Flame Retardancy, Thermal and Mechanical Properties of Novel Intumescent Flame Retardant/MXene/Poly(Vinyl Alcohol) Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Specimens
2.2.1. Synthesis of MXene (Ti3C2Tx) Nanosheets
2.2.2. Preparation of Poly(vinylphosphonic Acid)
2.2.3. Preparation of MXene/PVA Nanocomposites
2.3. Methods
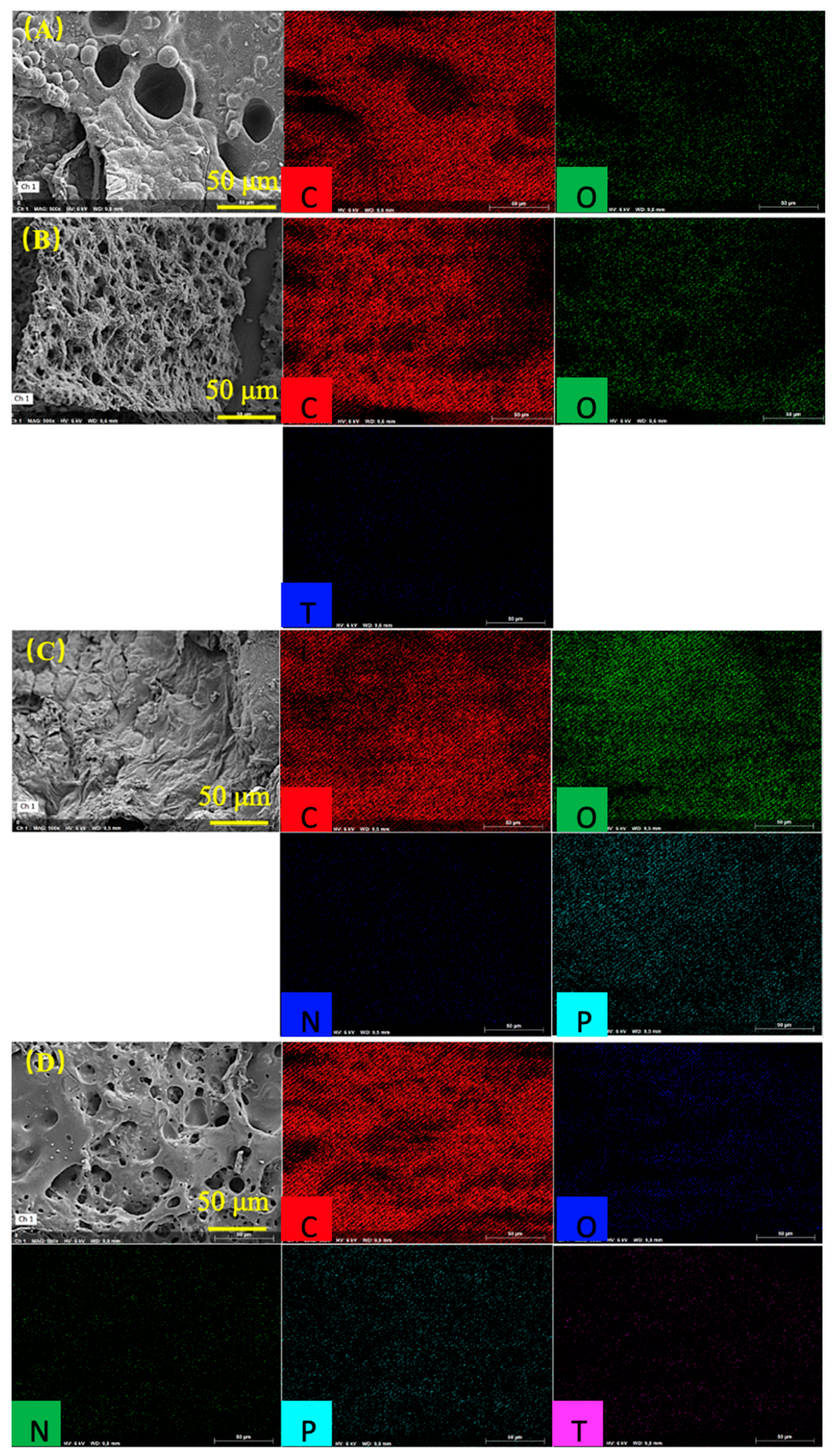
2.3.1. Morphology and Elemental Analysis
2.3.2. Flame-Retardant Properties Measurement
2.3.3. Thermal Stability Properties Measurement
2.3.4. Mechanical Properties Measurement
3. Results and Discussion
3.1. Characterization MXene and the Dispersion of MXene in the Nanocomposites
3.2. Flame-Retardant Properties
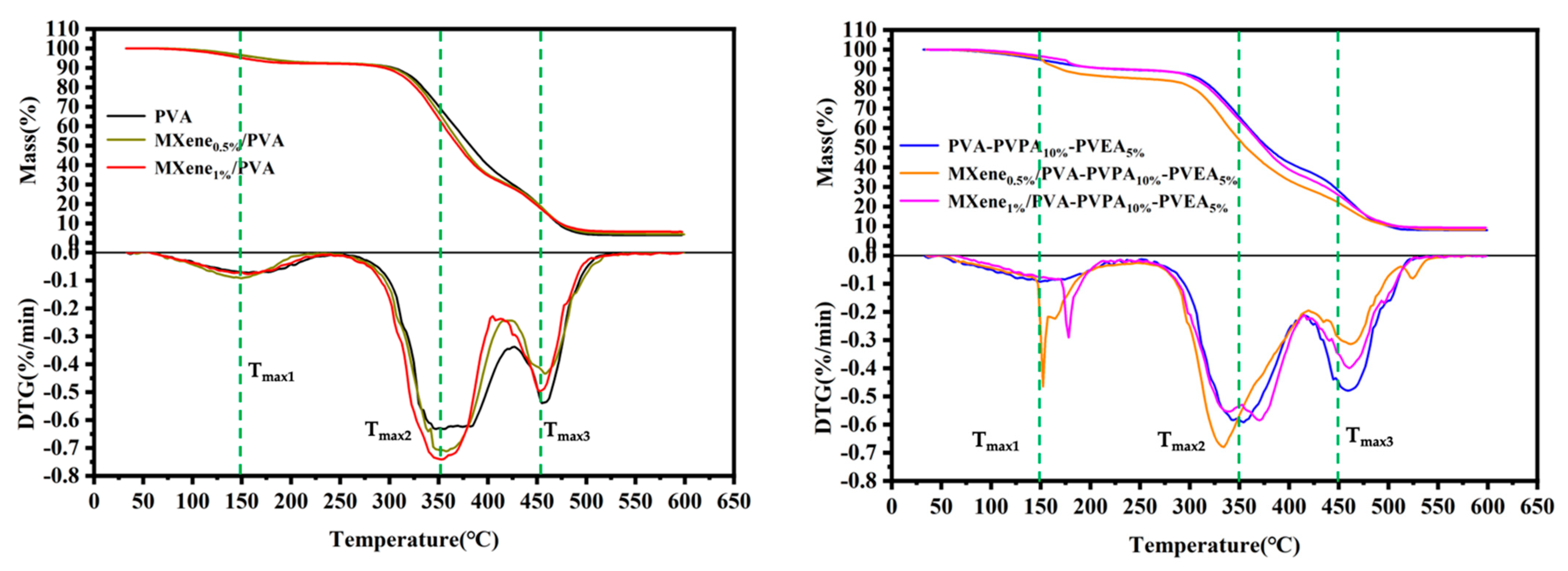
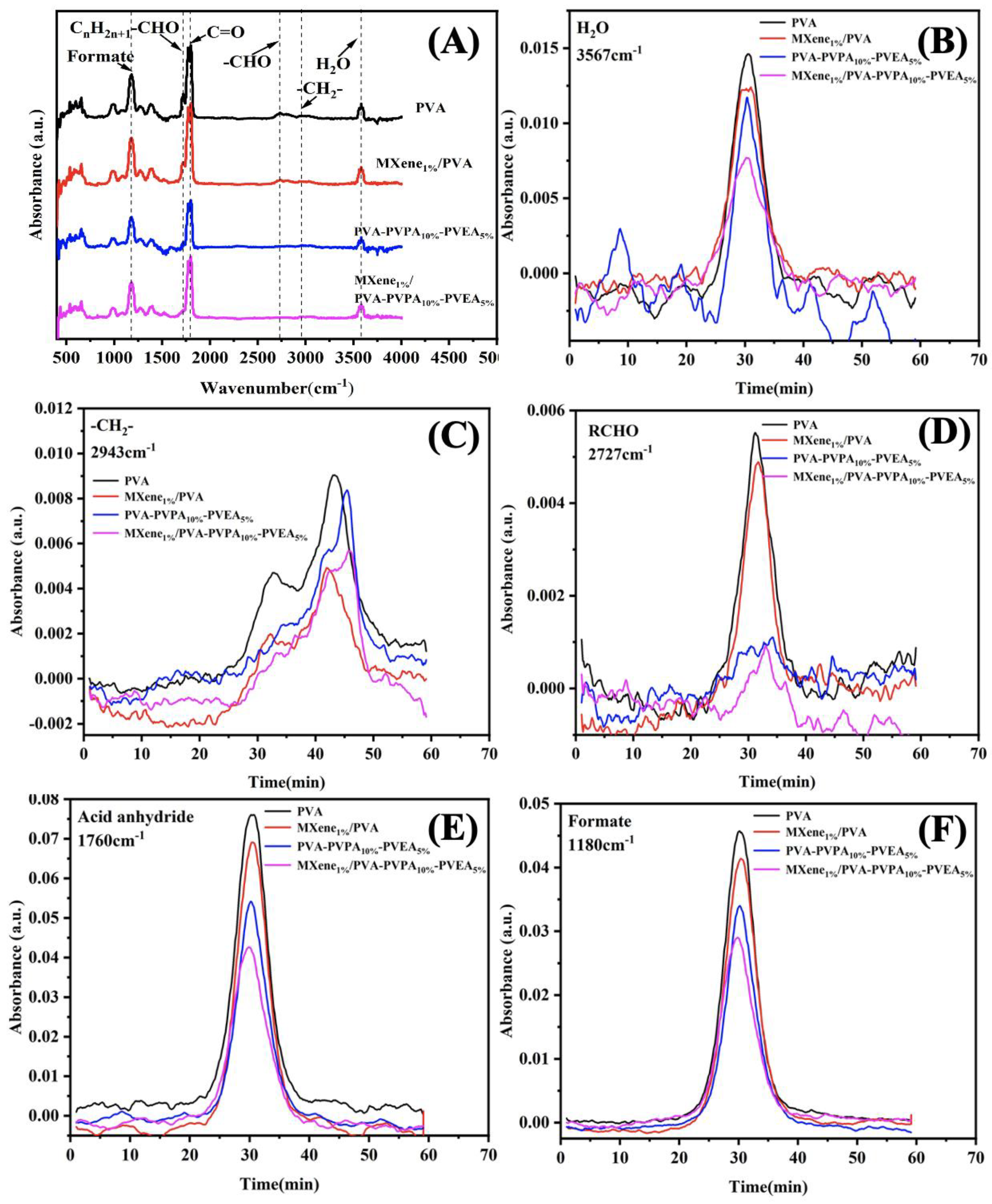
3.3. Thermal Properties
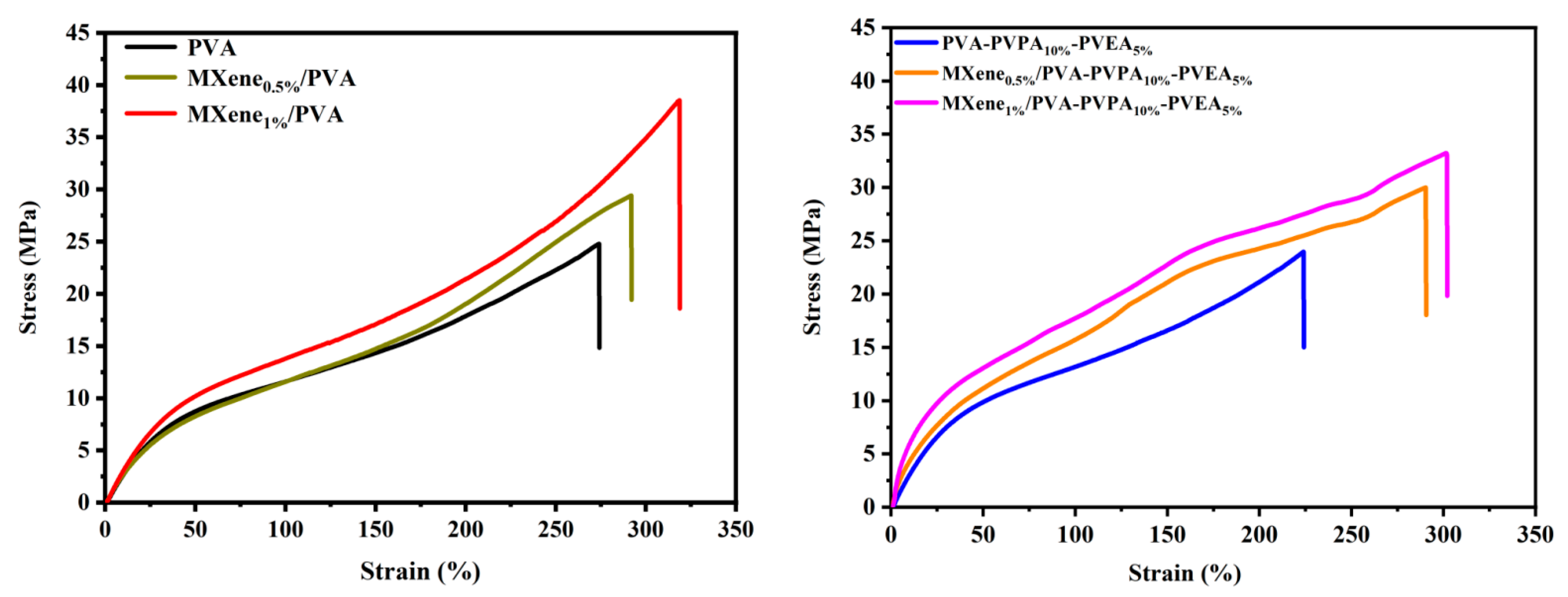
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelaziz, M.; Ghannam, M.M. Influence of titanium chloride addition on the optical and dielectric properties of PVA films. Phys. B 2010, 405, 958–964. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Guseinov, S.S.; Barannikov, V.P.; Zakharov, A.G. Thermal stability of polyvinyl alcohol/nanocrystalline cellulose composites. Carbohydr. Polym. 2015, 130, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Effect of separate zinc, copper and graphene oxides nanofillers on electrical properties of PVA based composite strips. J. Electron. Mater. 2019, 48, 1116–1121. [Google Scholar] [CrossRef]
