Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications
Abstract
:1. Introduction
2. Fluorescent Nanomaterials for Bioimaging
3. Nano-Drug Delivery Systems
3.1. Nano-Vehicles for Anticancer Drugs
3.2. Nanostructured Materials as Drug Delivery Vehicles for Antioxidant Drugs
4. Antimicrobial Materials
5. Gene Therapy
6. Biosensors
7. Tissue Engineering
8. Agriculture and Food Industry
9. Risks of Exposure to Nanomaterials
10. Global Market and Future of Nanomaterials
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Nada, A.A.; Sayegh, S.; Roualdes, S.; Renard, A.; Iatsunskyi, I.; Coy, E.; Bechelany, M. Simultaneous hydrogen and oxygen evolution reactions using free-standing nitrogen-doped-carbon–Co/CoOx nanofiber electrodes decorated with palladium nanoparticles. J. Mater. Chem. A 2021, 9, 17724–17739. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, V.; Kirubanandam, S.; Barhoum, A. Engineered nanomaterials: Nanofabrication and surface functionalization. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 305–340. [Google Scholar] [CrossRef]
- Cremers, V.; Rampelberg, G.; Barhoum, A.; Walters, P.; Claes, N.; de Oliveira, T.M.; Van Assche, G.; Bals, S.; Dendooven, J.; Detavernier, C. Oxidation barrier of Cu and Fe powder by Atomic Layer Deposition. Surf. Coat. Technol. 2018, 349, 1032–1041. [Google Scholar] [CrossRef]
- Hammani, S.; Moulai-Mostefa, N.; Samyn, P.; Bechelany, M.; Dufresne, A.; Barhoum, A. Morphology, Rheology and Crystallization in Relation to the Viscosity Ratio of Polystyrene/Polypropylene Polymer Blends. Materials 2020, 13, 926. [Google Scholar] [CrossRef]
- Barhoum, A.; Van Lokeren, L.; Rahier, H.; Dufresne, A.; Van Assche, G. Roles of in situ surface modification in controlling the growth and crystallization of CaCO3 nanoparticles, and their dispersion in polymeric materials. J. Mater. Sci. 2015, 50, 7908–7918. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Khattab, T.; Gätjen, L.; Wilken, R. Colored, photocatalytic, antimicrobial and UV-protected viscose fibers decorated with Ag/Ag2CO3 and Ag/Ag3PO4 nanoparticles. Cellulose 2019, 26, 5437–5453. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Salah, A.; Rizk, M.S.; Moustafa, H.; Bechelany, M.; Barhoum, A. Carbon-based Nanosensors for Salicylate Determination in Pharmaceutical Preparations. Electroanalysis 2019, 31, 778–789. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.; Mahmoud, S.; Abdel-Ghani, N.; El Nashar, R.; Bechelany, M.; Barhoum, A. Polyvinyl Chloride Modified Carbon Paste Electrodes for Sensitive Determination of Levofloxacin Drug in Serum, Urine, and Pharmaceutical Formulations. Sensors 2021, 21, 3150. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly Imprinted Electrochemical Sensor-Based Fe2O3@MWCNTs for Ivabradine Drug Determination in Pharmaceutical Formulation, Serum, and Urine Samples. Front. Bioeng. Biotechnol. 2021, 9, 648704. [Google Scholar] [CrossRef] [PubMed]
- Parikha Mehrotra, Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2018, 19, e1800256. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef]
- Tan, K.X.; Barhoum, A.; Pan, S.; Danquah, M.K. Risks and toxicity of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Central Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Said, M.M.; Rehan, M.; El-Sheikh, S.M.; Zahran, M.K.; Abdel-Aziz, M.S.; Bechelany, M.; Barhoum, A. Multifunctional Hydroxyapatite/Silver Nanoparticles/Cotton Gauze for Antimicrobial and Biomedical Applications. Nanomaterials 2021, 11, 429. [Google Scholar] [CrossRef]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fabrication of Nanostructures with Bottom-up Approach and Their Utility in Diagnostics, Therapeutics, and Others. In Environmental, Chemical and Medical Sensors; Springer: Berlin/Heidelberg, Germany, 2017; pp. 167–198. [Google Scholar] [CrossRef]
- Sawy, A.M.; Barhoumbb, A.; Gaber, S.A.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalilaf, S.G.A. Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef]
- Barhoum, A.; Van Assche, G.; Rahier, H.; Fleisch, M.; Bals, S.; Delplancked, M.-P.; Leroux, F.; Bahnemann, D. Sol-gel hot injection synthesis of ZnO nanoparticles into a porous silica matrix and reaction mechanism. Mater. Des. 2017, 119, 270–276. [Google Scholar] [CrossRef]
- Barhoum, A.; Melcher, J.; Van Assche, G.; Rahier, H.; Bechelany, M.; Fleisch, M.; Bahnemann, D. Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: Porous microparticles versus nonporous nanoparticles. J. Mater. Sci. 2016, 52, 2746–2762. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 10. [Google Scholar] [CrossRef]
- Malik, N.; Arfin, T.; Khan, A.U. Graphene nanomaterials: Chemistry and pharmaceutical perspectives. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, T., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 373–402. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Wan, B.; Gu, Y.; Li, X. Optically Active Nanomaterials for Bioimaging and Targeted Therapy. Front. Bioeng. Biotechnol. 2019, 7, 320. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Health Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Jeon, S.; You, D.G.; Park, J.H.; Kwon, I.C.; Koo, H.; Kim, K. Inorganic Nanoparticles for Image-Guided Therapy. Bioconjug. Chem. 2017, 28, 124–134. [Google Scholar] [CrossRef]
- Snipstad, S.; Hak, S.; Baghirov, H.; Sulheim, E.; Mørch, Y.; Lélu, S.; Von Haartman, E.; Bäck, M.; Nilsson, K.P.R.; Klymchenko, A.S.; et al. Labeling nanoparticles: Dye leakage and altered cellular uptake. Cytom. Part A 2016, 91, 760–766. [Google Scholar] [CrossRef]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef]
- Rees, P.; Wills, J.W.; Brown, R.; Barnes, C.M.; Summers, H.D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 2019, 10, 2341. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The Smart Targeting of Nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: Versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef]
- Kher, G.; Trehan, S.; Misra, A. Antisense Oligonucleotides and RNA Interference. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 325–386. [Google Scholar] [CrossRef]
- Cremers, G.A.O.; Rosier, B.J.H.M.; Brillas, R.R.; Albertazzi, L.; de Greef, T.F.A. Efficient Small-Scale Conjugation of DNA to Primary Antibodies for Multiplexed Cellular Targeting. Bioconjug. Chem. 2019, 30, 2384–2392. [Google Scholar] [CrossRef]
- Gao, J.; Yao, X.; Chen, Y.; Gao, Z.; Zhang, J. Near-Infrared Light-Induced Self-Powered Aptasensing Platform for Aflatoxin B1 Based on Upconversion Nanoparticles-Doped Bi2S3 Nanorods. Anal. Chem. 2020, 93, 677–682. [Google Scholar] [CrossRef]
- Yu, Z.; Eich, C.; Cruz, L.J. Recent Advances in Rare-Earth-Doped Nanoparticles for NIR-II Imaging and Cancer Theranostics. Front. Chem. 2020, 8, 496. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Lanthanide-Doped Upconversion Nanoparticles for Super-Resolution Microscopy. Front. Chem. 2021, 8, 619377. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; Barhoum, A.; El-Sherbiny, S.; Morsy, F.; El-Midany, A.A.-H.; Rahier, H. Preparation of superhydrophobic nanocalcite crystals using Box–Behnken design. Arab. J. Chem. 2019, 12, 1479–1486. [Google Scholar] [CrossRef]
- Rehan, M.; Khattab, T.A.; Barohum, A.; Gätjen, L.; Wilken, R. Development of Ag/AgX (X = Cl, I) nanoparticles toward antimicrobial, UV-protected and self-cleanable viscose fibers. Carbohydr. Polym. 2018, 197, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Lim, W.Q.; Phua, S.Z.F.; Xu, H.V.; Sreejith, S.; Zhao, Y. Recent advances in multifunctional silica-based hybrid nanocarriers for bioimaging and cancer therapy. Nanoscale 2015, 8, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem. Commun. 2014, 50, 14071–14081. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Saha, A.; Maity, A.; Ray, S.C.; Jana, N.R. Carbon Nanoparticle-based Fluorescent Bioimaging Probes. Sci. Rep. 2013, 3, srep01473. [Google Scholar] [CrossRef] [PubMed]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Theories of nanoparticle and nanostructure formation in liquid phase. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 597–619. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L. Physicochemical characterization of nanomaterials: Size, morphology, optical, magnetic, and electrical properties. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 279–304. [Google Scholar] [CrossRef]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Liquid-phase synthesis of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.; Lau, S. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef]
- Jhonsi, M.A. Carbon Quantum Dots for Bioimaging. In State of the Art in Nano-Bioimaging; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Subramaniyan, S.A.; Bella, A.P.; Johnson, P.M.; Kim, A.R.; Shim, K.S.; Yoo, D.J. Simple Fluorescence Turn-On Chemosensor for Selective Detection of Ba2+ Ion and Its Live Cell Imaging. Anal. Chem. 2019, 91, 10095–10101. [Google Scholar] [CrossRef]
- Hubbs, A.F.; Sargent, L.M.; Porter, D.W.; Sager, T.M.; Chen, B.T.; Frazer, D.G.; Castranova, V.; Sriram, K.; Nurkiewicz, T.R.; Reynolds, S.H.; et al. Nanotechnology: Toxicologic Pathology. Toxicol. Pathol. 2013, 41, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of Core Diameter, Surface Coating, and PEG Chain Length on the Biodistribution of Persistent Luminescence Nanoparticles in Mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture). Angew. Chemie Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef]
- Khanbeigi, R.A.; Abelha, T.F.; Woods, A.; Rastoin, O.; Harvey, R.D.; Jones, M.-C.; Forbes, B.; Green, M.A.; Collins, H.; Dailey, L.A. Surface Chemistry of Photoluminescent F8BT Conjugated Polymer Nanoparticles Determines Protein Corona Formation and Internalization by Phagocytic Cells. Biomacromolecules 2015, 16, 733–742. [Google Scholar] [CrossRef]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Du, T.; Zhao, C.; Lai, L.; Luo, S.; Selke, M.; Rehman, F.U.; Li, X.; Sun, Y.; Jiang, H.; Wang, X. Rapid and multimodal in vivo bioimaging of cancer cells through in situ biosynthesis of Zn&Fe nanoclusters. Nano Res. 2017, 10, 2626–2632. [Google Scholar] [CrossRef]
- Feng, L.; Liu, L.; Lv, F.; Bazan, G.C.; Wang, S. Preparation and Biofunctionalization of Multicolor Conjugated Polymer Nanoparticles for Imaging and Detection of Tumor Cells. Adv. Mater. 2014, 26, 3926–3930. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Prabakaran, D.; Maroli, N.; Kim, A.R.; Park, B.-H.; Han, M.-K.; Ramesh, T.; Ponpandian, S.; Yoo, D.J. Mitochondria-targeted acridine-based dual-channel fluorescence chemosensor for detection of Sn4+ and Cr2O72-ions in water and its application in discriminative detection of cancer cells. J. Hazard. Mater. 2021, 419, 126409. [Google Scholar] [CrossRef]
- Rhim, W.-K.; Kim, M.; Hartman, K.L.; Kang, K.W.; Nam, J.-M. Radionuclide-labeled nanostructures for In Vivo imaging of cancer. Nano Converg. 2015, 2, 10. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Jiang, T.; Ran, G.; Song, Q. Cadmium induced aggregation of orange–red emissive carbon dots with enhanced fluorescence for intracellular imaging. J. Hazard. Mater. 2021, 427, 128092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yu, X.; Peng, S.; Luo, Y.; Li, J.; Lu, L. Construction of nanomaterials as contrast agents or probes for glioma imaging. J. Nanobiotechnol. 2021, 19, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, F.; Kuo, C.W.; Chen, B.-C.; Chen, P. Recent advances in the use of fluorescent nanoparticles for bioimaging. Nanomedicine 2019, 14, 1759–1769. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Hong, Y.; Tang, B.Z. Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging. Mater. Horizons 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Caponetti, V.; Trzcinski, J.W.; Cantelli, A.; Tavano, R.; Papini, E.; Mancin, F.; Montalti, M. Self-Assembled Biocompatible Fluorescent Nanoparticles for Bioimaging. Front. Chem. 2019, 7, 168. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Huang, P. Graphene-based nanomaterials for bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242–254. [Google Scholar] [CrossRef]
