On-Surface Thermal Stability of a Graphenic Structure Incorporating a Tropone Moiety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Details
2.2. Experimental Methods
2.3. Sample Preparation
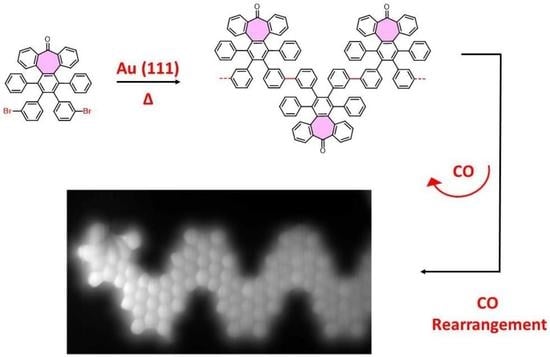
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, M.; Xiao, Y.; Liu, J.; Lu, W.; Fu, L. Controllable Fabrication of Nanostructured Graphene Towards Electronics. Adv. Electron. Mater. 2016, 2, 1500456. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Nguyen, V.H. Promising Applications of Graphene and Graphene-Based Nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 023002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wu, S.; Yang, R.; Zhang, G. Graphene: Nanostructure Engineering and Applications. Front. Phys. 2017, 12, 127206. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Li, L.S. Solution chemistry Approach to Graphene Nanostructures. Ph.D Thesis, Indiana University, Bloomington, IN, USA, 2013. [Google Scholar]
- Gourdon, A. On-Surface Covalent Coupling in Ultrahigh Vacuum. Angew. Chem. Int. Ed. 2008, 47, 6950–6953. [Google Scholar] [CrossRef]
- Gross, L.; Mohn, F.; Moll, N.; Liljeroth, P.; Meyer, G. The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy. Science 2009, 325, 1110–1114. [Google Scholar] [CrossRef] [Green Version]
- Méndez, J.; López, M.F.; Martín-Gago, J.A. On-Surface Synthesis of Cyclic Organic Molecules. Chem. Soc. Rev. 2011, 40, 4578–4590. [Google Scholar] [CrossRef]
- Clair, S.; de Oteyza, D.G. Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis. Chem. Rev. 2019, 119, 4717–4776. [Google Scholar] [CrossRef]
- Chen, Z.; Narita, A.; Müllen, K. Graphene Nanoribbons: On-Surface Synthesis and Integration into Electronic Devices. Adv. Mater. 2020, 32, 2001893. [Google Scholar] [CrossRef]
- Durr, R.A.; Haberer, D.; Lee, Y.-L.; Blackwell, R.; Kalayjian, A.M.; Marangoni, T.; Ihm, J.; Louie, S.G.; Fischer, F.R. Orbitally Matched Edge-Doping in Graphene Nanoribbons. J. Am. Chem. Soc. 2018, 140, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, G.D.; Tsai, H.-Z.; Omrani, A.A.; Marangoni, T.; Wu, M.; Rizzo, D.J.; Rodgers, G.F.; Cloke, R.R.; Durr, R.A.; Sakai, Y.; et al. Atomically Precise Graphene Nanoribbon Heterojunctions from a Single Molecular Precursor. Nat. Nanotechnol. 2017, 12, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sánchez, C.; Dienel, T.; Nicolaï, A.; Kharche, N.; Liang, L.; Daniels, C.; Meunier, V.; Liu, J.; Feng, X.; Müllen, K.; et al. On-Surface Synthesis and Characterization of Acene-Based Nanoribbons Incorporating Four-Membered Rings. Chem.-Eur. J. 2019, 25, 12074–12082. [Google Scholar] [CrossRef]
- Liu, M.; Liu, M.; She, L.; Zha, Z.; Pan, J.; Li, S.; Li, T.; He, Y.; Cai, Z.; Wang, J.; et al. Graphene-like Nanoribbons Periodically Embedded with Four- and Eight-Membered Rings. Nat. Commun. 2017, 8, 14924. [Google Scholar] [CrossRef] [Green Version]
- Di Giovannantonio, M.; Eimre, K.; Yakutovich, A.V.; Chen, Q.; Mishra, S.; Urgel, J.I.; Pignedoli, C.A.; Ruffieux, P.; Müllen, K.; Narita, A.; et al. On-Surface Synthesis of Antiaromatic and Open-Shell Indeno[2,1-b]Fluorene Polymers and Their Lateral Fusion into Porous Ribbons. J. Am. Chem. Soc. 2019, 141, 12346–12354. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Hou, I.C.-Y.; Eimre, K.; Pignedoli, C.A.; Ruffieux, P.; Narita, A.; Fasel, R. On-Surface Synthesis of Polyazulene with 2,6-Connectivity. Chem. Commun. 2019, 55, 13466–13469. [Google Scholar] [CrossRef]