- Arain, M.F.; Wang, M.; Chen, J.; Zhang, H. Study on PVA fiber surface modification for strain-hardening cementitious composites (PVA-SHCC). Constr. Build. Mater. 2019, 197, 107–116. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of cellulose nanocrystals and lignin nanoparticles on mechanical, antioxidant and water vapour barrier properties of glutaraldehyde crosslinked PVA films. Polymers 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A. The development of Speek/PVA/Teos blend membrane for proton exchange membrane fuel cells. Electrochim. Acta. 2018, 271, 127–136. [Google Scholar] [CrossRef]
- Yan, X.; Fang, J.; Zhu, C.; Li, J.; Qi, D. Design and characterization of ramie fiber-reinforced composites with flame retardant surface layer including iron oxide and expandable graphite. J. Polym. Eng. 2021, 41, 576–584. [Google Scholar] [CrossRef]
- Mrinal, B. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Kandola, B.K.; Williams, K.V.; Ebdon, J.R. Organo-Inorganic hybrid intumescent fire retardant coatings for thermoplastics based on poly(vinylphosphonic acid). Molecules 2020, 25, 688. [Google Scholar] [CrossRef] [Green Version]
- Lanzinger, D.; Salzinger, S.; Soller, B.S.; Rieger, B. Poly(vinylphosphonate)s as macromolecular flame retardants for polycarbonate. Ind. Eng. Chem. Res. 2015, 54, 1703–1712. [Google Scholar] [CrossRef]
- Akihiro, N.; Hiroshi, S.; Katsuichi, N.; Tomoo, S. Effects of polyethylenepolyamine base multi-branched high molecular weight nonionic surface active agents on fluidity of highly-loaded coal-water slurry. J. Jpn. Oil. Chem. Soc. 1986, 35, 188–192. [Google Scholar]