- Yadav, V.; Roy, S.; Singh, P.; Khan, Z.; Jaiswal, A. 2D MoS2-Based Nanomaterials for Therapeutic, Bioimaging, and Biosensing Applications. Small 2018, 15, e1803706. [Google Scholar] [CrossRef]
- Zhao, W.; Li, A.; Zhang, A.; Zheng, Y.; Liu, J. Recent Advances in Functional-Polymer-Decorated Transition-Metal Nanomaterials for Bioimaging and Cancer Therapy. ChemMedChem 2018, 13, 2134–2149. [Google Scholar] [CrossRef]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-Activated Nanoparticles: A Toolbox for Bioimaging, Therapeutics, and Neuromodulation. Accounts Chem. Res. 2020, 53, 2692–2704. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Cheng, D.; Wu, C.; Lu, Q.; Yang, W.; Zhu, X.; Yin, P.; Liu, M.; Li, H.; et al. Group IV nanodots: Synthesis, surface engineering and application in bioimaging and biotherapy. J. Mater. Chem. B 2020, 8, 10290–10308. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene oxide and its derivatives as promising in-vitro bio-imaging platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Gavel, P.K. Low molecular weight self-assembling peptide-based materials for cell culture, antimicrobial, anti-inflammatory, wound healing, anticancer, drug delivery, bioimaging and 3D bioprinting applications. Soft Matter 2020, 16, 10065–10095. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2021, 68, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Beziere, N.; del Pino, P.; Pelaz, B.; Estrada, G.; Tian, F.; Ntziachristos, V.; de la Fuente, J.M.; Cui, D. Gold Nanoprisms as Optoacoustic Signal Nanoamplifiers for In Vivo Bioimaging of Gastrointestinal Cancers. Small 2012, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Tang, K.-C.; Chung, M.-F.; Cheng, S.-H.; Huang, C.-M.; Chu, C.-H.; Chou, P.-T.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; et al. Enhanced Plasmonic Resonance Energy Transfer in Mesoporous Silica-Encased Gold Nanorod for Two-Photon-Activated Photodynamic Therapy. Theranostics 2014, 4, 798–807. [Google Scholar] [CrossRef]
- Yadav, A.; Rao, C.; Verma, N.C.; Mishra, P.M.; Nandi, C.K. Magnetofluorescent Nanoprobe for Multimodal and Multicolor Bioimaging. Mol. Imaging 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Liu, F.; Collot, M.; Anton, N. Dye-Loaded Nanoemulsions: Biomimetic Fluorescent Nanocarriers for Bioimaging and Nanomedicine. Adv. Health Mater. 2020, 10, e2001289. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Tan, H.W.; Zheng, W.; Liang, Z.-L.; Yu, F.-Y.; Wu, D.-D.; Yao, Y.; Zhong, Q.-H.; Yan, R.; Lau, A.T.Y. Cadmium telluride quantum dot-exposed human bronchial epithelial cells: A further study of the cellular response by proteomics. Toxicol. Res. 2019, 8, 994–1001. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Shin, J.; Wu, J.; Omstead, D.T.; Kiziltepe, T.; Littlepage, L.E.; Bilgicer, B. Engineering peptide-targeted liposomal nanoparticles optimized for improved selectivity for HER2-positive breast cancer cells to achieve enhanced in vivo efficacy. J. Control. Release 2020, 322, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- du Toit, L.; Pillay, V.; Choonara, Y.; Pillay, S.; Harilall, S.-L. Patenting of Nanopharmaceuticals in Drug Delivery: No Small Issue. Recent Patents Drug Deliv. Formul. 2007, 1, 131–142. [Google Scholar] [CrossRef]
- Narducci, D. An Introduction to Nanotechnologies: What’s in it for Us? Veter-Res. Commun. 2007, 31, 131–137. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug Delivery with Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 15. [Google Scholar] [CrossRef]
- Marcelo, G.; Kaplan, E.; Tarazona, M.P.; Mendicuti, F. Interaction of gold nanoparticles with Doxorubicin mediated by supramolecular chemistry. Colloids Surf. B Biointerfaces 2015, 128, 237–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Walker, J.B.; Minic, Z.; Liu, F.; Goshgarian, H.; Mao, G. Transporter protein and drug-conjugated gold nanoparticles capable of bypassing the blood-brain barrier. Sci. Rep. 2016, 6, 25794. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.; Raza, A.; Ghafoor, S.; Naeem, A.; Naz, S.S.; Riaz, S.; Ahmed, W.; Rana, N.F. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. 2016, 91, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Sodium alginate–polyvinyl alcohol–bovin serum albumin coated Fe3O4 nanoparticles as anticancer drug delivery vehicle: Doxorubicin loading and in vitro release study and cytotoxicity to HepG2 and L02 cells. Mater. Sci. Eng. C 2017, 79, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Prabha, G.; Raj, V. Formation and characterization of β-cyclodextrin (β-CD)-polyethyleneglycol (PEG)-polyethyleneimine (PEI) coated Fe3O4 nanoparticles for loading and releasing 5-Fluorouracil drug. Biomed. Pharmacother. 2016, 80, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Matranga, C.; Tan, S.; Alba, N.; Cui, X.T. Carbon nanotube nanoreservior for controlled release of anti-inflammatory dexamethasone. Biomaterials 2011, 32, 6316–6323. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.A.; Patel, S.; Sousa, A.A.; Patel, V.; Molinolo, A.A.; Ji, Y.; Leapman, R.D.; Gutkind, J.S.; Rusling, J.F. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine 2010, 5, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Ruzycka, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.; Wojewódzka, M.; Kruszewski, M.; Grudzinski, I. Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Roozbahani, M.; Kharaziha, M.; Emadi, R. pH sensitive dexamethasone encapsulated laponite nanoplatelets: Release mechanism and cytotoxicity. Int. J. Pharm. 2017, 518, 312–319. [Google Scholar] [CrossRef]
- Gurdag, S.; Khandare, J.; Stapels, S.; Matherly, L.H.; Kannan, R.M. Activity of Dendrimer−Methotrexate Conjugates on Methotrexate-Sensitive and -Resistant Cell Lines. Bioconjug. Chem. 2006, 17, 275–283. [Google Scholar] [CrossRef]
- Cirstoiu-Hapca, A.; Buchegger, F.; Lange, N.; Bossy, L.; Gurny, R.; Delie, F. Benefit of anti-HER2-coated paclitaxel-loaded immuno-nanoparticles in the treatment of disseminated ovarian cancer: Therapeutic efficacy and biodistribution in mice. J. Control. Release 2010, 144, 324–331. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.S.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomed. NanotechnoL. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Franch, A.; Castell, M.; Perez-Cano, F.J.; Bräuer, R.; Pohlers, D.; Gajda, M.; Siskos, A.P.; Katsila, T.; Tamvakopoulos, C.; et al. Liposomal encapsulation enhances and prolongs the anti-inflammatory effects of water-soluble dexamethasone phosphate in experimental adjuvant arthritis. Arthritis Res. Ther. 2010, 12, R147. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Contreras-Sandoval, A.; Haeri, A.; Hagen, T.L.T.; Navarro-Blasco, I.; Koning, G.; Garrido, M.J. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J. Control. Release 2015, 210, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Y.; Kuang, G.; Liu, S.; Zhou, D.; Chen, X.; Jing, X.; Huang, Y. Pt(iv) prodrug-backboned micelle and DCA loaded nanofibers for enhanced local cancer treatment. J. Mater. Chem. B 2017, 5, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, G.; Liu, D.; Xie, Z.; Huang, Y.; Wang, X.; Wu, W.; Jing, X. Inhibition of orthotopic secondary hepatic carcinoma in mice by doxorubicin-loaded electrospun polylactide nanofibers. J. Mater. Chem. B 2012, 1, 101–109. [Google Scholar] [CrossRef]
- Adeel, M.; Duzagac, F.; Canzonieri, V.; Rizzolio, F. Self-Therapeutic Nanomaterials for Cancer Therapy: A Review. ACS Appl. Nano Mater. 2020, 3, 4962–4971. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Gong, Y.-K.; Winnik, F.M. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale 2011, 4, 360–368. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, L. Applications of Nanoparticles for Anticancer Drug Delivery: A Review. J. Nanosci. Nanotechnol. 2015, 15, 4753–4773. [Google Scholar] [CrossRef]
- Wen, R.; Umeano, A.C.; Kou, Y.; Xu, J.; Farooqi, A.A. Nanoparticle systems for cancer vaccine. Nanomedicine 2019, 14, 627–648. [Google Scholar] [CrossRef] [PubMed]
- James, H.P.; John, R.; Alex, A.; Anoop, K. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Cohen, B.E.; Bangham, A.D. Diffusion of Small Non-Electrolytes across Liposome Membranes. Nature 1972, 236, 173–174. [Google Scholar] [CrossRef]
- Thanou, M. Nanoparticles for Drug and Gene Delivery. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1686–1691. [Google Scholar] [CrossRef]
- Vahed, S.Z.; Fathi, N.; Samiei, M.; Dizaj, S.M.; Sharifi, S. Targeted cancer drug delivery with aptamer-functionalized polymeric nanoparticles. J. Drug Target. 2018, 27, 292–299. [Google Scholar] [CrossRef]
- Gugulothu, D.; Barhoum, A.; Afzal, S.M.; Venkateshwarlu, B.; Uludag, H. Structural Multifunctional Nanofibers and their Emerging Applications. In Handbook of Nanofibers; Springer Nature Switzerland AG: Cham, Switzerland, 2018; pp. 1–41. [Google Scholar] [CrossRef]
- Bubakir, M.M.; Li, H.; Barhoum, A.; Yang, W. Advances in Melt Electrospinning Technique. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 125–156. [Google Scholar] [CrossRef]
- Sasikala, A.R.K.; Unnithan, A.R.; Yun, Y.-H.; Park, C.H.; Kim, C.S. An implantable smart magnetic nanofiber device for endoscopic hyperthermia treatment and tumor-triggered controlled drug release. Acta Biomater. 2016, 31, 122–133. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A Smart Hyperthermia Nanofiber with Switchable Drug Release for Inducing Cancer Apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Ankegowda, V.M.; Kollur, S.P.; Prasad, S.K.; Pradeep, S.; Dhramashekara, C.; Jain, A.S.; Prasad, A.; Srinivasa, C.; Sridhara Setty, P.; Gopinath, S.M.; et al. Phyto-Mediated Synthesis of Silver Nanoparticles Using Terminalia chebula Fruit Extract and Evaluation of Its Cytotoxic and Antimicrobial Potential. Molecules 2020, 25, 5042. [Google Scholar] [CrossRef]
- Gagliardi, A.; Cosco, D.; Udongo, B.P.; Dini, L.; Viglietto, G.; Paolino, D. Design and Characterization of Glyceryl Monooleate-Nanostructures Containing Doxorubicin Hydrochloride. Pharmaceutics 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Faisalina, A.; Sonvico, F.; Colombo, P.; Amirul, A.; Wahab, H.; Majid, M. Docetaxel-Loaded Poly(3HB-co-4HB) Biodegradable Nanoparticles: Impact of Copolymer Composition. Nanomaterials 2020, 10, 2123. [Google Scholar] [CrossRef]
- Nicosia, A.; Cavallaro, G.; Costa, S.; Utzeri, M.A.; Cuttitta, A.; Giammona, G.; Mauro, N. Carbon Nanodots for On Demand Chemophotothermal Therapy Combination to Elicit Necroptosis: Overcoming Apoptosis Resistance in Breast Cancer Cell Lines. Cancers 2020, 12, 3114. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; He, M.; Yang, Y.; Ma, M.; Guo, Z.; Zhao, M.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Reversing P-Glycoprotein-Associated Multidrug Resistance of Breast Cancer by Targeted Acid-Cleavable Polysaccharide Nanoparticles with Lapatinib Sensitization. ACS Appl. Mater. Interfaces 2020, 12, 51198–51211. [Google Scholar] [CrossRef] [PubMed]