- Fan, Q.; Martin-Jimenez, D.; Ebeling, D.; Krug, C.K.; Brechmann, L.; Kohlmeyer, C.; Hilt, G.; Hieringer, W.; Schirmeisen, A.; Gottfried, J.M. Nanoribbons with Nonalternant Topology from Fusion of Polyazulene: Carbon Allotropes beyond Graphene. J. Am. Chem. Soc. 2019, 141, 17713–17720. [Google Scholar] [CrossRef]
- Hieulle, J.; Carbonell-Sanromà, E.; Vilas-Varela, M.; Garcia-Lekue, A.; Guitián, E.; Peña, D.; Pascual, J.I. On-Surface Route for Producing Planar Nanographenes with Azulene Moieties. Nano Lett. 2018, 18, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Urgel, J.I.; Di Giovannantonio, M.; Segawa, Y.; Ruffieux, P.; Scott, L.T.; Pignedoli, C.A.; Itami, K.; Fasel, R. Negatively Curved Warped Nanographene Self-Assembled on Metal Surfaces. J. Am. Chem. Soc. 2019, 141, 13158–13164. [Google Scholar] [CrossRef]
- Liu, J.; Mishra, S.; Pignedoli, C.A.; Passerone, D.; Urgel, J.I.; Fabrizio, A.; Lohr, T.G.; Ma, J.; Komber, H.; Baumgarten, M.; et al. Open-Shell Nonbenzenoid Nanographenes Containing Two Pairs of Pentagonal and Heptagonal Rings. J. Am. Chem. Soc. 2019, 141, 12011–12020. [Google Scholar] [CrossRef]
- Kawasumi, K.; Zhang, Q.; Segawa, Y.; Scott, L.T.; Itami, K. A Grossly Warped Nanographene and the Consequences of Multiple Odd-Membered-Ring Defects. Nat. Chem. 2013, 5, 739–744. [Google Scholar] [CrossRef]
- Luo, J.; Xu, X.; Mao, R.; Miao, Q. Curved Polycyclic Aromatic Molecules That Are π-Isoelectronic to Hexabenzocoronene. J. Am. Chem. Soc. 2012, 134, 13796–13803. [Google Scholar] [CrossRef]
- Márquez, I.R.; Fuentes, N.; Cruz, C.M.; Puente-Muñoz, V.; Sotorrios, L.; Marcos, M.L.; Choquesillo-Lazarte, D.; Biel, B.; Crovetto, L.; Gómez-Bengoa, E.; et al. Versatile Synthesis and Enlargement of Functionalized Distorted Heptagon-Containing Nanographenes. Chem. Sci. 2017, 8, 1068–1074. [Google Scholar] [CrossRef] [Green Version]
- Cruz, C.M.; Castro-Fernández, S.; Maçôas, E.; Cuerva, J.M.; Campaña, A.G. Undecabenzo[7]Superhelicene: A Helical Nanographene Ribbon as a Circularly Polarized Luminescence Emitter. Angew. Chem. Int. Ed. 2018, 57, 14782–14786. [Google Scholar] [CrossRef]
- Cruz, C.M.; Márquez, I.R.; Castro-Fernández, S.; Cuerva, J.M.; Maçôas, E.; Campaña, A.G. A Triskelion-Shaped Saddle–Helix Hybrid Nanographene. Angew. Chem. Int. Ed. 2019, 58, 8068–8072. [Google Scholar] [CrossRef]
- Medel, M.A.; Tapia, R.; Blanco, V.; Miguel, D.; Morcillo, S.P.; Campaña, A.G. Octagon-Embedded Carbohelicene as a Chiral Motif for Circularly Polarized Luminescence Emission of Saddle-Helix Nanographenes. Angew. Chem. Int. Ed. 2021, 60, 6094–6100. [Google Scholar] [CrossRef]
- Márquez, I.R.; Castro-Fernández, S.; Millán, A.; Campaña, A.G. Synthesis of Distorted Nanographenes Containing Seven- and Eight-Membered Carbocycles. Chem. Commun. 2018, 54, 6705–6718. [Google Scholar] [CrossRef]
- Pun, S.H.; Miao, Q. Toward Negatively Curved Carbons. Acc. Chem. Res. 2018, 51, 1630–1642. [Google Scholar] [CrossRef]