- Opwis, K.; Wego, A.; Bahners, T.; Schollmeyer, E. Permanent flame retardant finishing of textile materials by a photochemical immobilization of vinyl phosphonic acid. Polym. Degrad. Stab. 2011, 96, 393–395. [Google Scholar] [CrossRef]
- Williams, K.; Ebdon, J.R.; Kandola, B.K. Intumescent fire-retardant coatings for plastics based on poly(vinylphosphonic acid): Improving water resistance with comonomers. J. Appl. Polym. Sci. 2020, 137, 47601. [Google Scholar] [CrossRef] [Green Version]
- Li, J. Improvement of interfacial adhesion in PP/PS blends enhanced with polyethylene-polyamine surface treated carbon fiber. Adv. Mat. Res. 2015, 1061–1062, 170–174. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, F.; Zhang, N.; Zhang, H. Few-layer MXene Ti3C2Tx (T = F, O, or OH) saturable absorber for visible bulk laser. Opt. Mater. Express. 2019, 9, 1795–1802. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, S.; Liang, W.; Luo, S.; He, Z.; Ge, Y.; Wang, H.; Cao, R.; Zhang, F.; Wen, Q.; et al. Broadband nonlinear photonics in few-layer MXene Ti3C2Tx (T = F, O, or OH). Laser. Photonics. Rev. 2018, 12, 1700229. [Google Scholar] [CrossRef]
- Levitt, A.; Zhang, J.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene-Based fibers, yarns, and fabrics for wearableenergy storage devices. Adv. Funct. Mater. 2020, 30, 2000739. [Google Scholar] [CrossRef]
- Ahn, S.; Han, T.H.; Maleski, K.; Song, J.; Kim, Y.H.; Park, M.H.; Zhou, H.; Yoo, S.; Gogotsi, Y.; Lee, T.W. A 2D titanium carbide MXene flexible electrode for high-efficiency light-emitting diodes. Adv. Mater. 2020, 32, 2000919. [Google Scholar] [CrossRef]