- Tieu, T.; Wojnilowicz, M.; Huda, P.; Thurecht, K.J.; Thissen, H.; Voelcker, N.H.; Cifuentes-Rius, A. Nanobody-displaying porous silicon nanoparticles for the co-delivery of siRNA and doxorubicin. Biomater. Sci. 2020, 9, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Dorjsuren, B.; Chaurasiya, B.; Ye, Z.; Liu, Y.; Li, W.; Wang, C.; Shi, D.; Evans, C.E.; Webster, T.J.; Shen, Y. Cetuximab-Coated Thermo-Sensitive Liposomes Loaded with Magnetic Nanoparticles and Doxorubicin for Targeted EGFR-Expressing Breast Cancer Combined Therapy. Int. J. Nanomed. 2020, 15, 8201–8215. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.-M.; Tseng, S.-H.; Chou, M.-Y. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int. J. Nanomed. 2015, 10, 3663–3685. [Google Scholar] [CrossRef]
- Fernandes, C.; Oliveira, C.; Benfeito, S.; Soares, P.; Garrido, J.; Borges, F. Nanotechnology and antioxidant therapy: An emerging approach for neurodegenerative diseases. Curr. Med. Chem. 2014, 21, 4311–4327. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and Nanotechnology: Promises and Limits of Potentially Disruptive Approaches in the Treatment of Central Nervous System Diseases. Adv. Health Mater. 2019, 9, e1901589. [Google Scholar] [CrossRef]
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for Cancer Targeting: Current and Future Directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Yehya, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K. Anti-oxidant activities of biopolymeric nanoparticles: Boon or bane. J. Pharm. Res. 2014, 8, 871. [Google Scholar]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial Nanoparticle Antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar] [CrossRef]
- Yusof, F.; Ismail, N. Antioxidants effects of Platinum Nanoparticles: A Potential Alternative Treatment to Lung Diseases. J. Appl. Pharm. Sci. 2015, 5, 140–145. [Google Scholar] [CrossRef]
- Watanabe, A.; Kajita, M.; Kim, J.; Kanayama, A.; Takahashi, K.; Mashino, T.; Miyamoto, Y. In vitro free radical scavenging activity of platinum nanoparticles. Nanotechnology 2009, 20, 455105. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Hackett, D.; Tchounwou, P.B. Genotoxicity study of silver nanoparticles in bone marrow cells of Sprague–Dawley rats. Food Chem. Toxicol. 2015, 85, 52–60. [Google Scholar] [CrossRef]
- Saikia, J.P.; Paul, S.; Konwar, B.K.; Samdarshi, S.K. Nickel oxide nanoparticles: A novel antioxidant. Colloids Surf. B Biointerfaces 2010, 78, 146–148. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2018, 11, 37–44. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuca, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Khan, M.S.; Vishakante, G.D.; Siddaramaiah, H. Gold nanoparticles: A paradigm shift in biomedical applications. Adv. Colloid Interface Sci. 2013, 199–200, 44–58. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–Based Silica Particles as Flavonoid Carrier for Drug Delivery Applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zimmerman, M.C.; Yang, R.; Tong, J.; Vinogradov, S.; Kabanov, A.V. Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free. Radic. Biol. Med. 2010, 49, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Yi, X.; Luxenhofer, R.; Banks, W.A.; Jordan, R.; Zimmerman, M.C.; Kabanov, A.V. Conjugates of Superoxide Dismutase 1 with Amphiphilic Poly(2-oxazoline) Block Copolymers for Enhanced Brain Delivery: Synthesis, Characterization and Evaluation in Vitro and in Vivo. Mol. Pharm. 2012, 10, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Scherpereel, A.; Wiewrodt, R.; Ng, K.; Sweitzer, T.; Arguiri, E.; Shuvaev, V.; Solomides, C.C.; Albelda, S.M.; Muzykantov, V.R. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L283–L292. [Google Scholar] [CrossRef]
- Brynskikh, A.M.; Zhao, Y.; Mosley, R.L.; Li, S.; Boska, M.D.; Klyachko, N.L.; Kabanov, A.V.; Gendelman, H.E.; Batrakova, E.V. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine 2010, 5, 379–396. [Google Scholar] [CrossRef]
- Williams, S.R.; Lepene, B.S.; Thatcher, C.D.; Long, T.E. Synthesis and Characterization of Poly(ethylene glycol)−Glutathione Conjugate Self-Assembled Nanoparticles for Antioxidant Delivery. Biomacromolecules 2008, 10, 155–161. [Google Scholar] [CrossRef]
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control Release 2010, 146, 276–290. [Google Scholar] [CrossRef]
- Wegmann, J.; Krucker, M.; Bachmann, S.; Fischer, G.; Zeeb, D.; Lienau, A.; Glaser, T.; Runge, F.; Lüddecke, E.; Albert, K. Characterization of Lycopene Nanoparticles Combining Solid-State and Suspended-State NMR Spectroscopy. J. Agric. Food Chem. 2002, 50, 7510–7514. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Romano, M.R.; Brullé, L.; Touil, Y.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and Antiradical SiO2 Nanoparticles Covalently Functionalized with Gallic Acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tang, H.; Lu, M.; Gao, C.; Wang, F.; Ding, Y.; Zhang, S.; Yang, M. Preparation of nanosilica-immobilized antioxidant and the antioxidative behavior in low density polyethylene. Polym. Degrad. Stab. 2016, 135, 1–7. [Google Scholar] [CrossRef]
- Du, L.; Suo, S.; Wang, G.; Jia, H.; Liu, K.J.; Zhao, B.; Liu, Y. Mechanism and Cellular Kinetic Studies of the Enhancement of An-tioxidant Activity by Using Surface-Functionalized Gold Nanoparticles. Chem. Eur. J. 2013, 19, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Aktas, N. Preparation and characterization of monodisperse, mesoporous natural poly (tannic acid)–silica nanoparticle composites with antioxidant properties. Microporous Mesoporous Mater. 2016, 226, 316–324. [Google Scholar] [CrossRef]
- Arriagada, F.; Correa, O.; Gunther, G.; Nonell, S.; Mura, F.; Olea-Azar, C.; Morales, J. Morin Flavonoid Adsorbed on Mesoporous Silica, a Novel Antioxidant Nanomaterial. PLoS ONE 2016, 11, e0164507. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Kim, C.K.; Jeong, H.-G.; Soh, M.; Kim, T.; Choi, I.-Y.; Ki, S.-K.; Kim, D.Y.; Yang, W.; Hyeon, T.; et al. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res. 2017, 10, 2743–2760. [Google Scholar] [CrossRef]
- Joseph, A.; Wood, T.; Chen, C.-C.; Corry, K.; Snyder, J.M.; Juul, S.E.; Parikh, P.; Nance, E. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy. Nano Res. 2018, 11, 5670–5688. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Komarek, M.; Lubasová, D.; Sanetrnik, F.; Maryska, J. Preparation of Antibacterial Nanofibre/Nanoparticle Covered Composite Yarns. J. Nanomater. 2016, 2016, 7565972. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef]
- Shahriary, M.; Veisi, H.; Hekmati, M.; Hemmati, S. In situ green synthesis of Ag nanoparticles on herbal tea extract (Stachys lavandulifolia)-modified magnetic iron oxide nanoparticles as antibacterial agent and their 4-nitrophenol catalytic reduction activity. Mater. Sci. Eng. C 2018, 90, 57–66. [Google Scholar] [CrossRef]
- Youssef, A.M.; Moustafa, H.A.; Barhoum, A.; Hakim, A.E.-F.A.A.; Dufresne, A. Evaluation of the Morphological, Electrical and Antibacterial Properties of Polyaniline Nanocomposite Based on Zn/Al-Layered Double Hydroxides. ChemistrySelect 2017, 2, 8553–8566. [Google Scholar] [CrossRef]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Nanomaterials History, Classification, Unique Properties, Production and Market. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135167. [Google Scholar]
- Nnaji, C.O.; Jeevanandam, J.; Chan, Y.S.; Danquah, M.K.; Pan, S.; Barhoum, A. Engineered nanomaterials for wastewater treatment: Current and future trends. In Fundamentals of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 129–168. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Smetana, A.B.; Klabunde, K.J.; Marchin, G.R.; Sorensen, C.M. Biocidal Activity of Nanocrystalline Silver Powders and Particles. Langmuir 2008, 24, 7457–7464. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Nakamura, N.; Komine, T. Continuous-sterilization system that uses photosemiconductor powders. Appl. Environ. Microbiol. 1988, 54, 1330–1333. [Google Scholar] [CrossRef]
- Kim, B.; Kim, D.; Cho, D.; Cho, S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere 2003, 52, 277–281. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, A.P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ortíz, N.; De la Rosa-García, S.; González-Gómez, W.; Soria-Castro, M.; Quintana, P.; Oskam, G.; Ortega-Morales, B. Antifungal Coatings Based on Ca(OH)2 Mixed with ZnO/TiO2 Nanomaterials for Protection of Limestone Monuments. ACS Appl. Mater. Interfaces 2013, 5, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Nanoparticles as Antimicrobial Agents: Their Toxicity and Mechanisms of Action. J. Nanosci. Nanotechnol. 2014, 14, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Mageshwari, K.; Sathyamoorthy, R. Flower-shaped CuO Nanostructures: Synthesis, Characterization and Antimicrobial Activity. J. Mater. Sci. Technol. 2013, 29, 909–914. [Google Scholar] [CrossRef]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Measurement of fungi by an indirect conductimetric assay. Lett. Appl. Microbiol. 2003, 37, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Di Gianvincenzo, P.; Marradi, M.; Martínez-Ávila, O.M.; Bedoya, L.M.; Alcami, J.; Penadés, S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg. Med. Chem. Lett. 2010, 20, 2718–2721. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.K.; Che, C.-M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef]
- Zheng, Y.; Cloutier, P.; Hunting, D.J.; Sanche, L. Radiosensitization by Gold Nanoparticles: Comparison of DNA Damage Induced by Low and High-Energy Electrons. J. Biomed. Nanotechnol. 2008, 4, 469–473. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017, 497, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, J.; Gu, W.; Sun, C.; Wu, D.; Yang, T.; Yang, G. Photocatalytic Inactivation Efficiency of Anatase Nano-TiO2 Sol on the H9N2 Avian Influenza Virus. Photochem. Photobiol. 2010, 86, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Baiocco, P.; Ilari, A.; Ceci, P.; Orsini, S.; Gramiccia, M.; Di Muccio, T.; Colotti, G. Inhibitory Effect of Silver Nanoparticles on Trypanothione Reductase Activity and Leishmania infantum Proliferation. ACS Med. Chem. Lett. 2010, 2, 230–233. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.; Bagirova, M.; Ustundag, C.B.; Kaya, C.; Rafailovich, M. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int. J. Nanomed. 2011, 6, 2705–2714. [Google Scholar] [CrossRef]
- Nadhman, A.; Nazir, S.; Khan, M.I.; Arooj, S.; Bakhtiar, M.; Shahnaz, G.; Yasinzai, M. PEGylated silver doped zinc oxide nanoparticles as novel photosensitizers for photodynamic therapy against Leishmania. Free. Radic. Biol. Med. 2014, 77, 230–238. [Google Scholar] [CrossRef]
- Saad, A.H.A.; Soliman, M.I.; Azzam, A.M. Antiparasitic Activity of Silver and Copper Oxide Nanoparticles against Entamoeba histolytica and Cryptosporidium parvum Cysts. J. Egypt. Soc. Parasitol. 2015, 45, 593–602. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Marimuthu, S. Copper nanoparticles synthesized by polyol process used to control hematophagous parasites. Parasitol. Res. 2011, 109, 1403–1415. [Google Scholar] [CrossRef]
- Barhoum, A.; Rehan, M.F.; Rahier, H.; Bechelany, M.; Van Assche, G. Seed-Mediated Hot-Injection Synthesis of Tiny Ag Nanocrystals on Nanoscale Solid Supports and Reaction Mechanism. ACS Appl. Mater. Interfaces 2016, 8, 10551–10561. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef]
- Barhoum, A.; Rahier, H.; Benelmekki, M.; Van Assche, G. Recent trends in nanostructured particles: Synthesis, functionalization, and applications. In Fundamentals of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 605–639. [Google Scholar] [CrossRef]
- Rastogi, A.; Singh, P.; Haraz, F.A.; Barhoum, A. Chapter 19—Biological Synthesis of Nanoparticles: An Environmentally Benign Approach. Available online: https://www.sciencedirect.com/science/article/pii/B9780323512558000239 (accessed on 21 April 2019).
