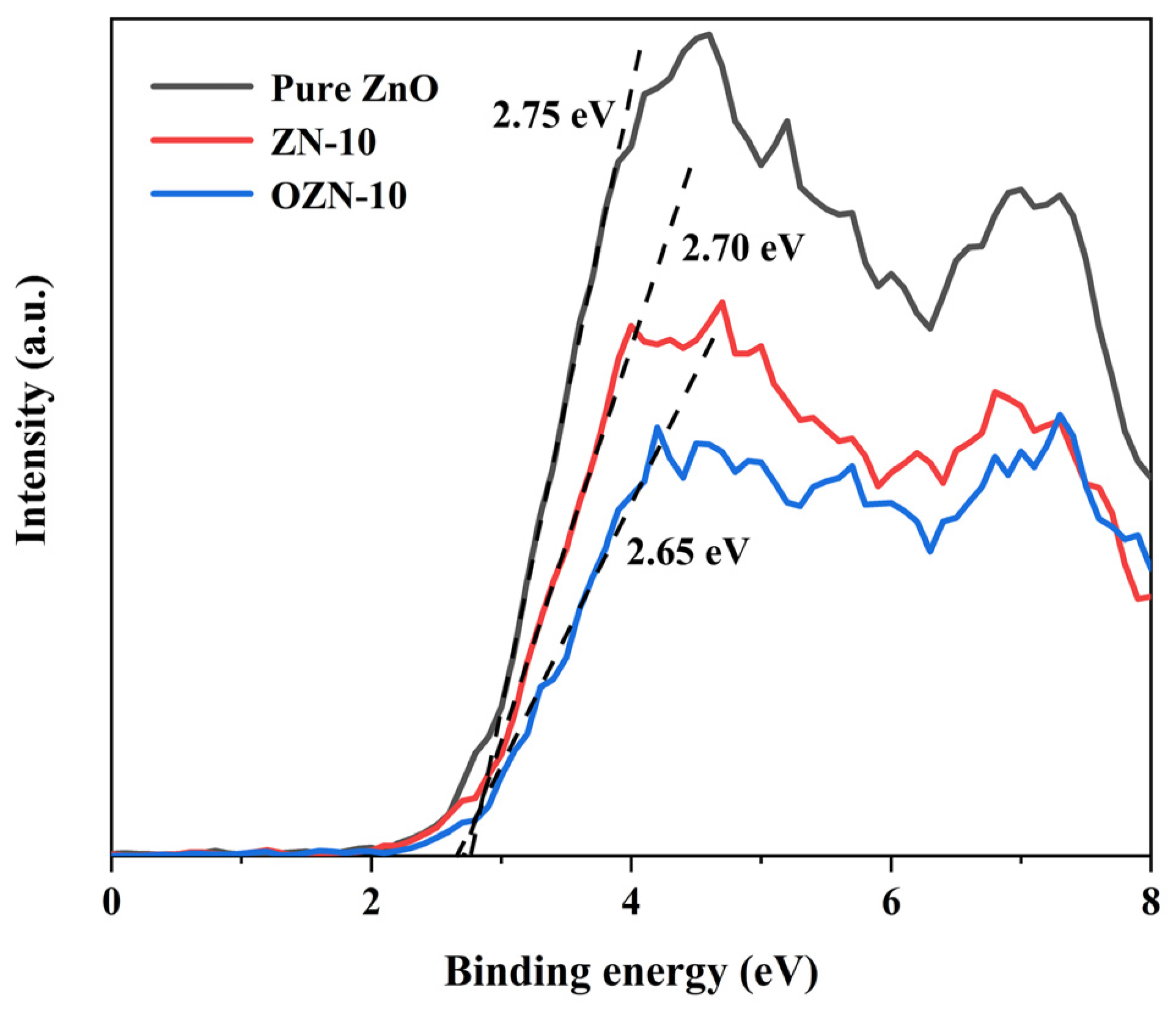
3.5. Photoelectric Chemical Performance Test and EPR Analysis
3.6. Chemical Potential Energy and Free Radical Experiments
3.7. Photocatalytic Mechanism of OZN Materials
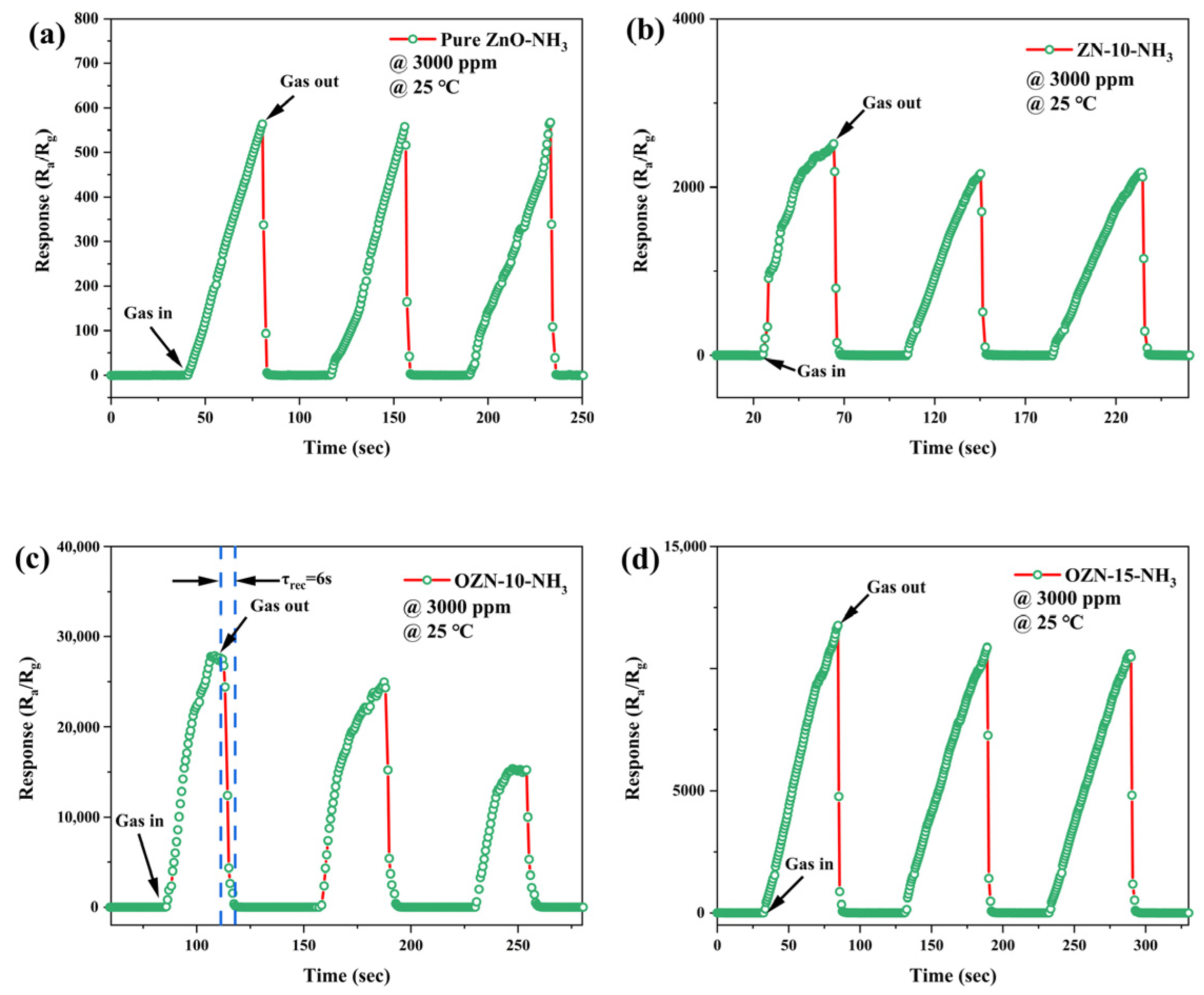
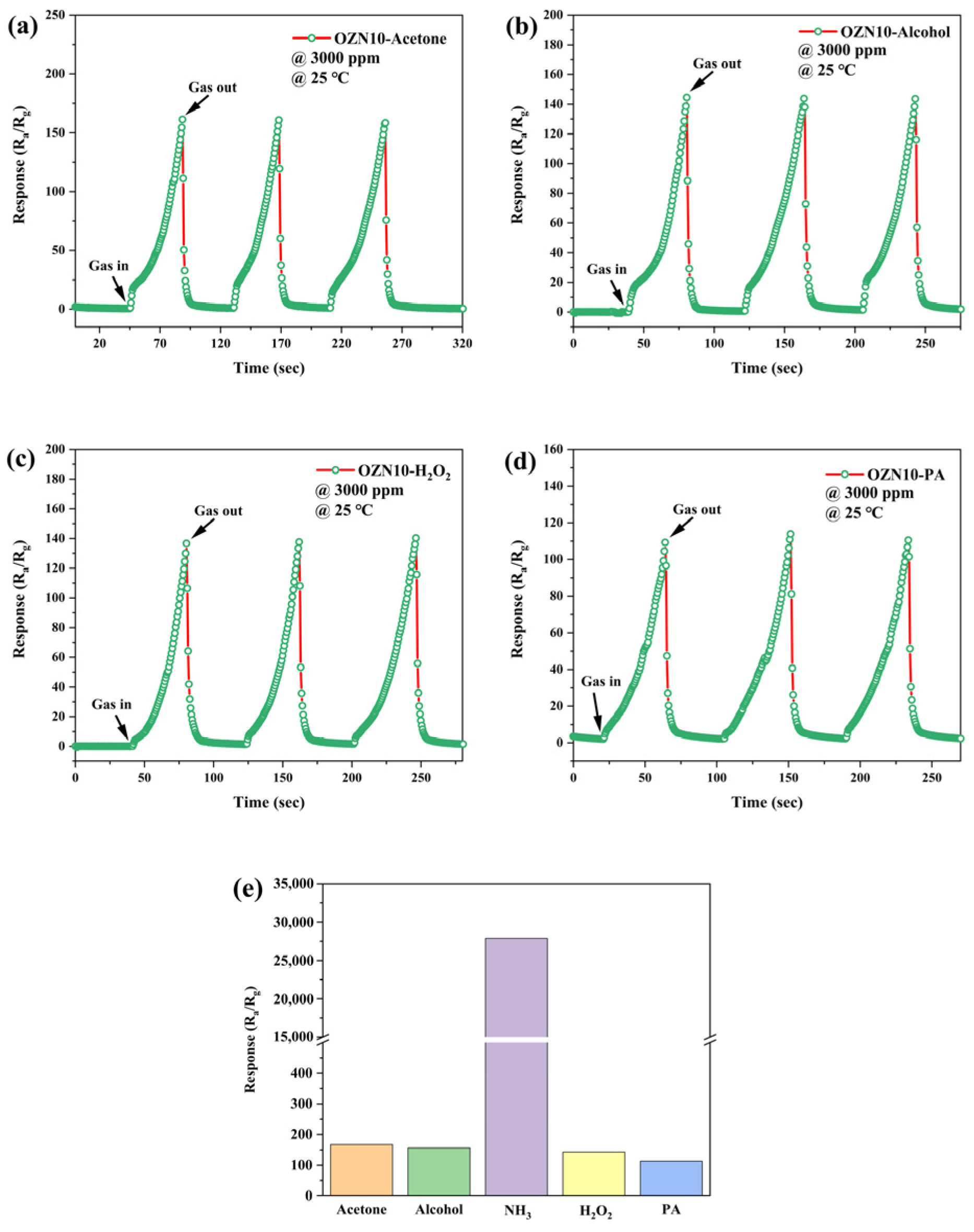
3.8. Analysis of Gas Sensitive Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karthikeyan, V.; Padmanaban, A.; Dhanasekaran, T. Snthesis and characterization of ZnO/NiO and its photocatalytic activity. Mech. Mater. Sci. Eng. J. 2018, 9. [Google Scholar] [CrossRef]
- Si, Q.; Guo, W.; Wang, H. Difunctional Carbon Quantum Dots/g-C3N4 with In-plane Electron Buffer for Intense Tetracycline Degradation under Visible Light: Tight Adsorption and Smooth Electron Transfer. Appl. Catal. B Environ. 2021, 299, 120694. [Google Scholar] [CrossRef]
- Yin, Z.; Qi, S.; Deng, S. Bi2MoO6 /TiO2 heterojunction modified with Ag quantum dots: A novel photocatalyst for the efficient degradation of tetracycline hydrochloride. J. Alloys Compd. 2021, 888, 161582. [Google Scholar] [CrossRef]
- Wang, L.; Cai, P.R.; Liu, Z.Z. Role of Carbon Quantum Dots on Nickel Titanate to Promote Water Oxidation Reaction under Visible Light Illumination. J. Colloids Interface Sci. 2022, 607, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xie, S.; Zhou, W. Water Pollution and Health Impact in China: A Mini Review. Open Environ. J. 2008, 2, 1–5. [Google Scholar] [CrossRef]
- Sun, J.; Dong, S.; Feng, J. Enhanced sunlight photocatalytic performance of Sn-doped ZnO for Methylene Blue degradation. J. Mol. Catal. A Chem. 2011, 335, 145–150. [Google Scholar] [CrossRef]
