Optical and Physicochemical Characterizations of a Cellulosic/CdSe-QDs@S-DAB5 Film
Abstract
:1. Introduction
2. Materials and Methods
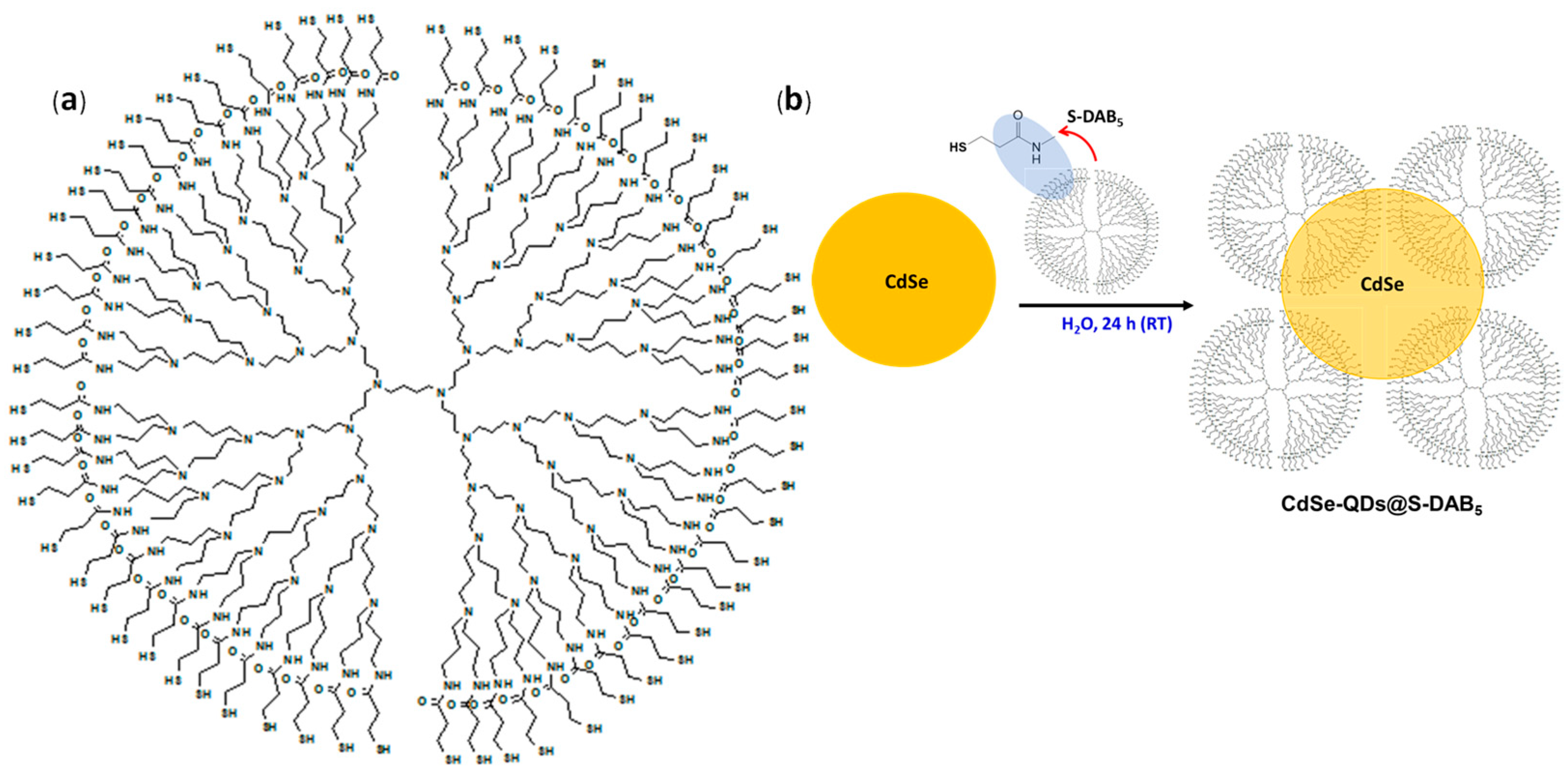
2.1. Preparation of CdSe QDs and Thiolated DAB Generation 5 Dendrimer
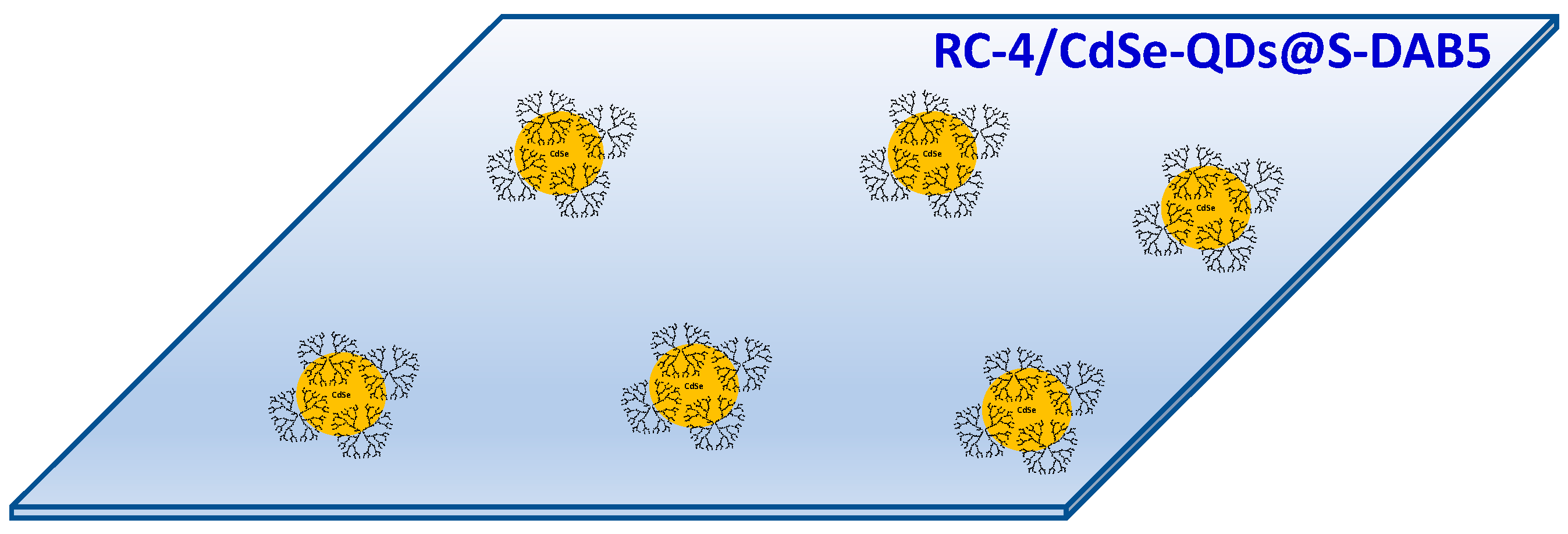
2.2. Preparation of the Cellulosic Films Modified with CdSe-QDs@ S-DAB5 Nanoparticles
2.3. Surface Analysis: FE-SEM Microscopy and XPS Spectroscopy
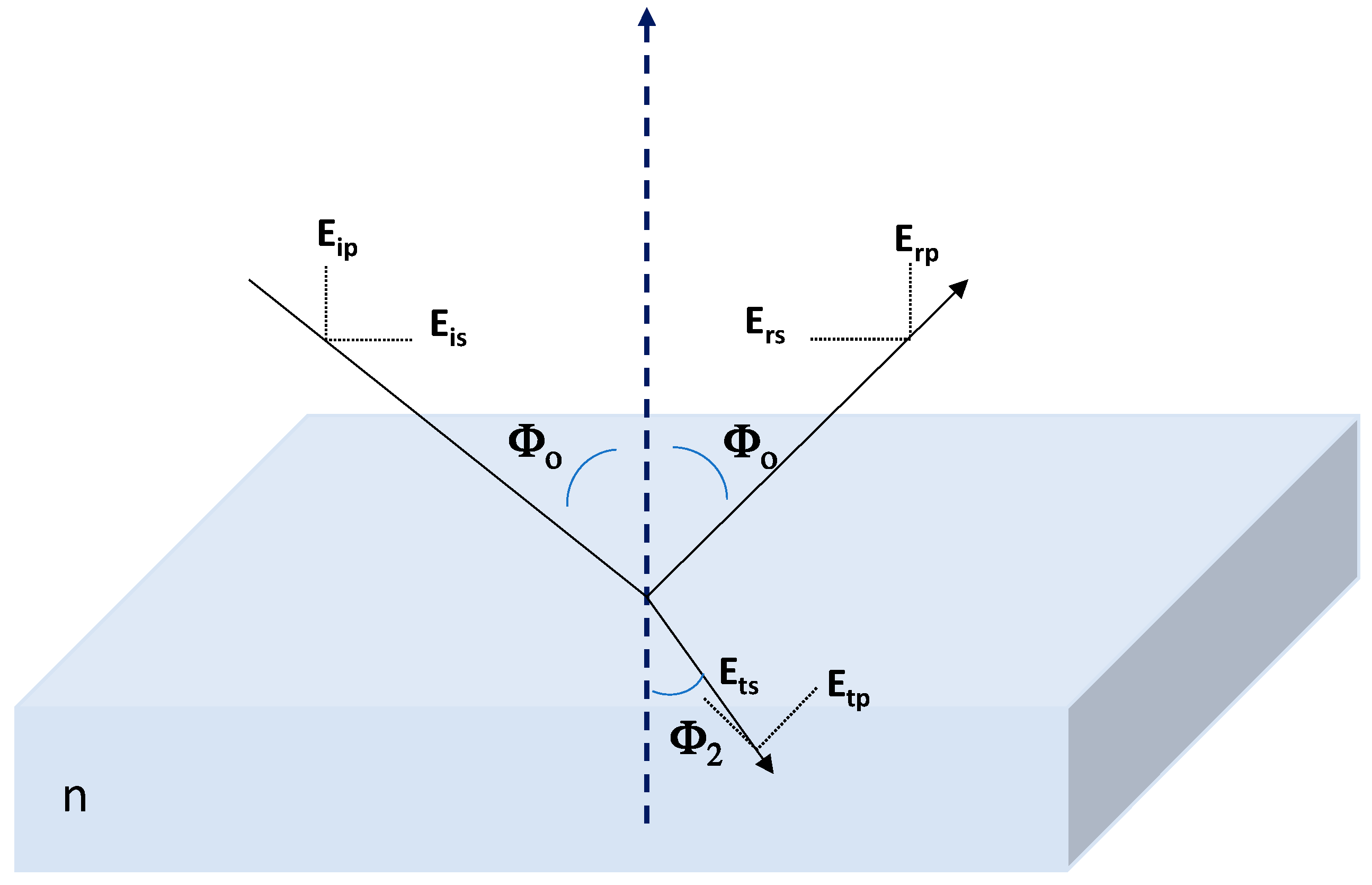
2.4. Optical Characterization
2.5. Impedance Spectroscopy Measurements
3. Results and Discussion
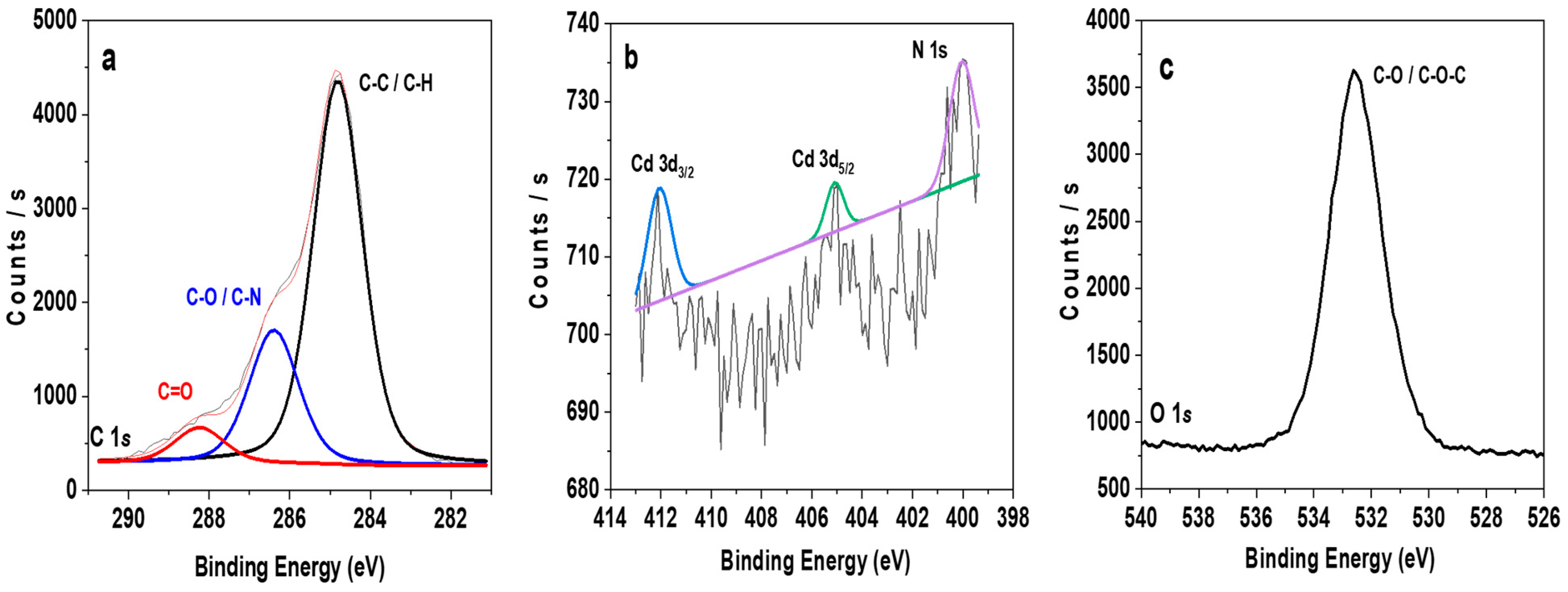
3.1. Surface and Bulk Analysis
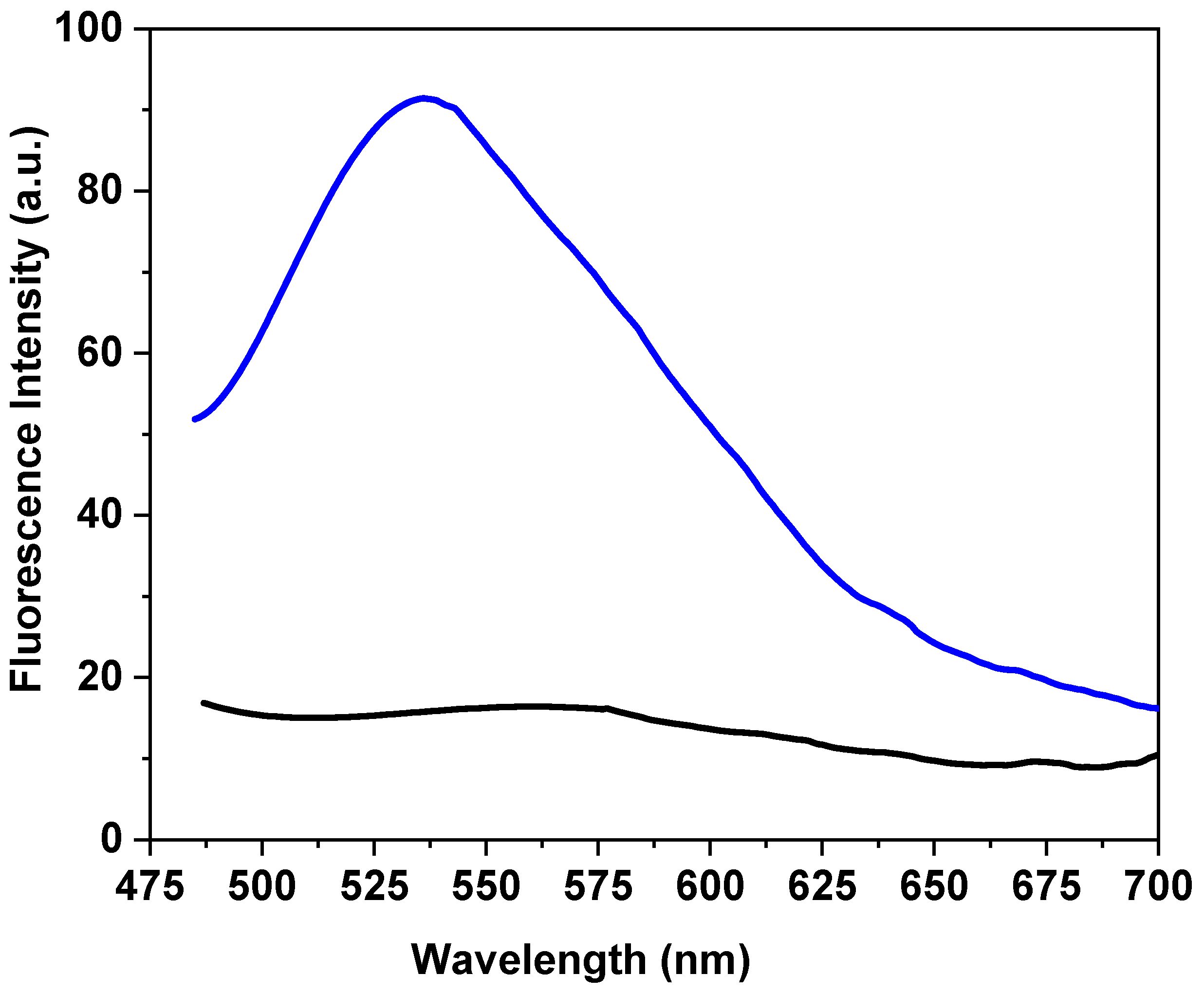
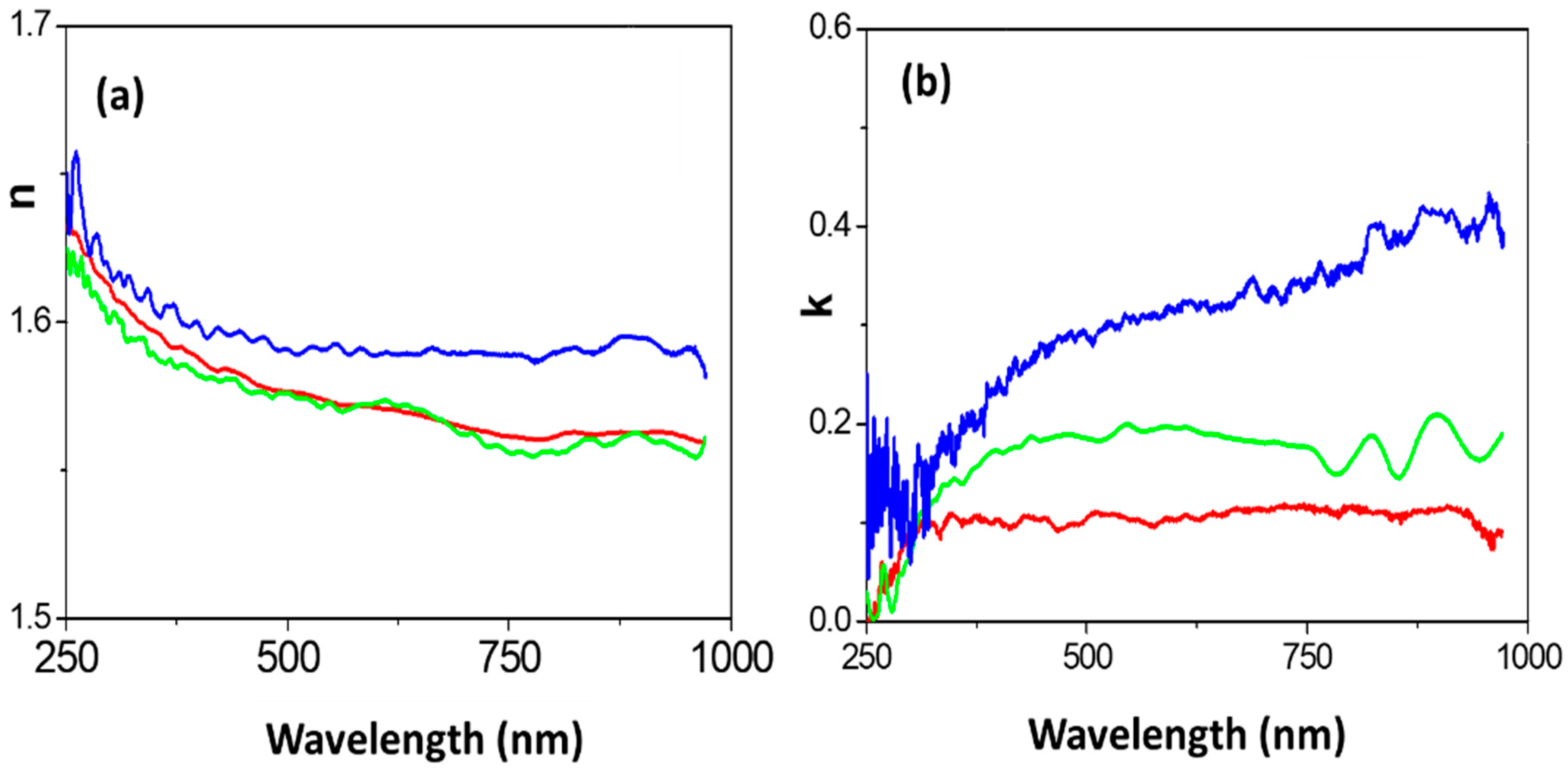
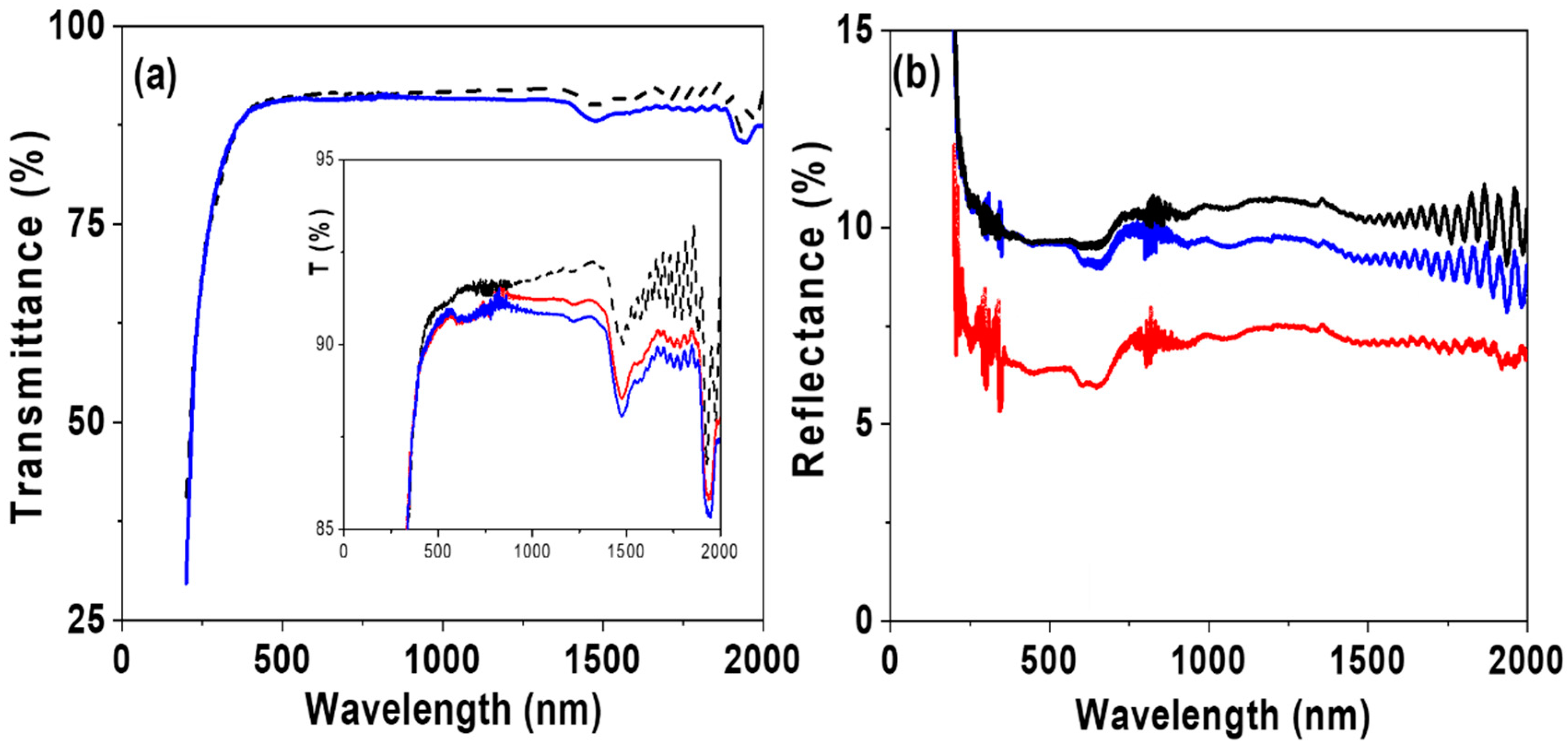
3.2. Optical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ekimov, A.; Onushchenko, A.A. Quantum size effect in the optical spectra of semiconductor microcrystals. Sov. Phys. Semicond. 1982, 16, 775–778. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 6, 2260–2345. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.J.; Ahmed, T.; Gautam, P.; Sadhu, A.; Lien, D.-H.; Chen, S.-C.; Chueh, Y.-L.; Kuo, H.-C. Recent Advances in Two-Dimensional Quantum Dots and Their Applications. Nanomaterials 2021, 11, 1549. [Google Scholar] [CrossRef]
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Klimov, V.I. Spectral and Dynamical Properties of Multiexcitons in Semiconductor Nanocrystals. Annu. Rev. Phys. Chem. 2007, 58, 635–673. [Google Scholar] [CrossRef]
