Synthesis, Characterization, and Supercapacitor Performance of a Mixed-Phase Mn-Doped MoS2 Nanoflower
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Manganese Doped MoS2 (Mn-MoS2) Nanoflowers
2.3. Preparation of Working Electrodes
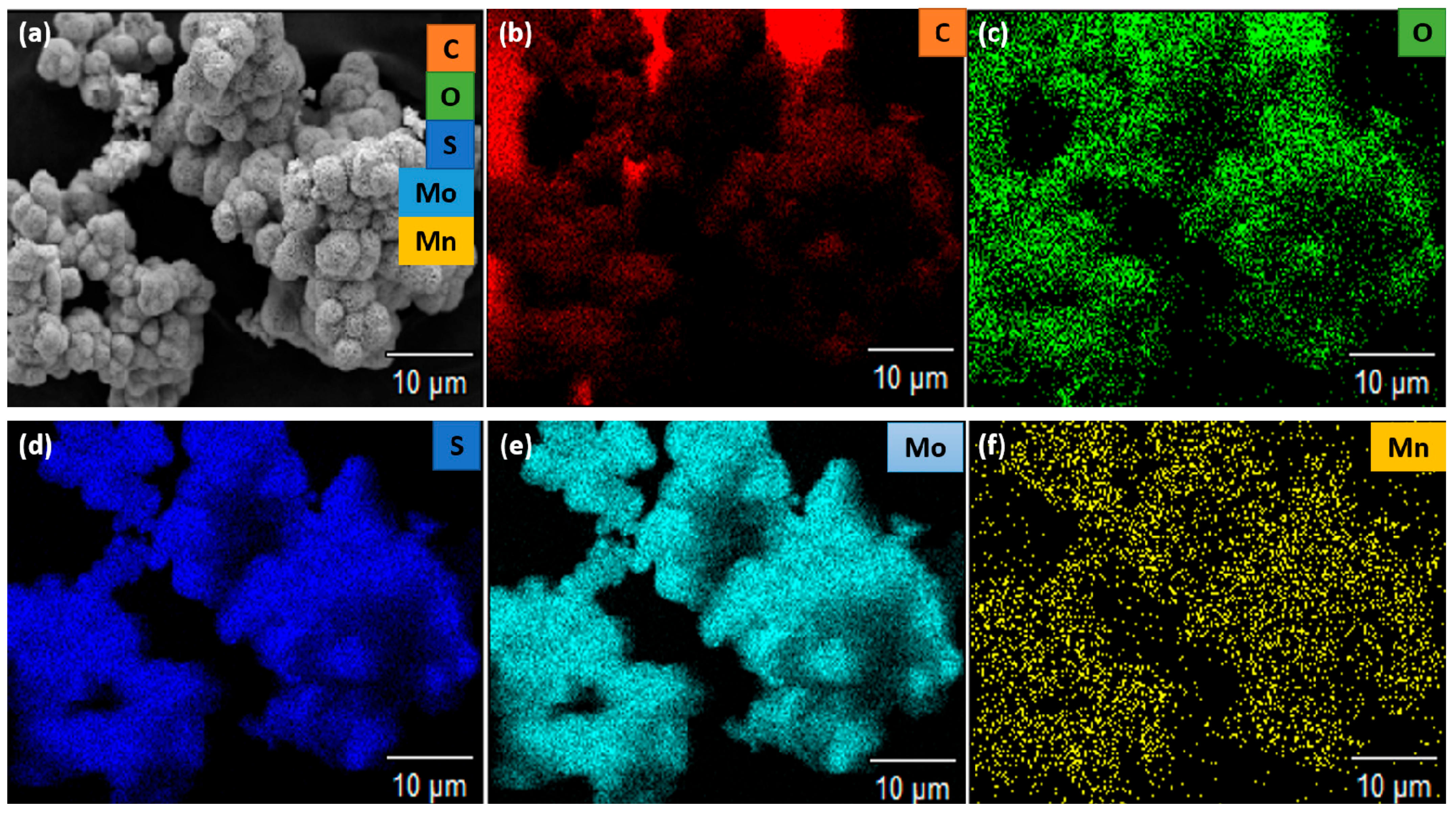
2.4. Characterizations
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mothkuri, S.; Chakrabarti, S.; Gupta, H.; Padya, B.; Rao, T.N.; Jain, P.K. Synthesis of MnO2 nano-flakes for high performance supercapacitor application. Mater. Today Proc. 2018, 26, 142–147. [Google Scholar] [CrossRef]
- Aihemaitituoheti, R.; Alhebshi, N.A.; Abudula, T. Effects of Precursors and Carbon Nanotubes on Electrochemical Properties of Electrospun Nickel Oxide Nanofibers-Based Supercapacitors. Molecules 2021, 26, 5656. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [Green Version]
- Bello, I.T.; Oladipo, O.A.; Adedokun, O.; Dhlamini, M.S. Recent advances on the preparation and electrochemical analysis of MoS 2 -based materials for supercapacitor applications: A mini-review. Mater. Today Commun. 2020, 25, 101664. [Google Scholar] [CrossRef]
- Bello, I.T.; Adio, S.A.; Oladipo, A.O.; Adedokun, O.; Mathevula, L.E.; Dhlamini, M.S. Molybdenum sulfide-based supercapacitors: From synthetic, bibliometric, and qualitative perspectives. Int. J. Energy Res. 2021, 45, 12665–12692. [Google Scholar] [CrossRef]
- Chia, X.; Eng, A.Y.S.; Ambrosi, A.; Tan, S.M.; Pumera, M. Electrochemistry of Nanostructured Layered Transition-Metal Dichalcogenides. Chem. Rev. 2015, 115, 11941–11966. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Chen, Y.; Yu, M.; Sun, T.; Lu, L.; Xie, T.; Zhang, Y.; Wu, Y. Hydrothermal synthesis of layered molybdenum sulfide/N-doped graphene hybrid with enhanced supercapacitor performance. Carbon N. Y. 2016, 99, 35–42. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Zhang, J.Z.; Wang, L.L.; Mo, Y.P. One-step preparation of layered molybdenum disulfide/multi-walled carbon nanotube composites for enhanced performance supercapacitor. Energy 2014, 67, 234–240. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Han, Y.; Li, J.; Yang, L.; Benamara, M.; Chen, L.; Zhu, H. Two-Dimensional Water-Coupled Metallic MoS2 with Nanochannels for Ultrafast Supercapacitors. Nano Lett. 2017, 17, 1825–1832. [Google Scholar] [CrossRef]