- Cruz, C.M.; Márquez, I.R.; Mariz, I.F.A.; Blanco, V.; Sánchez-Sánchez, C.; Sobrado, J.M.; Martín-Gago, J.A.; Cuerva, J.M.; Maçôas, E.; Campaña, A.G. Enantiopure Distorted Ribbon-Shaped Nanographene Combining Two-Photon Absorption-Based Upconversion and Circularly Polarized Luminescence. Chem. Sci. 2018, 9, 3917–3924. [Google Scholar] [CrossRef] [Green Version]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Marangoni, T.; Haberer, D.; Rizzo, D.J.; Cloke, R.R.; Fischer, F.R. Heterostructures through Divergent Edge Reconstruction in Nitrogen-Doped Segmented Graphene Nanoribbons. Chem.-Eur. J. 2016, 22, 13037–13040. [Google Scholar] [CrossRef] [Green Version]
- Shekhirev, M.; Zahl, P.; Sinitskii, A. Phenyl Functionalization of Atomically Precise Graphene Nanoribbons for Engineering Inter-Ribbon Interactions and Graphene Nanopores. ACS Nano 2018, 12, 8662–8669. [Google Scholar] [CrossRef]
- Vo, T.H.; Perera, U.G.E.; Shekhirev, M.; Mehdi Pour, M.; Kunkel, D.A.; Lu, H.; Gruverman, A.; Sutter, E.; Cotlet, M.; Nykypanchuk, D.; et al. Nitrogen-Doping Induced Self-Assembly of Graphene Nanoribbon-Based Two-Dimensional and Three-Dimensional Metamaterials. Nano Lett. 2015, 15, 5770–5777. [Google Scholar] [CrossRef] [PubMed]
- Bebensee, F.; Svane, K.; Bombis, C.; Masini, F.; Klyatskaya, S.; Besenbacher, F.; Ruben, M.; Hammer, B.; Linderoth, T. Adsorption and Dehydrogenation of Tetrahydroxybenzene on Cu(111). Chem. Commun. 2013, 49, 9308–9310. [Google Scholar] [CrossRef] [PubMed]
- Ruiz del Árbol, N.; Palacio, I.; Otero-Irurueta, G.; Martínez, J.I.; de Andrés, P.L.; Stetsovych, O.; Moro-Lagares, M.; Mutombo, P.; Svec, M.; Jelínek, P.; et al. On-Surface Bottom-Up Synthesis of Azine Derivatives Displaying Strong Acceptor Behavior. Angew. Chem. Int. Ed. 2018, 57, 8582–8586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzmann, G.; Frenking, G.; Steiner, B. Thermal- and Electron Impact-Induced Decarbonylation of Tropones: A Comparison of Neutral and Radical-Cationic Pericyclic Reaction Mechanisms. J. Chem. Soc. Perkin Trans. 2 1984, 1943–1948. [Google Scholar] [CrossRef]
- Dastan, A.; Kilic, H.; Saracoglu, N. One Hundred Years of Benzotropone Chemistry. Beilstein J. Org. Chem. 2018, 14, 1120–1180. [Google Scholar] [CrossRef] [PubMed]
- McNamara, O.A.; Maguire, A.R. The Norcaradiene–Cycloheptatriene Equilibrium. Tetrahedron 2011, 67, 9–40. [Google Scholar] [CrossRef]
- West, R.; Kusuda, K.; Rao, V.N.M. Octachlorocycloheptatriene and Related Compounds. J. Am. Chem. Soc. 1971, 93, 3627–3632. [Google Scholar] [CrossRef]
- Kato, M.; Mitsuda, M.; Shibuya, T.; Furuichi, K. Synthesis of Benzo[1,2:4,5]Dicycloheptene-1,9-Dione via 6,7,12,13- Tetrahydro-7,12-Methano-3<I>H</I>-Cycloheptacyclodecene-3,14-Dione and Its Protonation Behavior. Bull. Chem. Soc. Jpn. 1991, 64, 2081–2087. [Google Scholar] [CrossRef] [Green Version]
- Mato, M.; Herlé, B.; Echavarren, A.M. Cyclopropanation by Gold- or Zinc-Catalyzed Retro-Buchner Reaction at Room Temperature. Org. Lett. 2018, 20, 4341–4345. [Google Scholar] [CrossRef]