- Liu, C.; Wei, X.; Hao, S.; Zong, B.; Chen, X.; Li, Z.; Mao, S. Label-Free, fast response, and simply operated silver ion detection with a Ti3C2Tx MXene field-effect transistor. Anal. Chem. 2021, 93, 8010–8018. [Google Scholar] [CrossRef]
- Soleymaniha, M.; Shahbazi, M.A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting role of MXene nanosheets in biomedical sciences: Therapeutic and biosensing innovations. Adv. Healthc. Mater. 2019, 8, 1801137. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Li, W. High-Thermal-Stability and High-Thermal-Conductivity Ti3C2Tx MXene/Poly(vinyl alcohol) (PVA) composites. ACS Omega. 2018, 3, 2609–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolčiak, P.; Ali, A.; Hassan, M.K.; Helal, M.I.; Tanvir, A.; Popelka, A.; Al-Maadeed, M.A.; Krupa, I.; Mahmoud, K.A. 2D Ti3C2Tx (MXene)-reinforced polyvinyl alcohol (PVA) nanofibers with enhanced mechanical and electrical properties. PLoS ONE 2017, 12, e0183705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Yin, X.; Li, X.; Li, M.; Liang SZhang, L.; Cheng, L. Lightweight Ti2CTx MXene/Poly(vinyl alcohol) composite foams for electromagnetic wave shielding with absorption-dominated feature. ACS Appl. Mater. Interfaces 2019, 11, 10198–10207. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yin Yuen, A.C.; Zhang, Z.C.; Shen, L.L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Seiler, L.; Loiseau, J.; Leising, F.; Boustingorry, P.; Harrisson, S.; Destarac, M. Acceleration and improved control of aqueous RAFT/MADIX polymerization of vinylphosphonic acid in the presence of alkali hydroxides. Polym. Chem. 2017, 8, 3825–3832. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Xu, X.; Liu, L.; Yu, B.; Maluk, C.; Huang, G.; Wang, H.; Song, P. Bioinspired, highly adhesive, nanostructured polymeric coatings for superhydrophobic fire-extinguishing thermal insulation foam. ACS Nano 2021, 15, 11667–11680. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Li, L.; Wang, Y.; Wang, J.; Xiong, J.; Du, S.; Zhang, P.; Shi, X.; Yu, J. MXene/polymer nanocomposites: Preparation, properties, and applications. Polym. Rev. 2021, 61, 80–115. [Google Scholar] [CrossRef]
- Ning, H.; Ma, Z.; Zhang, Z.; Zhang, D.; Wang, Y. A novel multifunctional flame retardant MXene/nanosilica hybrid for poly(vinyl alcohol) with simultaneously improved mechanical properties. New J. Chem. 2021, 45, 4292–4302. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Y.; Xu, W.; Huang, D.; Wang, Z.; Hong, M. Fabrication and thermal stability of two-dimensional carbide Ti3C2 nanosheets. Ceram. Inter. 2016, 42, 8419–8424. [Google Scholar] [CrossRef]
- Holland, B.; Hay, J. The thermal degradation of poly(vinyl acetate) measured by thermal analysis–Fourier transform infrared spectroscopy. Polymer 2002, 43, 2207–2211. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Z.; Xu, B.; Ren, H.; Jiang, Z.; Zhu, P. A facile and effective flame-retardant coating for cotton fabric with α-aminodiphosphonate siloxane. Polym. Degrad. Stab. 2020, 180, 109312. [Google Scholar] [CrossRef]