- Chan, P.Y.; Majid, S.R. Metal oxide-based electrode materials for supercapacitor applications. In Advanced Materials and Their Applications–Micro to Nano Scale; One Central Press: Altrincham, UK, 2012; pp. 13–30. ISBN 978-1-910086-21-6. [Google Scholar]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Soon, J.M.; Loh, K.P. Electrochemical double-layer capacitance of MoS2 nanowall films. Electrochem. Solid-State Lett. 2007, 10, 250–254. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Yang, Y. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Zhai, S.; Fan, Z.; Jin, K.; Zhou, M.; Zhao, H.; Zhao, Y.; Ge, F.; Li, X.; Cai, Z. Synthesis of zinc sulfide/copper sulfide/porous carbonized cotton nanocomposites for flexible supercapacitor and recyclable photocatalysis with high performance. J. Colloid Interface Sci. 2020, 575, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.F.; Yousef, A.K.M.; Hassan, A.; Hussain, S.; Ashiq, M.N.; Ul-Hassan, M.; Razaq, A. Significantly improved electrochemical characteristics of nickel sulfide nanoplates using graphene oxide thin film for supercapacitor applications. J. Energy Storage 2021, 33, 102091. [Google Scholar] [CrossRef]
- Zheng, L.; Teng, F.; Ye, X.; Zheng, H.; Fang, X. Photo/Electrochemical Applications of Metal Sulfide/TiO2 Heterostructures. Adv. Energy Mater. 2020, 10, 1–32. [Google Scholar] [CrossRef]
- Merki, D.; Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Chen, W. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Li, H.; Li, H.; Jiang, L.; Shi, Y.; Sun, Y.; Lu, G.; Zhang, Q.; Chen, X.; Zhang, H. Single-layer MoS2 phototransistors. ACS Nano 2012, 6, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Sha, C.; Lu, B.; Mao, H.; Cheng, J.; Pan, X.; Lu, J.; Ye, Z. 3D ternary nanocomposites of molybdenum disulfide/polyaniline/reduced graphene oxide aerogel for high performance supercapacitors. Carbon N. Y. 2016, 99, 26–34. [Google Scholar] [CrossRef]
- Xiao, J.; Choi, D.; Cosimbescu, L.; Koech, P.; Liu, J.; Lemmon, J.P. Exfoliated MoS2 nanocomposite as an anode material for lithium ion batteries. Chem. Mater. 2010, 22, 4522–4524. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Zheng, N.; Bu, X.; Feng, P. Synthetic design of crystalline inorganic chalcogenides exhibiting fast-ion conductivity. Nature 2003, 426, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, D.N.; Selvakumar, M. Active-defective activated carbon/MoS2 composites for supercapacitor and hydrogen evolution reactions. Appl. Surf. Sci. 2018, 453, 132–140. [Google Scholar] [CrossRef]
- Sangeetha, D.N.; Santosh, M.S.; Selvakumar, M. Flower-like carbon doped MoS2/Activated carbon composite electrode for superior performance of supercapacitors and hydrogen evolution reactions. J. Alloys Compd. 2020, 831, 154745. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, Y.; Wang, J.; Yang, W.; Xu, L.; Tian, X.; Shuang, Y. MoS2/MnO2 heterostructured nanodevices for electro-. Nano Res. 2018, 11, 4–7. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Ting, J.M. MoS2/MoOx-Nanostructure-Decorated Activated Carbon Cloth for Enhanced Supercapacitor Performance. ChemSusChem 2018, 11, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A.W. Characterization of MoS2-Graphene Composites for High-Performance Coin Cell Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 17388–17398. [Google Scholar] [CrossRef] [PubMed]