- Amelia, M.; Lincheneau, C.; Silvi, S.; Credi, A. Electrochemical properties of CdSe and CdTe quantum dots. Chem. Soc. Rev. 2012, 41, 5728–5743. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Chizhov, A.; Rumyantseva, M.; Gaskov, A. Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges. Nanomaterials 2021, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Jeong, B.G.; Chang, J.H.; Joung, J.F.; Yoon, S.-Y.; Lee, D.C.; Park, S.; Huh, J.; Yang, H.; Bae, W.K.; et al. Chemically resistant and thermally stable quantum dots prepared by shell encapsulation with cross-linkable block copolymer ligands. NPG Asia Mater. 2000, 12, 19. [Google Scholar] [CrossRef]
- Chen, K.-J.; Chen, H.-C.; Shih, M.-H.; Wang, C.-H.; Kuo, M.-Y.; Yang, Y.-C.; Lin, C.-C.; Kuo, H.-C. The Influence of the Thermal Effect on CdSe/ZnS Quantum Dots in Light-Emitting Diodes. J. Light. Technol. 2012, 30, 2256–2261. [Google Scholar] [CrossRef]
- Wang, N.; Koh, S.; Jeong, B.G.; Lee, D.; Kim, W.D.; Park, K.; Nam, M.K.; Lee, K.; Kim, Y.; Lee, B.-H.; et al. Highly luminescent silica-coated CdS/CdSe/CdS nanoparticles with strong chemical robustness and excellent thermal stability. Nanotechnology 2017, 28, 185603. [Google Scholar] [CrossRef]
- Tselikov, G.I.; Timoshenko, V.Y.; Golovan, L.A.; Plenge, J.; Shatalova, A.M.; Shandryuk, G.A.; Kutergina, I.Y.; Merekalov, A.S.; Rühl, E.; Talroze, R.V. Role of the Polymer Matrix on the Photoluminescence of Embedded CdSe Quantum Dots. ChemPhysChem 2015, 16, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chang, S.; Grover, C.P. Cryptography based on the absorption/emission features of multicolor semiconductor nanocrystal quantum dots. Opt. Express 2004, 12, 2925–2931. [Google Scholar] [CrossRef]
- Abitbol, T.; Gray, D. CdSe/ZnS QDs Embedded in Cellulose Triacetate Films with Hydrophilic Surfaces. Chem. Mater. 2007, 19, 4270–4276. [Google Scholar] [CrossRef]