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Upadhyaya, C.P.; Singh, A.; Abd-Elsalam, K.A.; Prasad, R. Applications of Silver Nanoparticles in Plant Protection. In Nanobiotechnology Applications in Plant Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 247–265. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Shaheen, M.S.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2013, 18, 356–363. [Google Scholar] [CrossRef]
- Belaiche, Y.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Tedjani, M.L.; Barhoum, A. Green synthesis and characterization of silver/silver oxide nanoparticles using aqueous leaves extract of artemisia herbaalba as reducing and capping agents. Rom. J. Mater. 2021, 51, 342–352. [Google Scholar]
- Fatima, S.; Ali, K.; Ahmed, B.; Al Kheraif, A.A.; Syed, A.; Elgorban, A.M.; Musarrat, J.; Lee, J. Titanium Dioxide Nanoparticles Induce Inhibitory Effects against Planktonic Cells and Biofilms of Human Oral Cavity Isolates of Rothia mucilaginosa, Georgenia sp. and Staphylococcus saprophyticus. Pharmaceutics 2021, 13, 1564. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Qais, F.A.; Ahmad, N.; Khan, A.; Alyousef, A.A.; Arshad, M.; Noor, S.; Khan, J.M.; Alam, P.; et al. Phyto-Mediated Synthesis of Porous Titanium Dioxide Nanoparticles From Withania somnifera Root Extract: Broad-Spectrum Attenuation of Biofilm and Cytotoxic Properties Against HepG2 Cell Lines. Front. Microbiol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Ilyas, M.; Waris, A.; Khan, A.U.; Zamel, D.; Yar, L.; Baset, A.; Muhaymin, A.; Khan, S.; Ali, A.; Ahmad, A. Biological synthesis of titanium dioxide nanoparticles from plants and microorganisms and their potential biomedical applications. Inorg. Chem. Commun. 2021, 133, 108968. [Google Scholar] [CrossRef]
- Balaraman, R.P.; Mendel, J.; Flores, L.; Choudhary, M. Nanoparticle Biosynthesis and Interaction with the Microbial Cell, Antimicrobial and Antibiofilm Effects, and Environmental Impact. In Nanomaterial Biointeractions at the Cellular, Organismal and System Levels; Springer: Cham, Switzerland, 2021; pp. 371–405. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Cadenas-Pliego, G.; Ávila-Orta, C.; Comparán-Padilla, V.; Lugo-Uribe, L.; Pérez-Alvarez, M.; Tavizón, S.; Santillán, G. Antimicrobial Property of Polypropylene Composites and Functionalized Copper Nanoparticles. Polymers 2021, 13, 1694. [Google Scholar] [CrossRef]
- Gharpure, S.; Akash, A.; Ankamwar, B. A Review on Antimicrobial Properties of Metal Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 3303–3339. [Google Scholar] [CrossRef]
- El-Sheikh, S.; El-Sherbiny, S.; Barhoum, A.; Deng, Y. Effects of cationic surfactant during the precipitation of calcium carbonate nano-particles on their size, morphology, and other characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 44–49. [Google Scholar] [CrossRef]
- Sharma, U.; Badyal, P.N.; Gupta, S. Polymeric nanoparticles drug delivery to brain: A review. Int. J. Pharmacol. 2015, 2, 60–69. [Google Scholar]
- Jun, A.S.; Larkin, D.F.P. Prospects for gene therapy in corneal disease. Eye 2003, 17, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Mann, M.J.; Morishita, R.; Kaneda, Y. Fusigenic viral liposome for gene therapy in cardiovascular diseases. Proc. Natl. Acad. Sci. USA 1996, 93, 11421–11425. [Google Scholar] [CrossRef] [PubMed]
- Caplen, N.; Gao, X.; Hayes, P.; Elaswarapu, R.; Fisher, G.; Kinrade, E.; Chakera, A.; Schorr, J.; Hughes, B.; Dorin, J.R. Gene therapy for cystic fibrosis in humans by liposome-mediated DNA transfer: The production of resources and the regulatory process. Gene Ther. 1994, 1, 139–147. [Google Scholar] [PubMed]
- Balazs, D.A.; Godbey, W. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2010, 2011, 326497. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Ji, L.; Tanaka, F.; Saito, Y.; Gopalan, B.; Branch, C.D.; Xu, K.; Atkinson, E.N.; Bekele, B.N.; Stephens, L.C.; et al. Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Ther. 2004, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Prateeksha; Gupta, V.K.; Chen, J.; Atanasov, A. Organic Nanoparticle-Based Combinatory Approaches for Gene Therapy. Trends Biotechnol. 2017, 35, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef]
- Nagamune, T. Biomolecular engineering for nanobio/bionanotechnology. Nano Converg. 2017, 4, 1–56. [Google Scholar] [CrossRef]
- Prabu, S.L.; Suriyaprakash, T.N.K.; Thirumurugan, R. Medicated Nanoparticle for Gene Delivery. In Advanced Technology for Delivering Therapeutics; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/52818 (accessed on 11 May 2017). [CrossRef]
- Jat, S.K.; Bhattacharya, J.; Sharma, M.K. Nanomaterial based gene delivery: A promising method for plant genome engineering. J. Mater. Chem. B 2020, 8, 4165–4175. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, F.M.; Saad, M.; Barhoum, A.; Bechelany, M.; Rizk, M.S. PVC membrane, coated-wire, and carbon-paste ion-selective electrodes for potentiometric determination of galantamine hydrobromide in physiological fluids. Mater. Sci. Eng. C 2018, 89, 140–148. [Google Scholar] [CrossRef] [PubMed]
- El Nashar, R.M.; Ghani, N.T.A.; El Gohary, N.A.; Barhoum, A.; Madbouly, A. Molecularly imprinted polymers based biomimetic sensors for mosapride citrate detection in biological fluids. Mater. Sci. Eng. C 2017, 76, 123–129. [Google Scholar] [CrossRef] [PubMed]
- El-Beshlawy, M.M.; Abdel-Haleem, F.M.; Barhoum, A. Molecularly Imprinted Potentiometric Sensor for Nanomolar Determination of Pioglitazone Hydrochloride in Pharmaceutical Formulations. Electroanalysis 2021, 33, 1244–1254. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Tang, C.K.; Vaze, A.; Shen, M.; Rusling, J.F. High-Throughput Electrochemical Microfluidic Immunoarray for Multiplexed Detection of Cancer Biomarker Proteins. ACS Sens. 2016, 1, 1036–1043. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Abraham, J.; Arunima, R.; Nimitha, K.; George, S.C.; Thomas, S. One-dimensional (1D) nanomaterials: Nanorods and nanowires. In Nanoscale Processing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 71–101. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2017, 14, 2433–2454. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Votruba, A.R.; Farokhzad, O.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Barhoum, A.; Xiaoqing, C.; Li, H.; Samyn, P. Cellulose Nanofibers: Fabrication and Surface Functionalization Techniques. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 409–449. [Google Scholar] [CrossRef]