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In situ MoS2 Decoration of Laser-Induced Graphene as Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459–10465. [Google Scholar] [CrossRef]
- Da Silveira Firmiano, E.G.; Rabelo, A.C.; Dalmaschio, C.J.; Pinheiro, A.N.; Pereira, E.C.; Schreiner, W.H.; Leite, E.R. Supercapacitor electrodes obtained by directly bonding 2D MoS2 on reduced graphene oxide. Adv. Energy Mater. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Z.; Zhang, W.; Li, Z. Controlled synthesis of Ni(OH)2/MoS2 nanohybrids for high-performance supercapacitors. Mater. Chem. Phys. 2018, 209, 291–297. [Google Scholar] [CrossRef]
- Hao, C.; Wen, F.; Xiang, J.; Wang, L.; Hou, H.; Su, Z.; Hu, W.; Liu, Z. Controlled incorporation of Ni(OH)2 nanoplates into flowerlike MoS2 nanosheets for flexible all-solid-state supercapacitors. Adv. Funct. Mater. 2014, 24, 6700–6707. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Yin, H.; Zhao, H.; Wang, D.; Tang, Z. Growth of polypyrrole ultrathin films on mos2 monolayers as high-performance supercapacitor electrodes. Adv. Mater. 2015, 27, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.H. Three-Dimensional Tubular MoS2/PANI Hybrid Electrode for High Rate Performance Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 28294–28302. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Jalili, R.; Wang, C.; Zheng, T.; Chao, Y.; Wallace, G.G. A robust free-standing MoS2/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) film for supercapacitor applications. Electrochim. Acta 2017, 235, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Zhang, G.; Cui, Y.; Sun, Y.; Qin, Q.; Zhang, J.; Zheng, W. One-step extended strategy for the ionic liquid-assisted synthesis of Ni3S4-MoS2 heterojunction electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 11278–11285. [Google Scholar] [CrossRef]
- Huang, F.; Meng, R.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; He, Y. One-step hydrothermal synthesis of a CoS2@MoS2 nanocomposite for high-performance supercapacitors. J. Alloys Compd. 2018, 742, 844–851. [Google Scholar] [CrossRef]
- Palanisamy, S.; Periasamy, P.; Subramani, K.; Shyma, A.P.; Venkatachalam, R. Ultrathin sheet structure Ni-MoS2 anode and MnO2/water dispersion graphene cathode for modern asymmetrical coin cell supercapacitor. J. Alloys Compd. 2018, 731, 936–944. [Google Scholar] [CrossRef]