- Shen, L. Biocompatible Polymer/Quantum Dots Hybrid Materials: Current Status and Future Developments. J. Funct. Biomater. 2011, 2, 355–372. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Feng, J. White-Light-Emitting Polymer Composite Film Based on Carbon Dots and Lanthanide Complexes. J. Phys. Chem. C 2015, 119, 7865–7872. [Google Scholar] [CrossRef]
- Zeng, J.; Yan, L. Metal-free transparent luminescent cellulose films. Cellulose 2015, 22, 729–736. [Google Scholar] [CrossRef]
- You, Y.; Zhang, H.; Liu, Y.; Lei, B. Transparent sunlight conversion film based on carboxymethyl cellulose and carbon dots. Carbohydr. Polym. 2016, 151, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.K.E.; Connell, R.; Held, J.; Pedersen, C.; Francis, L.; Hillmayer, M.A.; Ferry, V.E.; Kortshagen, U. Poly(methyl metacrylate) films with high concentrations of silicon quantum dots for visible transparent luminescent solar concentrators. ACS Appl. Mater. Interfaces 2020, 12, 4572–4578. [Google Scholar] [CrossRef] [PubMed]
- Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coatings 2021, 11, 1100. [Google Scholar] [CrossRef]
- Danial, W.H.; Md Bahri, N.F.; Abdul Majid, Z. Preparation, Marriage Chemistry and Applications of Graphene Quantum Dots–Nanocellulose Composite: A Brief Review. Molecules 2021, 26, 6158. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.K.; Handegård, Ø.S.; Hashimoto, A.; Nagao, T. Carbon Dot/Cellulose-Based Transparent Films for Efficient UV and High-Energy Blue Light Screening. ACS Sustain. Chem. Eng. 2021, 9, 9879–9890. [Google Scholar] [CrossRef]