- Mato, M.; García-Morales, C.; Echavarren, A.M. Generation of Gold(I) Carbenes by Retro-Buchner Reaction: From Cyclopropanes to Natural Products Synthesis. ChemCatChem 2019, 11, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yang, B.; Björk, J.; Zhong, Q.; Ju, H.; Zhang, J.; Cao, N.; Shi, Z.; Zhang, H.; Ebeling, D.; et al. Hierarchical Dehydrogenation Reactions on a Copper Surface. J. Am. Chem. Soc. 2018, 140, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Lin, H.; Zhang, J.; Zhang, H.; Li, Y.; Li, Q.; Chi, L. Mechanistic Investigations of the Au Catalysed C–H Bond Activations in on-Surface Synthesis. Phys. Chem. Chem. Phys. 2018, 20, 15901–15906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Perepichka, D.F.; Khaliullin, R.Z. Adatoms in the Surface-Confined Ullmann Coupling of Phenyl Groups. J. Phys. Chem. Lett. 2021, 12, 11061–11069. [Google Scholar] [CrossRef] [PubMed]
- Mio, M.J.; Kopel, L.C.; Braun, J.B.; Gadzikwa, T.L.; Hull, K.L.; Brisbois, R.G.; Markworth, C.J.; Grieco, P.A. One-Pot Synthesis of Symmetrical and Unsymmetrical Bisarylethynes by a Modification of the Sonogashira Coupling Reaction. Org. Lett. 2002, 4, 3199–3202. [Google Scholar] [CrossRef]
- Simonov, K.A.; Vinogradov, N.A.; Vinogradov, A.S.; Generalov, A.V.; Zagrebina, E.M.; Svirskiy, G.I.; Cafolla, A.A.; Carpy, T.; Cunniffe, J.P.; Taketsugu, T.; et al. From Graphene Nanoribbons on Cu(111) to Nanographene on Cu(110): Critical Role of Substrate Structure in the Bottom-Up Fabrication Strategy. ACS Nano 2015, 9, 8997–9011. [Google Scholar] [CrossRef]
- Di Giovannantonio, M.; Deniz, O.; Urgel, J.I.; Widmer, R.; Dienel, T.; Stolz, S.; Sánchez-Sánchez, C.; Muntwiler, M.; Dumslaff, T.; Berger, R.; et al. On-Surface Growth Dynamics of Graphene Nanoribbons: The Role of Halogen Functionalization. ACS Nano 2018, 12, 74–81. [Google Scholar] [CrossRef]
- Bronner, C.; Björk, J.; Tegeder, P. Tracking and Removing Br during the On-Surface Synthesis of a Graphene Nanoribbon. J. Phys. Chem. C 2015, 119, 486–493. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez, I.R.; Ruíz del Árbol, N.; Urgel, J.I.; Villalobos, F.; Fasel, R.; López, M.F.; Cuerva, J.M.; Martín-Gago, J.A.; Campaña, A.G.; Sánchez-Sánchez, C. On-Surface Thermal Stability of a Graphenic Structure Incorporating a Tropone Moiety. Nanomaterials 2022, 12, 488. https://doi.org/10.3390/nano12030488
Márquez IR, Ruíz del Árbol N, Urgel JI, Villalobos F, Fasel R, López MF, Cuerva JM, Martín-Gago JA, Campaña AG, Sánchez-Sánchez C. On-Surface Thermal Stability of a Graphenic Structure Incorporating a Tropone Moiety. Nanomaterials. 2022; 12(3):488. https://doi.org/10.3390/nano12030488
Chicago/Turabian StyleMárquez, Irene R., Nerea Ruíz del Árbol, José I. Urgel, Federico Villalobos, Roman Fasel, María F. López, Juan M. Cuerva, José A. Martín-Gago, Araceli G. Campaña, and Carlos Sánchez-Sánchez. 2022. "On-Surface Thermal Stability of a Graphenic Structure Incorporating a Tropone Moiety" Nanomaterials 12, no. 3: 488. https://doi.org/10.3390/nano12030488
APA StyleMárquez, I. R., Ruíz del Árbol, N., Urgel, J. I., Villalobos, F., Fasel, R., López, M. F., Cuerva, J. M., Martín-Gago, J. A., Campaña, A. G., & Sánchez-Sánchez, C. (2022). On-Surface Thermal Stability of a Graphenic Structure Incorporating a Tropone Moiety. Nanomaterials, 12(3), 488. https://doi.org/10.3390/nano12030488