- Ska, A.; Sr, B.; Dong, J. Tuning the interfacial electronic transitions of bi-dimensional nanocomposites (pGO/ZnO) towards photocatalytic degradation and energy application. Environ. Res. 2021, 204, 112050. [Google Scholar]
- Mishra, P.K.; Viji, P.; Dobnhal, R. Defects assisted photosensing and dye degradation of Ni/Ga co-doped ZnO: A theory added experimental investigation. J. Alloys Compd. 2022, 893, 162229. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.; Singh, N. Eliminating pesticide quinalphos from surface waters using synthesized GO-ZnO nanoflowers: Characterization, degradation pathways and kinetic study. Chemosphere 2021, 286, 131837. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Thayumanavan, A.; Ravichandran, K. Photocatalytic and antibacterial activities of eco-friendly green synthesized ZnO and NiO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 28, 2062–2068. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, W.; Li, X. Insights into the degradation mechanism of perfluorooctanoic acid under visible-light irradiation through fabricating flower-shaped Bi5O7I/ZnO n-n heterojunction microspheres. Chem. Eng. J. 2021, 420, 129934. [Google Scholar] [CrossRef]
- Song, M.; Qi, K.; Wen, Y. Rational design of novel three-dimensional reticulated Ag2O/ZnO Z-scheme heterojunction on Ni foam for promising practical photocatalysis. Sci. Total Environ. 2021, 793, 148519. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Walle, C. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Jun, P.; Wang, Z. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar]