- Campos, B.; Gelde, L.; Algarra, M.; da Silva, J.E.; Vázquez, M.; Benavente, J. Characterization of cellulose membranes modified with luminescent silicon quantum dots nanoparticles. Carbohydr. Polym. 2016, 151, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lai, C.; Marchewka, R.; Berry, R.M.; Tam, K.C. Use of CdS quantum dot-functionalized cellulose nanocrystal films for anti-counterfeiting applications. Nanoscale 2016, 8, 13288–13296. [Google Scholar] [CrossRef]
- Cuevas, A.; Campos, B.; Romero, R.; Algarra, M.; Vázquez, M.; Benavente, J. Eco-friendly modification of a regenerated cellulose based film by silicon, carbon and N-doped carbon quantum dots. Carbohydr. Polym. 2019, 206, 238–244. [Google Scholar] [CrossRef]
- Algarra, M.; Cuevas, A.L.; de Yuso, M.V.M.; Benavente, J. Insights into the formation of an emissive CdTe-quantum-dots/cellulose hybrid film. J. Colloid Interface Sci. 2020, 579, 714–722. [Google Scholar] [CrossRef]
- Ngoensawat, U.; Parnsubsakul, A.; Kaitphaiboonwet, S.; Wutikhun, T.; Sapcharoenkun, C.; Pienpinijtham, P.; Ekgasit, S. Luminescent nanohybrid of ZnO quantum dot and cellulose nanocrystal as anti-counterfeiting ink. Carbohydr. Polym. 2021, 15, 117864. [Google Scholar] [CrossRef]
- Chinthalapudi, N.; Kommaraju, V.V.D.; Kannan, M.K.; Nalluri, C.B.; Varanasi, S. Composites of cellulose nanofibers and silver nanoparticles for malachite green dye removal from water. Carbohydr. Polym. Technol. Appl. 2021, 2, 100098. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent advances in cellulose supported metal nanoparticles as green and sustainable catalysis for organic synthesis. Cellulose 2021, 28, 4545–4574. [Google Scholar] [CrossRef]