- Gugulothu, D.; Barhoum, A.; Nerella, R.; Ajmer, R.; Bechlany, M. Fabrication of Nanofibers: Electrospinning and Non-Electrospinning Techniques. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar] [CrossRef]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2021, 278, 118956. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.M.I.; Ali, A.S.M.; Al-Zeer, M.A.; Barhoum, A.; El-Hallouty, S.; Shousha, W.G.; Berg, J.; Kurreck, J.; Khalil, A.S.G. Spontaneous Formation of 3D Breast Cancer Tissues on Electrospun Chitosan/Poly(ethylene oxide) Nanofibrous Scaffolds. ACS Omega 2022, 7, 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of Nanomaterials in Dentistry: Interactions with the Oral Microenvironment, Clinical Applications, Hazards, and Benefits. ACS Nano 2015, 9, 2255–2289. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, Y.; Gao, J.; Xiao, Y.; Du, J. Dual Corona Vesicles with Intrinsic Antibacterial and Enhanced Antibiotic Delivery Capabilities for Effective Treatment of Biofilm-Induced Periodontitis. ACS Nano 2019, 13, 13645–13657. [Google Scholar] [CrossRef]
- Lombardi, V.R. Editorial [Exploring Neural-Immune System Interactions]. Curr. Immunol. Rev. 2012, 8, 37–38. [Google Scholar] [CrossRef]
- Bertram, L. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Iatsunskyi, I.; Coy, E.; Renard, A.; Salameh, C.; Weber, M.; Sayegh, S.; Nada, A.; Roualdes, S.; et al. Atomic layer deposition of Pd nanoparticles on self-supported carbon-Ni/NiO-Pd nanofiber electrodes for electrochemical hydrogen and oxygen evolution reactions. J. Colloid Interface Sci. 2020, 569, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Rasouli, R.; Yousefzadeh, M.; Rahier, H.; Bechelany, M. Nanofiber Technologies: History and Development. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–43. [Google Scholar] [CrossRef]
- Barhoum, A.; Favre, T.; Sayegh, S.; Tanos, F.; Coy, E.; Iatsunskyi, I.; Razzouk, A.; Cretin, M.; Bechelany, M. 3D Self-Supported Nitrogen-Doped Carbon Nanofiber Electrodes Incorporated Co/CoOx Nanoparticles: Application to Dyes Degradation by Electro-Fenton-Based Process. Nanomaterials 2021, 11, 2686. [Google Scholar] [CrossRef] [PubMed]
- Turky, A.O.; Barhoum, A.; Rashad, M.; Bechelany, M. Enhanced the structure and optical properties for ZnO/PVP nanofibers fabricated via electrospinning technique. J. Mater. Sci. Mater. Electron. 2017, 28, 17526–17532. [Google Scholar] [CrossRef]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquah, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Obeid, M.A.; Al Zoubi, M.S.; Charbe, N.B.; Chellappan, D.K.; Mishra, V.; Dureja, H.; Gupta, G.; Prasher, P.; Dua, K.; et al. Nanocelluloses in Sensing Technology. In Handbook of Nanocelluloses; Springer: Cham, Switzerland, 2021; pp. 1–30. [Google Scholar] [CrossRef]
- Xue, J.; Pisignano, D.; Xia, Y. Maneuvering the Migration and Differentiation of Stem Cells with Electrospun Nanofibers. Adv. Sci. 2020, 7, 2000735. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2016, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C 2014, 48, 651–662. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Bodnar, R.J. Chemokine Regulation of Angiogenesis during Wound Healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef]
- Mechanick, J.I. Practical aspects of nutritional support for wound-healing patients. Am. J. Surg. 2004, 188, 52–56. [Google Scholar] [CrossRef]
- Greer, N.; Foman, N.A.; Macdonald, R.; Dorrian, J.; Fitzgerald, P.; Rutks, I.; Wilt, T.J. Advanced Wound Care Therapies for Nonhealing Diabetic, Venous, and Arterial Ulcers. Ann. Intern. Med. 2013, 159, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Prabakaran, D.; Maroli, N.; Boguszewska-Czubara, A.; Masłyk, M.; Kim, A.R.; Chandrasekaran, B.; Yoo, D.J. Construction of a simple dual-channel fluorescence chemosensor for Cu2+ ion and GSSG detection and its mitochondria-targeting bioimaging applications. Anal. Chim. Acta 2021, 1181, 338896. [Google Scholar] [CrossRef] [PubMed]
- Randeria, P.S.; Seeger, M.A.; Wang, X.-Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Nanomaterials for delivery of nutrients and growth-promoting compounds to plants. In Nano-Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 177–226. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-Chitosan Nanoparticle Mediated Sustainable Approach To Enhance Seedling Growth in Maize by Mobilizing Reserved Food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2012, 9, 115–123. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, e0902. [Google Scholar] [CrossRef]
- Meurer, R.A.; Kemper, S.; Knopp, S.; Eichert, T.; Jakob, F.; Goldbach, H.E.; Schwaneberg, U.; Pich, A. Biofunctional Microgel-Based Fertilizers for Controlled Foliar Delivery of Nutrients to Plants. Angew. Chem. Int. Ed. 2017, 56, 7380–7386. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Singh, D.; Dwivedi, S. Nano-fertilizers: A Novel Way for Enhancing Nutrient Use Efficiency and Crop Productivity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3325–3335. [Google Scholar] [CrossRef]
- Preetha, P.S.; Balakrishnan, N. A Review of Nano Fertilizers and Their Use and Functions in Soil. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3117–3133. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and Their Smart Delivery System. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Subramanian, K.S.; Manikandan, A.; Thirunavukkarasu, M.; Rahale, C.S. Nano-fertilizers for Balanced Crop Nutrition. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 69–80. [Google Scholar]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Park, B. Nanotechnology and the packaging of food and other fast-moving consumer goods. In Trends in Packaging of Food, Beverages and Other Fast-Moving Consumer Goods (FMCG); Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 241–260. [Google Scholar] [CrossRef]
- Rossi, M.; Passeri, D.; Sinibaldi, A.; Angjellari, M.; Tamburri, E.; Sorbo, A.; Carata, E.; Dini, L. Nanotechnology for Food Packaging and Food Quality Assessment. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; pp. 149–204. [Google Scholar] [CrossRef]
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [CrossRef]
- Mu, L.; Droujinine, I.A.; Rajan, N.K.; Sawtelle, S.D.; Reed, M.A. Direct, Rapid, and Label-Free Detection of Enzyme–Substrate Interactions in Physiological Buffers Using CMOS-Compatible Nanoribbon Sensors. Nano Lett. 2014, 14, 5315–5322. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2015, 24, 15–28. [Google Scholar] [CrossRef]