- Li, X.; Song, J.; Liu, Y. Controlling oxygen vacancies and properties of ZnO. Curr. Appl. Phys. 2014, 14, 521–527. [Google Scholar] [CrossRef]
- Brillson, L.; Cox, J.; Gao, H. Native Point Defect Measurement and Manipulation in ZnO Nanostructures. Materials 2019, 12, 2242. [Google Scholar] [CrossRef] [Green Version]
- Anderson, V.; Chris, G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar]
- Zhu, P.; Yin, X.; Gao, X. Enhanced photocatalytic NO removal and toxic NO2 production inhibition over ZIF-8-derived ZnO nanoparticles with controllable amount of oxygen vacancies. Chin. J. Catal. 2021, 42, 175–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, M.; Yue, L. A room-temperature aniline sensor based on Ce doped ZnO porous nanosheets with abundant oxygen vacancies. J. Alloys Compd. 2021, 885, 160988. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Na, S.I. Fabrication and enhanced carbon monoxide gas sensing performance of p-CuO/n-TiO2 heterojunction device. Colloids Surf. A 2020, 612, 125962. [Google Scholar] [CrossRef]
- Yousaf, S.; Zulfiqar, S.; Din, M. Solar Light Irradiated Photocatalytic Activity of ZnO-NiO/rGO Nanocatalyst. J. Mater. Res. Technol. 2021, 12, 999–1009. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y. Glucose-assisted synthesis of hierarchical NiO-ZnO heterostructure with enhanced glycol gas sensing performance. Sens. Actuators B Chem. 2020, 329, 129167. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Q.; Ma, Y. Highly efficient photocatalytic hydrogen evolution and simultaneous formaldehyde degradation over Z-scheme ZnIn2S4-NiO/BiVO4 hierarchical heterojunction under visible light irradiation. Chem. Eng. J. 2021, 423, 130164. [Google Scholar] [CrossRef]