- Algarra, M.; Campos, B.; Alonso, B.; Miranda, M.; Martínez, Á.M.; Casado, C.; da Silva, J.E. Thiolated DAB dendrimers and CdSe quantum dots nanocomposites for Cd(II) or Pb(II) sensing. Talanta 2012, 88, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Esteves da Silva, J.C.G.; Algarra, M.; Campos, B.B. Synthesis and Analytical Applications of Quantum Dots Coated with Different Generations of DAB Dendrimers. In Advances in Nanocomposites-Synthesis, Characterization and Industrial Applications; Reddy, B., Ed.; IN-TECH: Rijeka, Croatia, 2011. [Google Scholar]
- Algarra, M.; Campos, B.; Miranda, M.; da Silva, J.C.E. CdSe quantum dots capped PAMAM dendrimer nanocomposites for sensing nitroaromatic compounds. Talanta 2011, 83, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Frechet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Lee, C.C.; A Mackay, J.; Frechet, J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Dias Martins, A.S.; Pallante, L.; Zizzi, E.A.; Miceli, M.; Ba̧tal, M.; Pinto Reis, C.; Deriu, M.A.; Klajnert-Maculewic, B. Noncovalent Interactions with PAMAM and PPI Dendrimers Promote the Cellular Uptake and Photodynamic Activity of Rose Bengal: The Role of the Dendrimer Structure. J. Med. Chem. 2021, 64, 15758–15771. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, R.; Zhelev, Z.; Kokuryo, D.; Spasov, L.; Aoki, I. Chemical nature and structure of organic coating of quantum dots is crucial for their application in imaging diagnostics. Int. J. Nanomed. 2011, 6, 1719–1732. [Google Scholar] [CrossRef] [Green Version]
- Campos, B.B.; Algarra, M.; Alonso, B.; Casado, C.M.; Jiménez, J.J.; Rodríguez-Castellón, E.; da Silva, J.E. Fluorescent sensor for Cr(VI) based in functionalized silicon quantum dots with dendrimers. Talanta 2015, 144, 862–867. [Google Scholar] [CrossRef]