- Jampilek, J.; Kos, J.; Kralova, K. Potential of Nanomaterial Applications in Dietary Supplements and Foods for Special Medical Purposes. Nanomaterials 2019, 9, 296. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Kumar, V.; Guleria, P.; Mehta, S.K. Nanosensors for food quality and safety assessment. Environ. Chem. Lett. 2017, 15, 165–177. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Vong, K.; Eda, S.; Kadota, Y.; Nasibullin, I.; Wakatake, T.; Yokoshima, S.; Shirasu, K.; Tanaka, K. An artificial metalloenzyme biosensor can detect ethylene gas in fruits and Arabidopsis leaves. Nat. Commun. 2019, 10, 5746. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.; Barba, F.; Putnik, P.; Kovačević, D.; Lorenzo, J. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Sankar, V.R.; Reddy, Y.D. Nanocochleate—A new approach in lipid drug delivery. Int. J. Pharm. Pharm. Sci. 2010, 2, 220–223. [Google Scholar]
- Sekhon, B. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef]
- Prakash, J.; Vignesh, K.; Anusuya, T.; Kalaivani, T.; Ramachandran, C.; Sudha, R.R.; Rubab, M.; Khan, I.; Elahi, F.; Oh, D.-H. Ap-plication of Nanoparticles in Food Preservation and Food Processing. J. Food Hyg. Saf. 2019, 34, 317–324. [Google Scholar]
- Ribeiro, T.; Baleizão, C.; Farinha, J.P.S. Functional Films from Silica/Polymer Nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Wang, P.-W.; Alalaiwe, A.; Lin, Z.-C.; Fang, J.-Y. Use of Lipid Nanocarriers to Improve Oral Delivery of Vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, S.; Dalai, S.; Sabat, D.; Singh, S.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of Al2O3Nanoparticles at Low Exposure Levels to a Freshwater Bacterial Isolate. Chem. Res. Toxicol. 2011, 24, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Lee, G.-H.; Yoon, C.; Jeong, U.; Kim, Y.; Cho, M.-H.; Kim, D.-W. Biodistribution and toxicity of spherical aluminum oxide nanoparticles. J. Appl. Toxicol. 2015, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Minigalieva, I.A.; Katsnelson, B.A.; Privalova, L.I.; Sutunkova, M.P.; Gurvich, V.B.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; Makeyev, O.H.; Panov, V.G.; et al. Combined Subchronic Toxicity of Aluminum (III), Titanium (IV) and Silicon (IV) Oxide Nanoparticles and Its Alleviation with a Complex of Bioprotectors. Int. J. Mol. Sci. 2018, 19, 837. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2010, 5, 43–54. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Ahamed, M.; Siddiqui, M.; Akhtar, M.; Ahmad, I.; Pant, A.B.; Alhadlaq, H. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010, 396, 578–583. [Google Scholar] [CrossRef]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitro 2009, 23, 1365–1371. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Lei, R.; Wu, C.; Yang, B.; Ma, H.; Shi, C.; Wang, Q.; Wang, Q.; Yuan, Y.; Liao, M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: A rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 2008, 232, 292–301. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Sharma, A.K.; Loeschner, K.; Jacobsen, N.R. Pulmonary toxicity of silver vapours, nanoparticles and fine dusts: A review. Regul. Toxicol. Pharmacol. 2020, 115, 104690. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.A.; Seckler, M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Cao, Y.; Gong, Y.; Liao, W.; Luo, Y.; Wu, C.; Wang, M.; Yang, Q. A review of cardiovascular toxicity of TiO2, ZnO and Ag nanoparticles (NPs). BioMetals 2018, 31, 457–476. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, V.D.; Prasad, S.V.; Banerjee, A.; Gopinath, M.; Murugesan, R.; Marotta, F.; Sun, X.-F.; Pathak, S. Health hazards of nanoparticles: Understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem. Toxicol. 2018, 42, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yathindranath, V.; Worden, M.; Thliveris, J.A.; Chu, S.; Parkinson, F.E.; Hegmann, T.; Miller, D.W. Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int. J. Nanomed. 2013, 8, 961–970. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.K.; Devey, M.; Hawkins, S.; Hails, L.; Davis, S.A.; Mann, S.; Chang, I.T.; Ingham, E.; Malhas, A.; Vaux, D.J.; et al. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials 2013, 34, 3559–3570. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Horie, M.; Kobayashi, N.; Shinohara, N.; Shimada, M. Inhalation Toxicity Assessment of Carbon-Based Nanoparticles. Acc. Chem. Res. 2012, 46, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Role of omics techniques in the toxicity testing of nanoparticles. J. Nanobiotechnol. 2017, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hamimed, S.; Abdeljelil, N.; Landoulsi, A.; Chatti, A.; Aljabali, A.A.A.; Barhoum, A. Bacterial Cellulose Nanofibers. In Handbook of Nanocelluloses; Barhoum, A., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Moodley, K.G.; Arumugam, V.; Barhoum, A. Nanocellulose-Based Materials for Wastewater Treatment. In Handbook of Nanocelluloses; Barhoum, A., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]




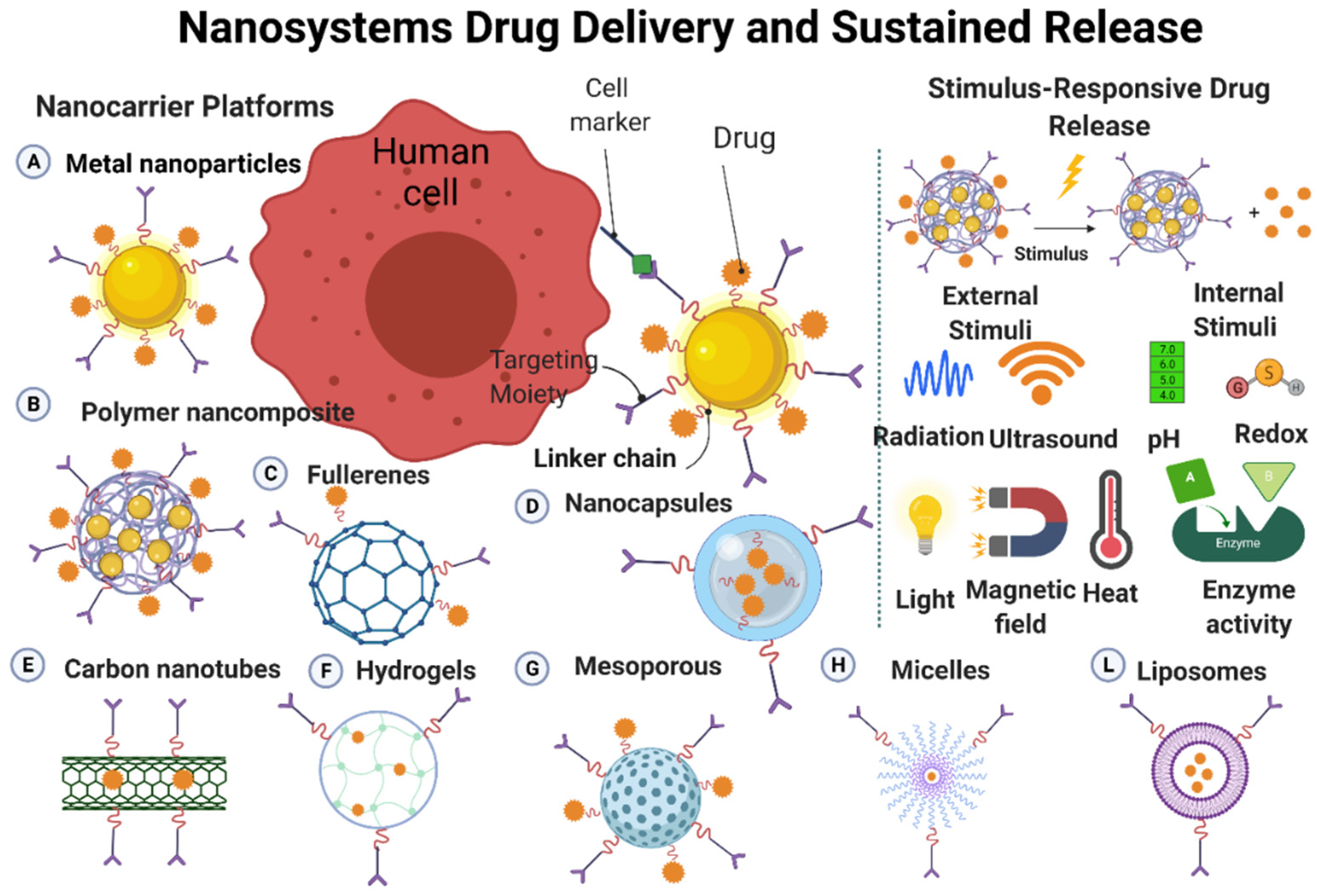

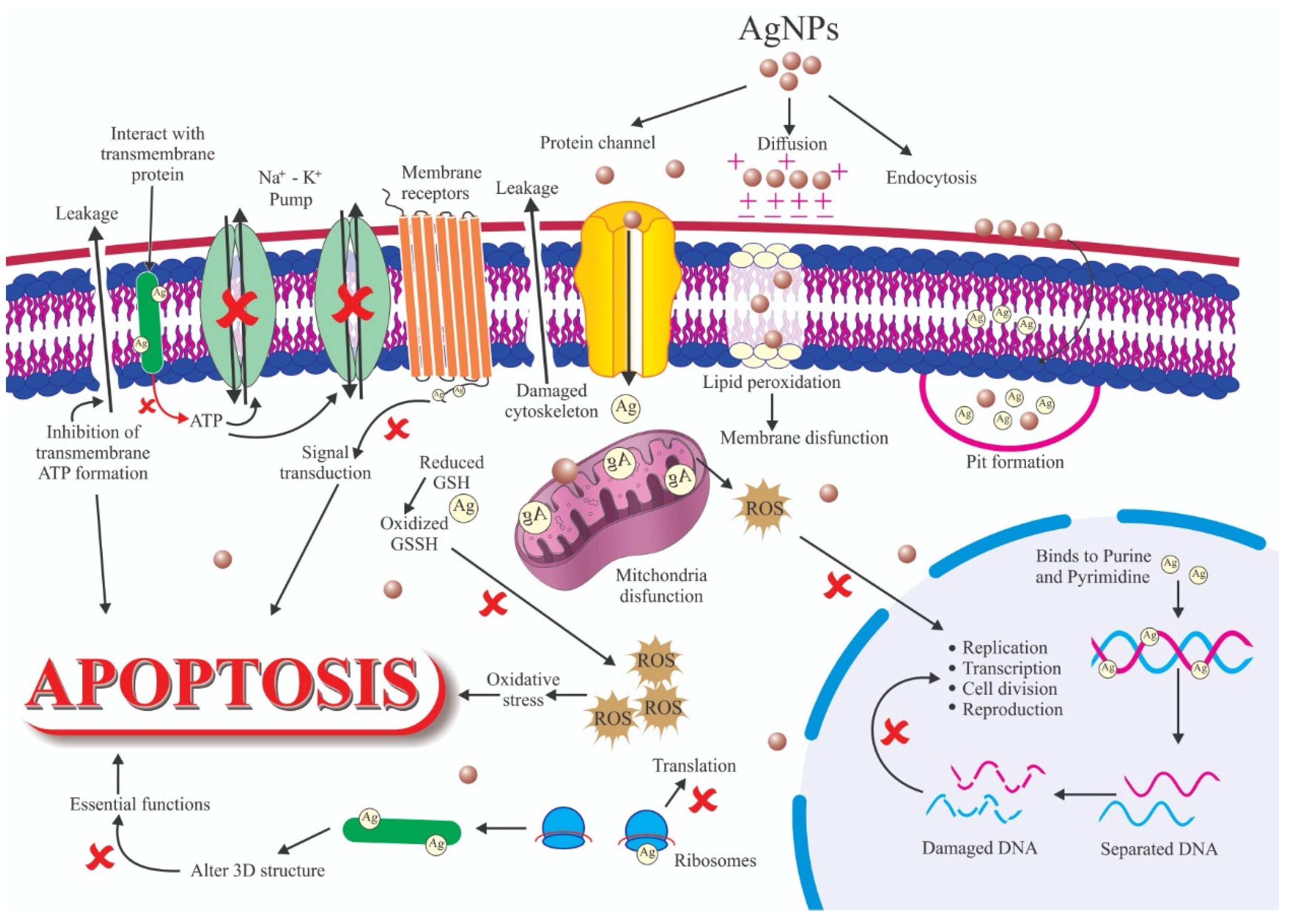

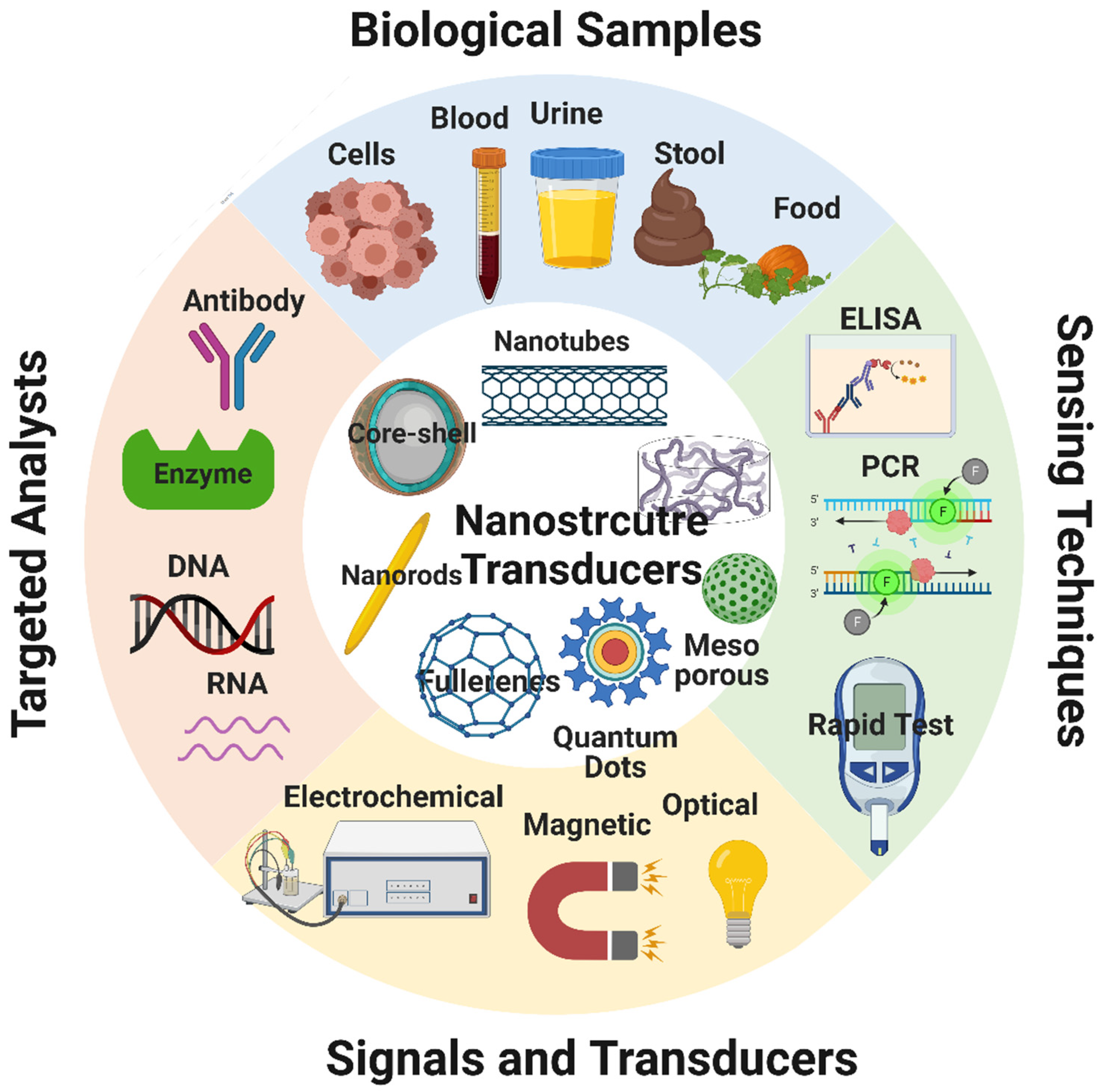


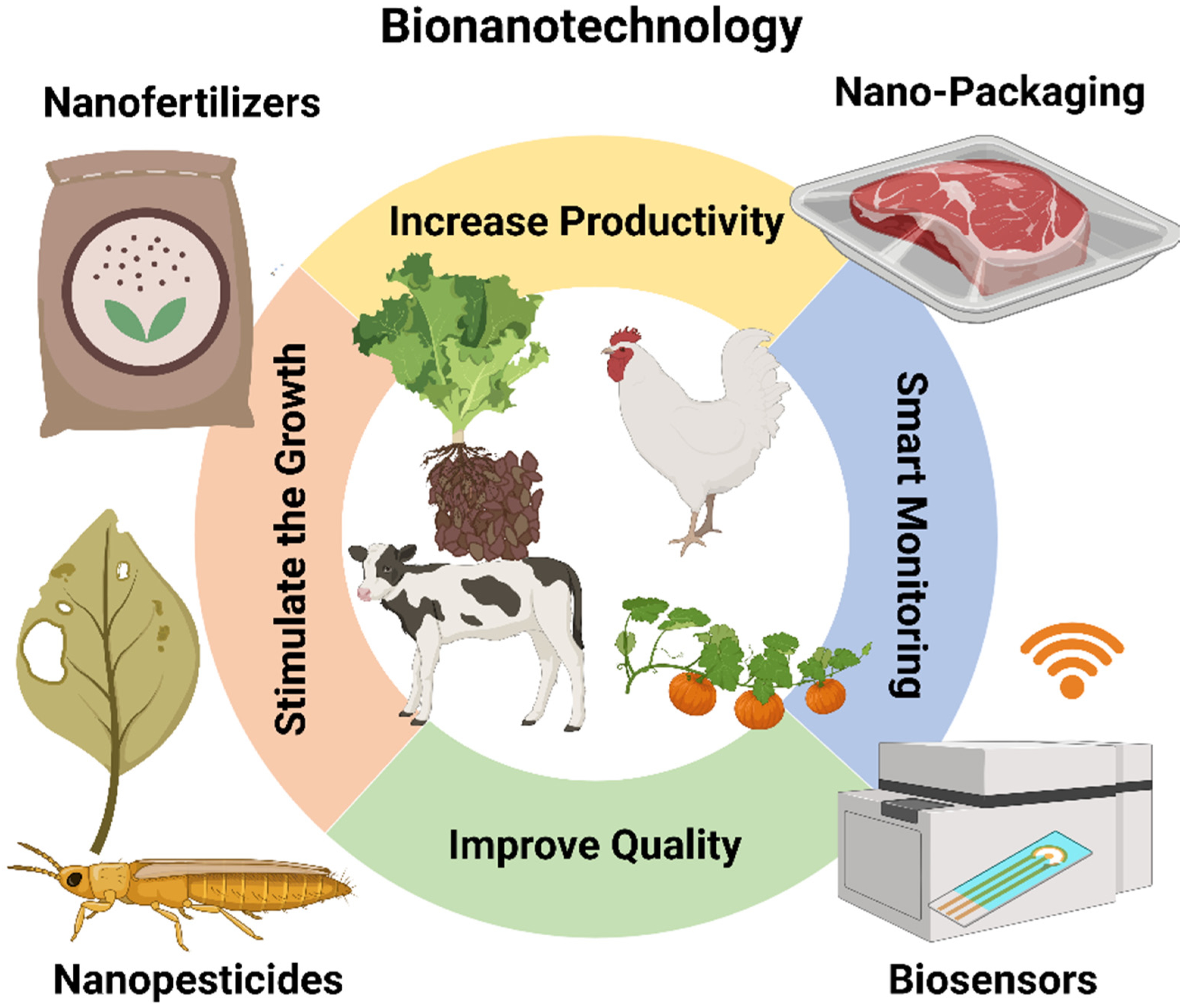
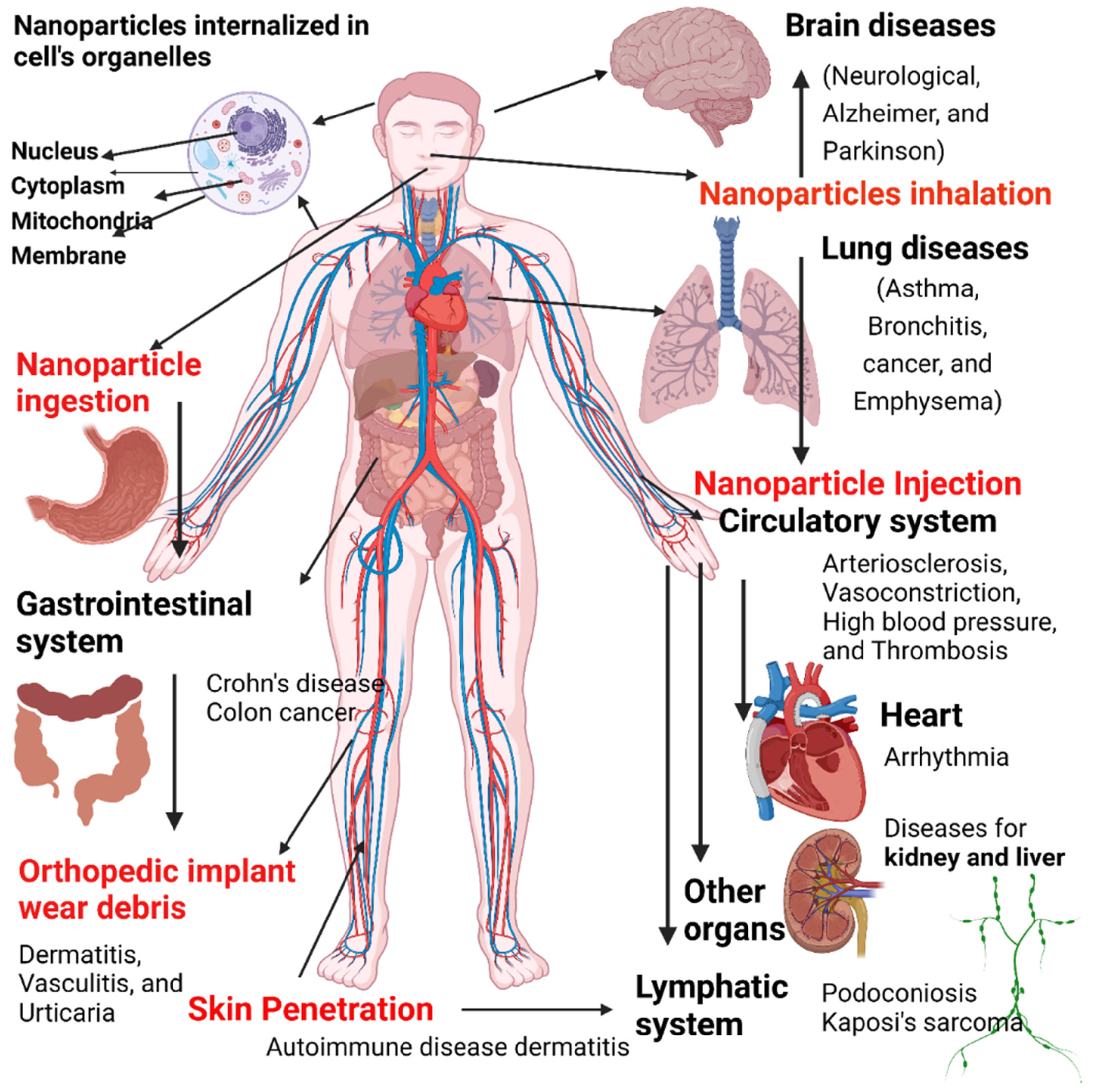
| Nanomaterial | Functionalization | Cell Lines | Refs |
|---|---|---|---|
| Graphene-based nanosheets | Surface functionalization by bio-compatible targeting ligands and coatings | MDA-MB-468 (MCF-7) | [70] |
| Molybdenum disulfide nanosheets | Chitosan; PLGA, PEG functionalization | Breast cancer cells (MDA-MB-468), HeLa uterine cancer cells, human lung cancer cells | [71] |
| Transition metal nanoparticles decorated with polymers | Polymer functionalization | Mice bearing 4T1 breast cancer cell xenografts | [72] |
| Lanthanide-activated nanoparticles | Doping with lanthanide | Cancer cells xenografted in mice | [73] |
| Group IV quantum dots | Surface functionalization | Various cancer cell types | [74] |
| Graphene oxide nanosheets | Surface functionalization | Tumor cells | [75] |
| Peptide-based nanoparticles | Chemical functionalization | Peptide-treated HeLa cells preloaded with Hg2+ | [76] |
| Silver nanoparticles | Aptamer conjugation | Leukemia cells, neural stem cells, kidney tissue, renal carcinoma cells | [77] |
| Gold nanoprisms | Conjugation with polyethylene glycol | Gastrointestinal carcinoma cells (HT 29) | [78] |
| Gold nanorods | Encasing by mesoporous silica | Carcinoma cells | [79] |
| Magnetofluroscentnanoprobe | Surface functionalization | Human Breast Cancer (MCF-7), HeLa cells | [80] |
| Dye-loaded nanoemulsions | Lipids conjugation with polyethylene glycol | Human colon cancer (HCT116), HeLa cells | [81] |