- Gu, J.; Yan, X.; Li, J.; Qian, Y.; Zhu, C.; Qi, D. Durable flame-retardant behavior of cotton textile with a water-based ammonium vinyl phosphonate. Polym. Degrad. Stab. 2021, 191, 109658. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, L.; Fu, L.; Yu, B.; Lv, Y.; Yang, F.; Song, P. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 2019, 378, 122267. [Google Scholar] [CrossRef]
- Pan, Y.; Fu, L.; Zhou, Q.; Wen, Z.; Lin, C.; Yu, J.; Wang, W.; Zhao, H. Flammability, thermal stability and mechanical properties of polyvinyl alcohol nanocomposites reinforced with delaminated Ti3C2Tx (MXene). Polym. Compos. 2019, 41, 210–218. [Google Scholar] [CrossRef]





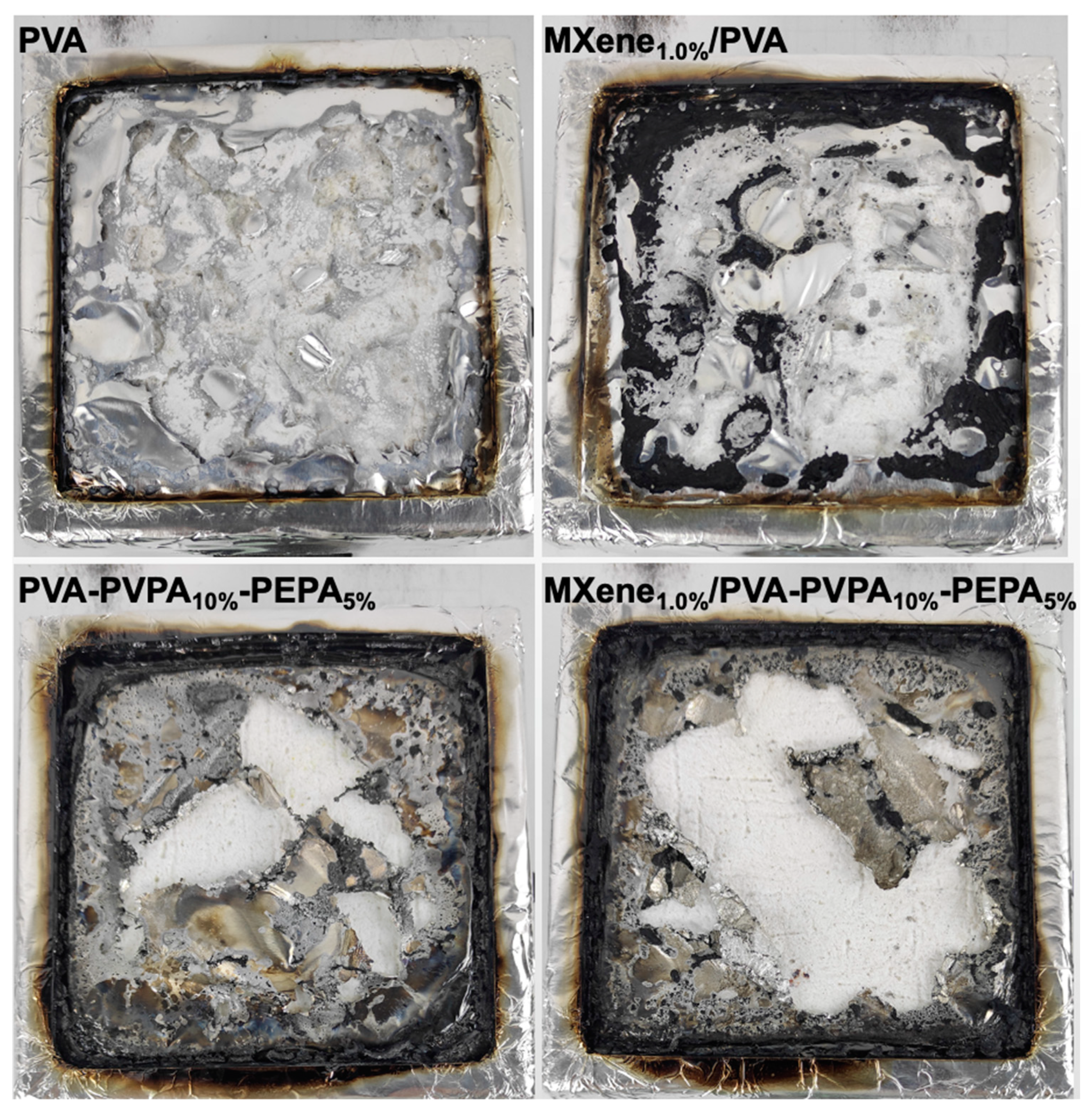

| Samples | PVA (wt%) | PVPA (wt%) | PEPA (wt%) | Ti3C2Tx (wt%) |
|---|---|---|---|---|
| PVA | 100.0 | 0.0 | 0.0 | 0.0 |
| MXene0.5%/PVA | 99.5 | 0.0 | 0.0 | 0.5 |
| MXene1.0%/PVA | 99.0 | 0.0 | 0.0 | 1.0 |
| PVA-PVPA10%-PEPA5% | 85.0 | 10.0 | 5.0 | 0.0 |
| MXene0.5%/PVA-PVPA10%-PEPA5% | 84.5 | 10.0 | 5.0 | 0.5 |
| MXene1.0%/PVA-PVPA10%-PEPA5% | 84.0 | 10.0 | 5.0 | 1.0 |
| Samples | LOI Value (%) | UL 94 | ||
|---|---|---|---|---|
| Rating | Dripping | Ignition of Cotton | ||
| PVA | 19.00 ± 0.19 | V2 | Yes | Yes |
| MXene0.5%/PVA | 19.50 ± 0.24 | V2 | Yes | Yes |
| MXene1.0%/PVA | 21.00 ± 0.28 | V1 | Yes | No |
| PVA-PVPA10%-PEPA5% | 25.50 ± 0.33 | V0 | No | No |
| MXene0.5%/PVA-PVPA10%-PEPA5% | 26.00 ± 0.38 | V0 | No | No |
| MXene1.0%/PVA-PVPA10%-PEPA5% | 27.50 ± 0.44 | V0 | No | No |