- Fernandes, T.; Fateixa, S.; Nogueira, H.I.S.; Daniel-Da-Silva, A.L.; Trindade, T. Dendrimer-Based Gold Nanostructures for SERS Detection of Pesticides in Water. Eur. J. Inorg. Chem. 2019, 2020, 1153–1162. [Google Scholar] [CrossRef]
- Serenko, O.; Skupov, K.; Bakirov, A.; Kuchkina, N.; Shifrina, Z.; Muzafarov, A. Porosity of Rigid Dendrimers in Bulk: Interdendrimer Interactions and Functionality as Key Factors. Nanomaterials 2021, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.; Campos, B.B.; Alonso, B.; Casado, C.M.; Da Silva, J.C.E.; Benavente, J. Inclusion of thiol DAB dendrimer/CdSe quantum dots based in a membrane structure: Surface and bulk membrane modification. Electrochim. Acta 2013, 89, 652–659. [Google Scholar] [CrossRef]
- Connolly, S.; Rao, S.N.; Fitzmaurice, D. Characterization of Protein Aggregated Gold Nanocrystals. J. Phys. Chem. B 2000, 104, 4765–4776. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons: Chichester, UK, 1995; Volume I. [Google Scholar]
- Tompkins, H.G.; McGahan, W.A. Spectroscopic Ellipsometry and Reflectometry: A User’s Guide; Wiley: Hoboken, NJ, USA, 1999; ISBN 978-0-471-18172-9. [Google Scholar]
- Ogieglo, W.; Wormeester, H.; Wessling, M.; Benes, N.E. Spectroscopic Ellipsometry Analysis of a Thin Film Composite Membrane Consisting of Polysulfone on a Porous α-Alumina Support. ACS Appl. Mater. Interfaces 2012, 4, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.L.; Yuso, M.D.V.M.D.; Vega, V.; González, A.S.; Prida, V.M.; Benavente, J. Influence of ALD Coating Layers on the Optical Properties of Nanoporous Alumina-Based Structures. Coatings 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Losurdo, M.; Bergmair, M.; Bruno, G.; Cattelan, D.; Cobet, C.; de Martino, A.; Fleischer, K.; Dohcevic-Mitrovic, Z.; Esser, N.; Galliet, M.; et