| Cadmium telluride quantum dots | Capping by shells | Human bronchial epithelial cells | [82] |
| Nanocarrier | Loaded Drug | Therapeutic Action | Ref |
|---|---|---|---|
| Metal-based nanoparticles | |||
| Gold nanoparticles | Doxorubicin | Anticancer effect in HeLa cells | [93] |
| Gold nanoparticles | Theophylline (THP), 1,3-dipropyl-8- cyclopentylxanthine (DPCPX) | Neuron reconstruction in vivo | [94] |
| Silver nanoparticles | Methotrexate-coated PEG | Anticancer effect in MCF-7 cells | [95] |
| Metal oxide-based nanoparticles | |||
| Fe3O4 nanoparticles | Doxorubicin | Anticancer effect in HeGP2 and Lo2 cells. | [96] |
| Fe3O4 nanoparticles | Fluorouracil | Anticancer effect in MCF-7 cells | [97] |
| Carbon-based nanoparticles | |||
| Multilayer carbon nanotubes | Dexamethasone | Anti-inflammatory effect in Highly-Aggressively Proliferating Immortalized cells (HAPI) | [98] |
| Single-layer carbon nanotubes | Cisplatin | Anticancer effect in head and neck squamous carcinoma in vivo and in vitro | [99] |
| Quantum dots | |||
| Ag–In–Zn–S quantum dots modified with 11-mercaptoundecanoic acid, L-cysteine, lipoic acid, and decorated with folic acid | Doxorubicin | Anticancer effect in A549 cells (human alveolar basal epithelial cells) | [100] |
| Nano-clays | |||
| Laponite nanoplates | Anionic dexamethasone | Anti-inflammatory effect in MG-63 osteoblast-like cells | [101] |
| Dendrimers | |||
| Poly-amido-amine dendrimers | Methotrexate | Anticancer effect in methotrexate -sensitive and resistant human acute lymphoblastoid leukemia (CCRF-CEM) and Chinese hamster ovary (CHO) cells | [102] |
| Polymeric nanoparticles | |||
| Poly-lactic acid | Paclitaxel | Anticancer effect in a mouse model of ovarian cancer in vivo. | [103] |
| Chitosan | Tacrine | Therapeutic effect in a rat model of Alzheimer’s disease in vivo (preclinical study) | [104] |
| Liposomes | |||
| Liposomes | Dexamethasone phosphate | Anti-inflammatory effect in a rat model of adjuvant-induced arthritis in vivo. | [105] |
| Liposomes | Cetuximab and oxaliplatin | Anticancer effect in mice xenografted with colon cancer cells in vivo | [106] |
| Nanofibers | |||
| Polyvinyl alcohol | PEG2000-Pt(IV) micelles and dichloroacetate | Anticancer effect in mice xenografted with cervical cancer cells in vivo | [107] |
| Polylactic acid electrospun nanofibers | Doxorubicin | Anticancer effect in mice with secondary hepatic carcinoma in vivo | [108] |
| Nanomaterial | Anticancer Drug | Targeted Cancer Cells | Refs |
|---|---|---|---|
| Silver nanoparticles | Terminaliachebula | Breast cancer cells (MCF-7) | [126] |
| Glycerylmonooleate nanostructures | Doxorubicin hydrochloride | Breast cancer cells (MCF-7, MDA-MB-231) | [127] |
| Poly (3HB-co-4HB) biodegradable nanoparticles | Docetaxel | Breast and prostate cancer cells | [128] |
| Carbon nanodots | Irinotecan | Breast cancer cells (MCF-7, MDA-MB-231) | [129] |
| Polysaccharide nanoparticles | Lapatinib | Breast cancer cells (MCF-7/ADR) | [130] |
| Fe3O4 nanoparticles | Doxorubicin | HepGP2 liver cancer cells and LO2 liver cells | [96] |
| Fe3O4 nanoparticles | Fluorouracil | Tumor cells and in vitro analysis | [97] |
| Porous silicon nanoparticles | Doxorubicin and siRNA | Prostate cancer cells | [131] |
| Thermosensitiveliposomes coated with cetuximab | Doxorubicin | EGFR-expressing breast cancer cells | [132] |
| Iron oxide nanoparticles | Cetuximab | A431 (epidermoid carcinoma) cell lines | [133] |
| Nanomaterial | Antioxidant Agent | Applications | Refs |
|---|---|---|---|
| Conjugates | Superoxide dismutase | Superoxide conversion to hydrogen peroxide | [150] |
| Conjugates | Superoxide dismutase | Enhancing drug delivery to the brain | [151] |
| Conjugates | Catalases | Hydrogen peroxide conversion to water | [152] |
| Nanozymes | Catalases | Hydrogen peroxide conversion to water | [153] |
| GSH-PEGDA oligomer nanoparticle | Glutathione peroxidase | Reduction of lipid hydroperoxides and conversion of hydrogen peroxides to water | [154] |
| Liposomes | Vitamins | ROS scavenging and upregulation of antioxidant molecules | [155] |
| Solid lipid nanoparticles | Carotenoids | Singlet oxygen quenching, formation of provitamin A carotenoids (free radical scavengers) | [156] |
| Liposomes | Lycopene | Singlet oxygen quenching, formation of provitamin A carotenoids (free radical scavengers) | [155] |
| Liposomes | Polyphenol flavonoid catechins | Free-radical scavengers, carcinogenic activity, inhibition of proinflammatory kinases | [157] |
| Quercetin nanosuspensions | Quecetin | Protection against LDL oxidation | [157] |
| Silica nanoparticles | Gallic acid | Rapid H-atom transfer to diphenyl picryl hydrazine | [158] |
| Silica nanoparticles | 3,4-di-tert-butyl-4- hydroxybenzoic acid | Improved thermal and oxidative stability of low-density polyethylene (LDPE) composites | [159] |
| PEG-coated silver nanoparticles | Salvianolic acid | Improved reactive oxygen species (ROS) scavenging and antioxidant activity in living cells | [160] |
| Mesoporous silica nanoparticles | Poly-tannic acid | Good antioxidant activity | [161] |
| Mesoporous silica nanoparticles | Morin | Potent quencher of singlet molecular oxygen (1O2), HO· scavenger | [162] |
| Ceria nanoparticles | Polyethylene glycol (PEG)-dendron phospholipids | Biocompatibility, reduction of cytotoxicity and oxidative stress | [163] |
| PLGA-PEG | Curcumin | Neuroprotection | [164] |
| Function | Mode of Action | Nanomaterial | Target Microorganism | Ref |
|---|---|---|---|---|
| Antibacterial | Interaction with DNA, resulting in DNA replication inhibition ROS production Interaction with sulfur-containing proteins, leading to the inhibition of the activity of several enzymes | Silver nanoparticles (Ag NPs) | Bacillus subtilis | [171] |
| Staphylococcus aureus | [172] | |||
| Methicillin-resistant coagulase-negative | [173] | |||
| staphylococci | [174] | |||
| Titanium oxide nanoparticles (TiO2 NPs) | Escherichia coli 0157:H7 | [175] | ||
| Staphylococcus aureus | [176] | |||
| Pseudomonas fluorescens | [177] | |||
| Copper oxide nanoparticles (CuO NPs) | Bacillus subtilis | [178] | ||
| Listeria monocytogenes | [179] | |||
| Antifungal | Disruption of the cell membrane integrity | Titanium oxide nanoparticles (TiO2 NPs) | Candida spp. | [180] |
| Penicillium expansum | [181] | |||
| Aspergillus niger spp. | [182] | |||
| Penicillium oxalicum | [183] | |||
| Siver nanoparticles (Ag NPs) | Candida spp. | [184] | ||
| Magnesium oxide nanoparticles (MgO NPs) | Saccharomyces cerevisiae | [185] | ||