- Falola, B.D.; Fan, L.; Wiltowski, T.; Suni, I.I. Electrodeposition of Cu-Doped MoS2 for Charge Storage in Electrochemical Supercapacitors. J. Electrochem. Soc. 2017, 164, D674–D679. [Google Scholar] [CrossRef]
- Shao, J.; Li, Y.; Zhong, M.; Wang, Q.; Luo, X.; Li, K.; Zhao, W. Enhanced-performance flexible supercapacitor based on Pt-doped MoS2. Mater. Lett. 2019, 252, 173–177. [Google Scholar] [CrossRef]
- Singha, S.S.; Rudra, S.; Mondal, S.; Pradhan, M.; Nayak, A.K.; Satpati, B.; Pal, P.; Das, K.; Singha, A. Mn incorporated MoS2 nanoflowers: A high performance electrode material for symmetric supercapacitor. Electrochim. Acta 2020, 338. [Google Scholar] [CrossRef]
- Sun, A.; Xie, L.; Wang, D.; Wu, Z. Enhanced energy storage performance from Co-decorated MoS2 nanosheets as supercapacitor electrode materials. Ceram. Int. 2018, 44, 13434–13438. [Google Scholar] [CrossRef]
- Rohith, R.; Manuraj, M.; Jafri, R.I.; Rakhi, R.B. Co-MoS2 nanoflower coated carbon fabric as a flexible electrode for supercapacitor. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, X.; Wang, H.; Liu, G.; Wang, G.; Zhang, H.; Zhao, H. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862. [Google Scholar] [CrossRef] [Green Version]
- Kushima, A.; Qian, X.; Zhao, P.; Zhang, S.; Li, J. Ripplocations in van der Waals layers. Nano Lett. 2015, 15, 1302–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singha, S.S.; Mondal, S.; Bhattacharya, T.S.; Das, L.; Sen, K.; Satpati, B.; Das, K.; Singha, A. Au nanoparticles functionalized 3D-MoS2 nanoflower: An efficient SERS matrix for biomolecule sensing. Biosens. Bioelectron. 2018, 119, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T.; et al. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, F.; Yang, S.; Li, Y.; Zhao, C.; Xu, M.; Zhang, Y.; Zeng, H. Robust ferromagnetism in Mn-doped MoS2 nanostructures. Appl. Phys. Lett. 2016, 109, 1–6. [Google Scholar] [CrossRef]
- Rasamani, K.D.; Alimohammadi, F.; Sun, Y. Interlayer-expanded MoS2. Mater. Today 2017, 20, 83–91. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, W.; Shen, R.; Ye, J.; Qin, Z.; Chao, Y. Three-dimensionally Ordered Macroporous Structure Enabled Nanothermite Membrane of Mn2O3/Al. Sci. Rep. 2016, 6, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groen, J.C.; Pérez-Ramírez, J. Critical appraisal of mesopore characterization by adsorption analysis. Appl. Catal. A Gen. 2004, 268, 121–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Z.; Dai, C.; Zhou, X.; Chen, W. Interfacial thermodynamics and kinetics of sorption of diclofenac on prepared high performance flower-like MoS2. J. Colloid Interface Sci. 2016, 481, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.B.; Alhebshi, N.A.; Anjum, D.H.; Alshareef, H.N. Nanostructured cobalt sulfide-on-fiber with tunable morphology as electrodes for asymmetric hybrid supercapacitors. J. Mater. Chem. A 2014, 2, 16190–16198. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhu, J.; Zhang, X.; San Hui, K.; Hui, K.N. 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials. J. Mater. Chem. A 2013, 1, 9046–9053. [Google Scholar] [CrossRef]
- Dubal, D.P.; Fulari, V.J.; Lokhande, C.D. Effect of morphology on supercapacitive properties of chemically grown β-Ni(OH)2 thin films. Microporous Mesoporous Mater. 2012, 151, 511–516. [Google Scholar] [CrossRef]
- Li, N.; Zhu, X.; Zhang, C.; Lai, L.; Jiang, R.; Zhu, J. Controllable synthesis of different microstructured MnO2 by a facile hydrothermal method for supercapacitors. J. Alloys Compd. 2017, 692, 26–33. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials–Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Element Line | Weight % | Norm. Wt.% | Atom % | Formula | Compnd % | Norm. Compnd. % |
|---|---|---|---|---|---|---|
| C K | 26.7 | 26.7 | 57.3 | C | 26.7 | 26.7 |
| O K | 16.5 | 16.5 | 26.4 | O | 16.5 | 16.5 |
| Mn K | 5.4 | 5.4 | 2.5 | Mn | 5.4 | 5.4 |
| Mo L | 51.4 | 51.4 | 13.8 | Mo | 51.4 | 51.4 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Current Density (A/g) | Specific Capacitance (F/g) | Energy Density (Wh/kg) | Power Density(W/kg) |
|---|---|---|---|
| 1 | 70.37 | 2.07 | 257.14 |
| 2 | 58.89 | 2.29 | 529.69 |
| 3 | 57.69 | 2.31 | 805.85 |
| 5 | 44.11 | 2.58 | 1454.52 |
| 10 | 29.90 | 3.14 | 4346.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, I.T.; Otun, K.O.; Nyongombe, G.; Adedokun, O.; Kabongo, G.L.; Dhlamini, M.S. Synthesis, Characterization, and Supercapacitor Performance of a Mixed-Phase Mn-Doped MoS2 Nanoflower. Nanomaterials 2022, 12, 490. https://doi.org/10.3390/nano12030490
Bello IT, Otun KO, Nyongombe G, Adedokun O, Kabongo GL, Dhlamini MS. Synthesis, Characterization, and Supercapacitor Performance of a Mixed-Phase Mn-Doped MoS2 Nanoflower. Nanomaterials. 2022; 12(3):490. https://doi.org/10.3390/nano12030490
Chicago/Turabian StyleBello, Ismaila T., Kabir O. Otun, Gayi Nyongombe, Oluwaseun Adedokun, Guy L. Kabongo, and Mokhotjwa S. Dhlamini. 2022. "Synthesis, Characterization, and Supercapacitor Performance of a Mixed-Phase Mn-Doped MoS2 Nanoflower" Nanomaterials 12, no. 3: 490. https://doi.org/10.3390/nano12030490
APA StyleBello, I. T., Otun, K. O., Nyongombe, G., Adedokun, O., Kabongo, G. L., & Dhlamini, M. S. (2022). Synthesis, Characterization, and Supercapacitor Performance of a Mixed-Phase Mn-Doped MoS2 Nanoflower. Nanomaterials, 12(3), 490. https://doi.org/10.3390/nano12030490