al. Spectroscopic ellipsometry and polarimetry for materials and systems analysis at the nanometer scale: State-of-the-art, potential, and perspectives. J. Nanopart. Res. 2009, 11, 1521–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logothetidis, S.; Gioti, M.; Gravalidis, C. Optical and electronic characterization on polymeric membranes. Synth. Met. 2003, 138, 369–374. [Google Scholar] [CrossRef]
- Vera, R.; Gelde, L.; Antico, E.; de Yuso, M.M.; Benavente, J.; Fontas, C. Tuning physicochemical, electrochemical and transport characteristics of polymer inclusion membrane by varying the counter-anion of the ionic liquid Aliquat 336. J. Membr. Sci. 2017, 529, 87–94. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of Impedance Spectroscopy. In Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; Barsoukov, E., Ed.; Wiley Online Library: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Fortunato, R.; Branco, L.C.; Afonso, C.A.M.; Benavente, J.; Crespo, J.G. Electrical impedance spectroscopy characterisation of supported ionic liquid membranes. J. Membr. Sci. 2006, 270, 42–49. [Google Scholar] [CrossRef]
- Vázquez, M.; Romero, V.; Fontas, C.; Antico, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical–chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.; Sobol, P.; Bomben, K.D. Handbook of X Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Praire, MN, USA, 1995. [Google Scholar]
- Zhao, M.; Cao, Y.; Liu, X.; Deng, J.; Li, D.; Gu, H. Effect of nitrogen atomic percentage on N+-bombarded MWCNTs in cytocompatibility and hemocompatibility. Nanoscale Res. Lett. 2014, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Benavente, J. Use of impedance spectroscopy for characterization of membranes and the effect of different modifications. In Membrane Modification: Technology and Applications; Hilal, N., Khayet, M., Wright, C.