| Candida albicans | [186] | |||
| Antiviral | Inhibition of virus attachment to the host cell membrane | Gold nanoparticles (Au NPs) | Human immunodeficiency virus | [187] |
| Influenza virus | [188] | |||
| Silver nanoparticles (Ag NPs) | Herpes simplex virus | [189] | ||
| Respiratory syncytial virus | [190] | |||
| Titanium oxide nanoparticles (TiO2 NPs) | Inactivation of bacteriophages | [191] | ||
| Inactivation of Qβ and T4 bacteriophages | [192] | |||
| Antiparasitic | Inhibition of promastigote proliferation and metabolic activity Generation of ROS that may inhibit parasitic infection | Silver nanoparticles (Ag NPs) | Leishmania tropica | [193] |
| Leishmania infantum | [194] | |||
| Entamoeba histolytica | [195] | |||
| Copper oxide nanoparticles (CuO NPs) | Entamoeba histolytica | [196] | ||
| Cryptosporidium parvum | [197] |
| Nanomaterial | Dimentionality | Key Benefits | Ref |
|---|---|---|---|
| Spherical metallic nanoparticles | Zero-dimensional (0D) | Immobilization of bio-receptors Improved analyte loading Strong catalytic characteristics | [226] |
| Spherical quantum dots | Zero-dimensional (0D) | Excellent fluorescence, Charge carrier quantum confinement size-adjusted band energy | [227] |
| Nanorods | One-dimensional (1D) | Excellent plasmonic materials Size-adjustable energy regulation to produce specific field responses | [228] |
| Nanowires (1D) | One-dimensional (1D) | Superior charge conduction Strong sensing characteristics | [228] |
| Carbon nanomaterials (1D and 2D) | One and two-dimensional (1D and 2D) | Superior charge conduction High functionalization potential | [229] |
| Agriculture | Food Processing | Food Packaging | Supplements | References |
|---|---|---|---|---|
| Detection of the specific molecule to estimate the enzyme-substrate interaction | Nanoencapsulation for bioavailability enhancement of nutraceuticals | Detection of foodborne chemicals and pathogens by fluorescent nanoparticles attached to antibodies | Nutrient absorption enhancement by nanosized powders | [274,275,276,277] |
| Delivery of pesticides and fertilizers through nanocapsules | Flavor enhancement using nanoencapsulation | Monitoring of temperature, moisture, and time using nanosensors | Cellulose nanocrystals function as drug carrier | [278,279,280,281] |
| Controlled delivery of growth hormones | Nanoparticles used as viscosifying agents | Ethylene detection by electrochemical nanosensors | Nutraceutical nanoencapsulation for enhancement of absorption and stability | [83,281,282,283] |
| Crop growth and soil condition monitoring using nanosensors | Replacement of meat cholesterol by plant-based steroid containing nanocapsules | Surface coated nanoparticles for antifungal and antimicrobial effect | Coiled nanoparticles (nano-cochleate) for cellular delivery of nutrients | [83,265,284,285] |
| Nanosensors for detection of plant and animal pathogens Vaccine delivery using nanocapsules | Removal of pathogens by selective binding of nanoparticles from food | Heat resistant films with silicate nanoparticles | Improvement of absorption by dispersing vitamin sprays to nanodroplets | [286,287,288,289] |
| Nanoparticle Type | Toxicity Mechanism | Applications | Refs |
|---|---|---|---|
| Aluminum oxide nanoparticles | Genotoxicity, changes in protein expression, oxidative stress, cell viability, mitochondrial function | Polymers, biomaterials, fuel cells, paints, textiles, and coatings | [290,291,292,293] |
| Gold nanoparticles | Non-toxic spherical core, relatively safe; lipid peroxidation, autophagy in lung fibroblasts | Contrast agents and drug carriers | [294,295] |
| Copper oxide nanoparticles | Oxidative damage (stress), cytotoxicity (cell membrane integrity), nephrotoxicity, genotoxicity, hepatotoxicity, and spleen toxicity | Antibacterial, semiconductors, heat transfer fluids, and contraceptive devices | [296,297,298,299] |
| Silver nanoparticles | Oxidative stress, genotoxicity, cell viability decrease, nephrotoxicity, cell membrane integrity, lung toxicity, and cardiovascular toxicity | Wound dressing, prostheses, coating for surgical instruments, and antibacterial agents | [300,301,302,303] |
| Zinc oxide nanoparticles | Mitochondrial dysfunction, genotoxicity, oxidative stress, hepatotoxicity, cell membrane integrity, cell viability, cardiovascular toxicity, inflammation, neurotoxicity, cytotoxicity, and reactive oxygen species production | Sunscreens, gas filters, UV detectors, wave filters, and body care products | [303,304,305,306] |
| Iron oxide nanoparticles | Neurotoxicity, mitochondrial function alterations, genotoxicity, lung toxicity, hepatotoxicity, reactive oxygen species production, cell viability, and endothelial permeability | Diagnostic agents and drug carriers | [307,308,309] |
| Titanium nanoparticles | Reactive oxygen species production, nephrotoxicity, genotoxicity, hepatotoxicity, immune function changes, lung toxicity, spleen toxicity, and cardiovascular toxicity | Coloring and pigment agents | [303,304] |
| Carbon-based nanoparticles and fullerenes | Cell membrane integrity, cell viability, bone toxicity, genotoxicity, hepatotoxicity, nephrotoxicity, spleen toxicity, cardiotoxicity, epigenetic toxicity, skin toxicity, carcinogenesis, neurotoxicity, and immunotoxicity | Drug carriers | [310,311,312,313] |
| Polymeric nanoparticles | Non-toxic, relatively safe, non-inflammatory, non-immunologic, and least toxic | Drug carriers | [314,315] |
| Nickel oxide nanoparticles | Apoptosis and lipid peroxidation increase | Antibacterial, antifungal, and cytotoxic | [316,317,318,319,320] |
| Cerium oxide nanoparticles | Apoptosis, cell membrane damage, p38-NRF2 signaling, and inflammation | Antimicrobial, corrosion protection, polishing, and solar cells | [321,322,323,324] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. https://doi.org/10.3390/nano12030457
Harish V, Tewari D, Gaur M, Yadav AB, Swaroop S, Bechelany M, Barhoum A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials. 2022; 12(3):457. https://doi.org/10.3390/nano12030457
Chicago/Turabian StyleHarish, Vancha, Devesh Tewari, Manish Gaur, Awadh Bihari Yadav, Shiv Swaroop, Mikhael Bechelany, and Ahmed Barhoum. 2022. "Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications" Nanomaterials 12, no. 3: 457. https://doi.org/10.3390/nano12030457
APA StyleHarish, V., Tewari, D., Gaur, M., Yadav, A. B., Swaroop, S., Bechelany, M., & Barhoum, A. (2022). Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials, 12(3), 457. https://doi.org/10.3390/nano12030457