| Samples | TTI (s) | pHRR (kW/m2) | FPI (s·m2/kW) | THR (MJ/m2) | TSR (m2/m2) | Residue Mass (%) |
|---|---|---|---|---|---|---|
| PVA | 9 | 1092 ± 3 | 0.008 ± 0.0002 | 16.7 ± 0.1 | 287.5 ± 0.3 | 0.3 ± 0.1 |
| MXene1.0%/PVA | 10 | 824 ± 3 | 0.012 ± 0.0001 | 16.5 ± 0.1 | 240.4 ± 0.4 | 4.1 ± 0.1 |
| PVA-PVPA10%-PEPA5% | 12 | 721 ± 6 | 0.017 ± 0.0004 | 16.1 ± 0.2 | 216.9 ± 0.7 | 8.1 ± 0.2 |
| MXene1.0%/PVA-PVPA10%-PEPA5% | 14 | 755 ± 7 | 0.019 ± 0.0005 | 12.4 ± 0.1 | 134.6 ± 0.8 | 9.5 ± 0.2 |
| Specimens | C | O | Ti | P | N | |
|---|---|---|---|---|---|---|
| PVA | Weight/% | 82.73 | 17.27 | / | / | / |
| Atmoic/% | 86.45 | 13.35 | / | / | / | |
| Error/% | 9.48 | 2.47 | / | / | / | |
| MXene1.0%/PVA | Weight/% | 60.14 | 16.16 | 23.70 | / | / |
| Atmoic/% | 76.89 | 15.51 | 7.60 | / | / | |
| Error/% | 6.94 | 2.28 | 4.19 | / | / | |
| PVA-PVPA10%-PEPA5% | Weight/% | 52.56 | 29.32 | / | 13.23 | 4.89 |
| Atmoic/% | 62.65 | 26.23 | / | 6.11 | 5.00 | |
| Error/% | 3.98 | 2.36 | / | 0.40 | 0.60 | |
| MXene1.0%/PVA-PVPA10%-PEPA5% | Weight/% | 67.68 | 10.87 | 15.06 | 6.39 | / |
| Atmoic/% | 82.44 | 9.94 | 4.60 | 3.02 | / | |
| Error/% | 7.69 | 1.57 | 2.89 | 0.32 | / | |
| Samples | T5% (°C) | Tmax1 (°C) | Δweight1 (%) | Tmax2 (°C) | Δweight2 (%) | Tmax3 (°C) | Δweight3 (%) | Char Residue at 600 °C (%) |
|---|---|---|---|---|---|---|---|---|
| PVA | 159.6 | 150.1 | 7.84 | 349.6 | 62.2 | 452.6 | 26.06 | 3.90 |
| MXene0.5%/PVA | 160.4 | 151.4 | 7.76 | 350.4 | 61.99 | 453.4 | 24.58 | 4.39 |
| MXene1.0%/PVA | 169.9 | 154.9 | 7.56 | 352.4 | 61.14 | 455.4 | 26.90 | 5.67 |