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Gelde, L.; Cuevas, A.L.; Benavente, J. Influence of Pore-Size/Porosity on Ion Transport and Static BSA Fouling for TiO2-Covered Nanoporous Alumina Membranes. Appl. Sci. 2021, 11, 5687. [Google Scholar] [CrossRef]
- Rene-Boisneuf, L.; Scaiano, J.C. Sensitivity versus Stability: Making Quantum Dots More Luminescent by Sulfur Photocuring without Compromising Sensor Response. Chem. Mater. 2008, 20, 6638–6642. [Google Scholar] [CrossRef]
- Available online: www.vaxasoftware.com (accessed on 10 December 2021).
- Hamizi, N.A.; Johan, M.R. Optical Properties of CdSe Quantum Dots via Non-TOP based Route. Int. J. Electrochem. Sci. 2012, 7, 8458–8467. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarra, M.; Cuevas, A.L.; Yuso, M.V.M.d.; Romero, R.; Alonso, B.; Casado, C.M.; Benavente, J. Optical and Physicochemical Characterizations of a Cellulosic/CdSe-QDs@S-DAB5 Film. Nanomaterials 2022, 12, 484. https://doi.org/10.3390/nano12030484
Algarra M, Cuevas AL, Yuso MVMd, Romero R, Alonso B, Casado CM, Benavente J. Optical and Physicochemical Characterizations of a Cellulosic/CdSe-QDs@S-DAB5 Film. Nanomaterials. 2022; 12(3):484. https://doi.org/10.3390/nano12030484
Chicago/Turabian StyleAlgarra, Manuel, Ana L. Cuevas, Ma Valle Martínez de Yuso, Rocío Romero, Beatriz Alonso, Carmen M. Casado, and Juana Benavente. 2022. "Optical and Physicochemical Characterizations of a Cellulosic/CdSe-QDs@S-DAB5 Film" Nanomaterials 12, no. 3: 484. https://doi.org/10.3390/nano12030484