- Indira, K.; Shanmugam, S.; Hari, A. Photocatalytic degradation of congo red dye using nickel–titanium dioxide nanoflakes synthesized by Mukia madrasapatna leaf extract. Environ. Res. 2021, 202, 111647. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Han, J.; Sun, L. NiO/ZnO composite decorated on rGO for detection of NO2. Sens. Actuators B Chem. 2021, 339, 129720. [Google Scholar] [CrossRef]
- Muhambihai, P.; Rama, V.; Subramaniam, P. Photocatalytic degradation of aniline blue, brilliant green and direct red 80 using NiO/CuO, CuO/ZnO and ZnO/NiO nanocomposites. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100360. [Google Scholar] [CrossRef]
- Nakate, U.T.; Yu, Y.T.; Park, S. High performance acetaldehyde gas sensor based on p-n heterojunction interface of NiO nanosheets and WO3 nanorods. Sens. Actuators B Chem. 2021, 23, 130264. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Pchoudhury, S. Microwave assisted synthesis of Co3O4 and NiO nanoplates and structural, optical, magnetic characterizations. Nano-Struct. Nano-Objects 2018, 14, 66–72. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M. Concentration of Ce3+ and Oxygen Vacancies in Cerium Oxide Nanoparticles. Chem. Mater. 2006, 18, 5144–5146. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Dong, G. NiO/ZnO p–n heterostructures and gas sensing properties for reduced operating temperature. RSC Adv. 2016, 110, 109091–109098. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Yang, L. Synergy of Ni dopant and oxygen vacancies in ZnO for efficient photocatalytic depolymerization of sodium lignosulfonate. Chem. Eng. J. 2020, 394, 125050. [Google Scholar] [CrossRef]