| PVA-PVPA10%-PEPA5% | 146.8 | 149.3 | 10.51 | 352.3 | 50.68 | 459.3 | 30.91 | 7.90 |
| MXene0.5%/PVA-PVPA10%-PEPA5% | 159.6 | 152.1 | 14.75 | 334.6 | 56.67 | 460.1 | 20.39 | 8.19 |
| MXene1.0%/PVA-PVPA10%-PEPA5% | 170.5 | 173.1 | 9.90 | 340.5 | 55.36 | 462.5 | 25.56 | 9.17 |
| Samples | Elongation at Break (%) | Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| PVA | 274.2 ± 3.5 | 24.8 ± 0.8 | 32.8 ± 0.3 |
| MXene0.5%/PVA | 292.0 ± 4.3 | 29.4 ± 1.3 | 35.2 ± 0.8 |
| MXene1.0%/PVA | 318.8 ± 4.7 | 38.2 ± 0.9 | 39.1 ± 1.1 |
| PVA-PVPA10%-PVEA5% | 224.2 ± 5.2 | 24.0 ± 3.6 | 40.5 ± 3.4 |
| MXene0.5%/PVA-PVPA10%-PVEA5% | 290.5 ± 5.4 | 30.0 ± 3.4 | 68.8 ± 5.1 |
| MXene1.0%/PVA-PVPA10%-PVEA5% | 301.9 ± 4.8 | 33.2 ± 2.7 | 92.2 ± 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Fang, J.; Gu, J.; Zhu, C.; Qi, D. Flame Retardancy, Thermal and Mechanical Properties of Novel Intumescent Flame Retardant/MXene/Poly(Vinyl Alcohol) Nanocomposites. Nanomaterials 2022, 12, 477. https://doi.org/10.3390/nano12030477
Yan X, Fang J, Gu J, Zhu C, Qi D. Flame Retardancy, Thermal and Mechanical Properties of Novel Intumescent Flame Retardant/MXene/Poly(Vinyl Alcohol) Nanocomposites. Nanomaterials. 2022; 12(3):477. https://doi.org/10.3390/nano12030477
Chicago/Turabian StyleYan, Xiaofei, Jie Fang, Jianjun Gu, Chenkai Zhu, and Dongming Qi. 2022. "Flame Retardancy, Thermal and Mechanical Properties of Novel Intumescent Flame Retardant/MXene/Poly(Vinyl Alcohol) Nanocomposites" Nanomaterials 12, no. 3: 477. https://doi.org/10.3390/nano12030477