- Samireh, M.A.; Hassanzadeh-Tabrizi, S.A. Facile synthesis and investigation of NiO–ZnO–Ag nanocomposites as efficient photocatalysts for degradation of methylene blue dye. Ceram. Int. 2019, 45, 14934–14942. [Google Scholar]
- Wang, X.; Xu, H.; Zhang, Y. Fructose-regulated ZnO Single-Crystal Nanosheets with Oxygen Vacancies for Photodegradation of High Concentration Pollutants and Photocatalytic Hydrogen Evolution. Ceram. Int. 2021, 47, 16170–16177. [Google Scholar] [CrossRef]
- Sahoo, P.; Sharma, A.; Padhan, S. Construction of ZnO@NiO heterostructure photoelectrodes for improved photoelectrochemical performance. Int. J. Hydrogen Energy 2021, 46, 36176–36188. [Google Scholar] [CrossRef]
- Xie, X.; Si, R.; Zheng, J. Synthesis of ZnO/NiO hollow spheres and their humidity sensing performance. J. Alloys Compd. 2021, 879, 160487. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, J. The Oxygen Vacancy Defect of ZnO/NiO Nanomaterials Improves Photocatalytic Performance and Ammonia Sensing Performance. Nanomaterials 2022, 12, 433. https://doi.org/10.3390/nano12030433
Zhang J, Li J. The Oxygen Vacancy Defect of ZnO/NiO Nanomaterials Improves Photocatalytic Performance and Ammonia Sensing Performance. Nanomaterials. 2022; 12(3):433. https://doi.org/10.3390/nano12030433
Chicago/Turabian StyleZhang, Jiaqi, and Jin Li. 2022. "The Oxygen Vacancy Defect of ZnO/NiO Nanomaterials Improves Photocatalytic Performance and Ammonia Sensing Performance" Nanomaterials 12, no. 3: 433. https://doi.org/10.3390/nano12030433





