Mollification of Doxorubicin (DOX)-Mediated Cardiotoxicity Using Conjugated Chitosan Nanoparticles with Supplementation of Propionic Acid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Chitosan Nanoparticles (CNPs) and DOX-Conjugated Chitosan Nanoparticles (DCNPs)
2.3. Drug Loading Efficacy
2.4. Characterization Studies
2.5. Experimental Animals
2.6. Experimental Design
2.7. Evaluation of ECG Alterations
2.8. Biochemical and Antioxidant Analyses
2.9. RNA Isolation and qPCR Analysis
2.10. Histological Examination
2.11. Statistical Analysis
3. Results and Discussion
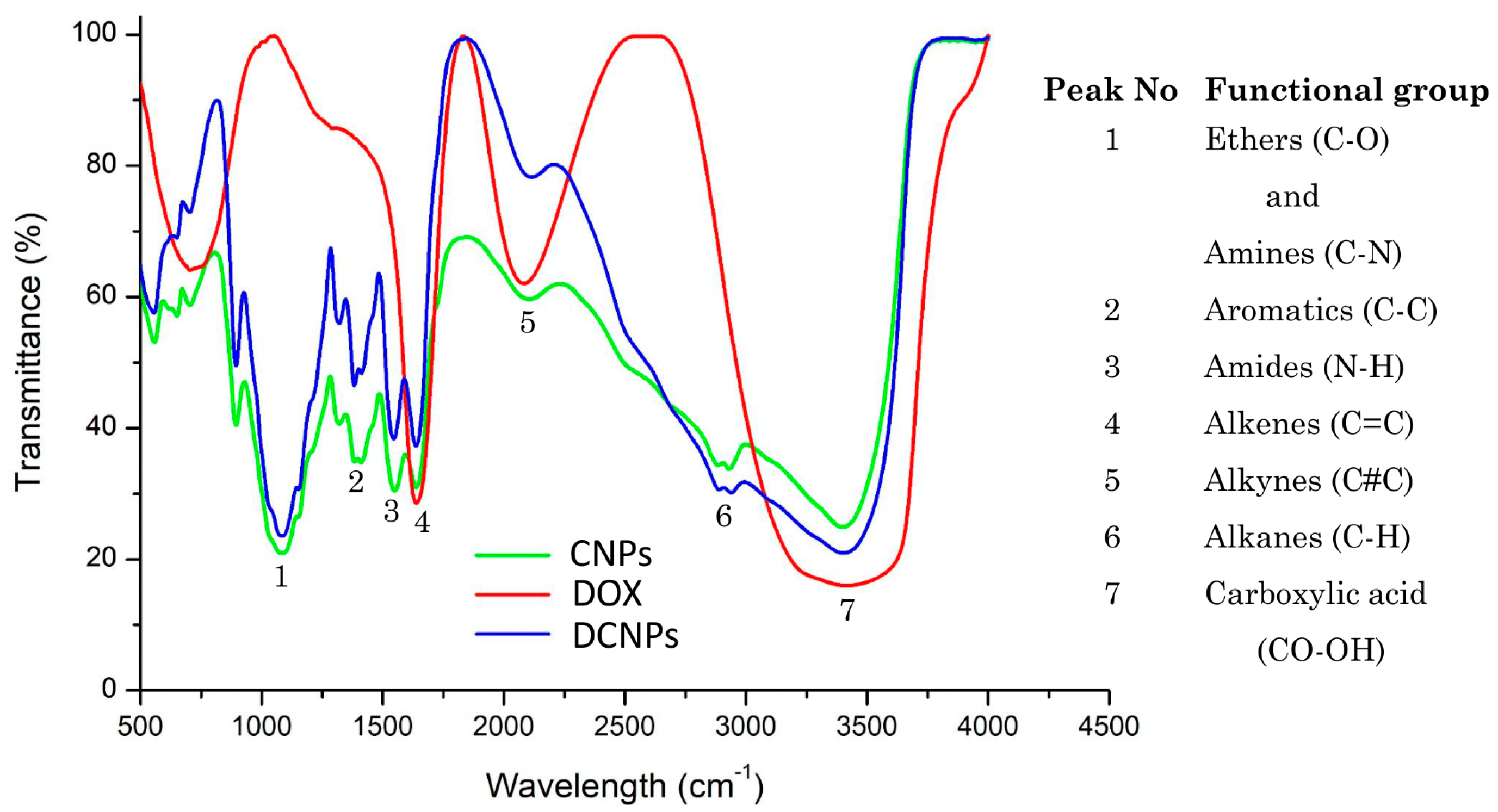
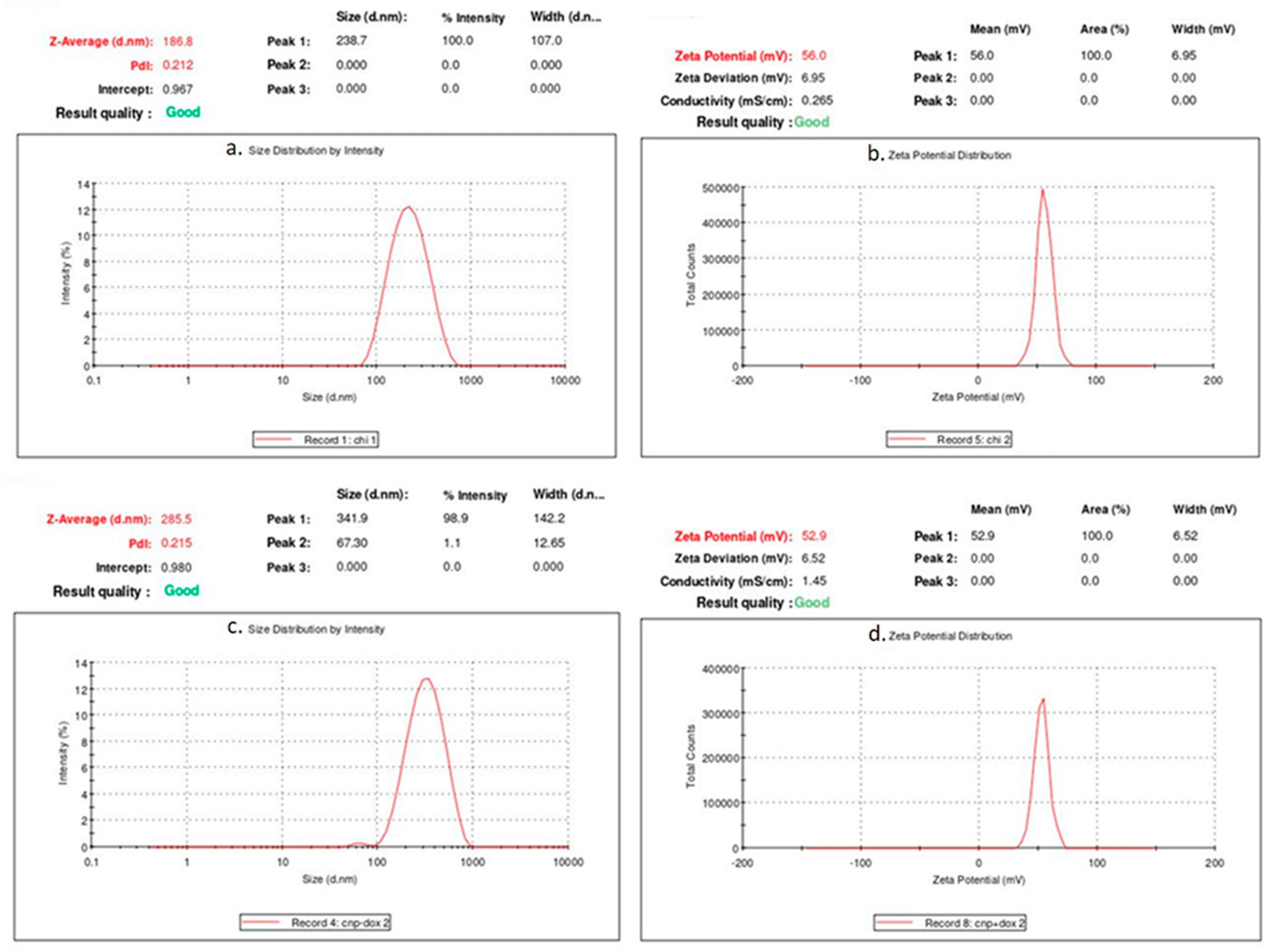
3.1. Characterization of CNPs and DCNPs
3.2. Changes in Body Weight
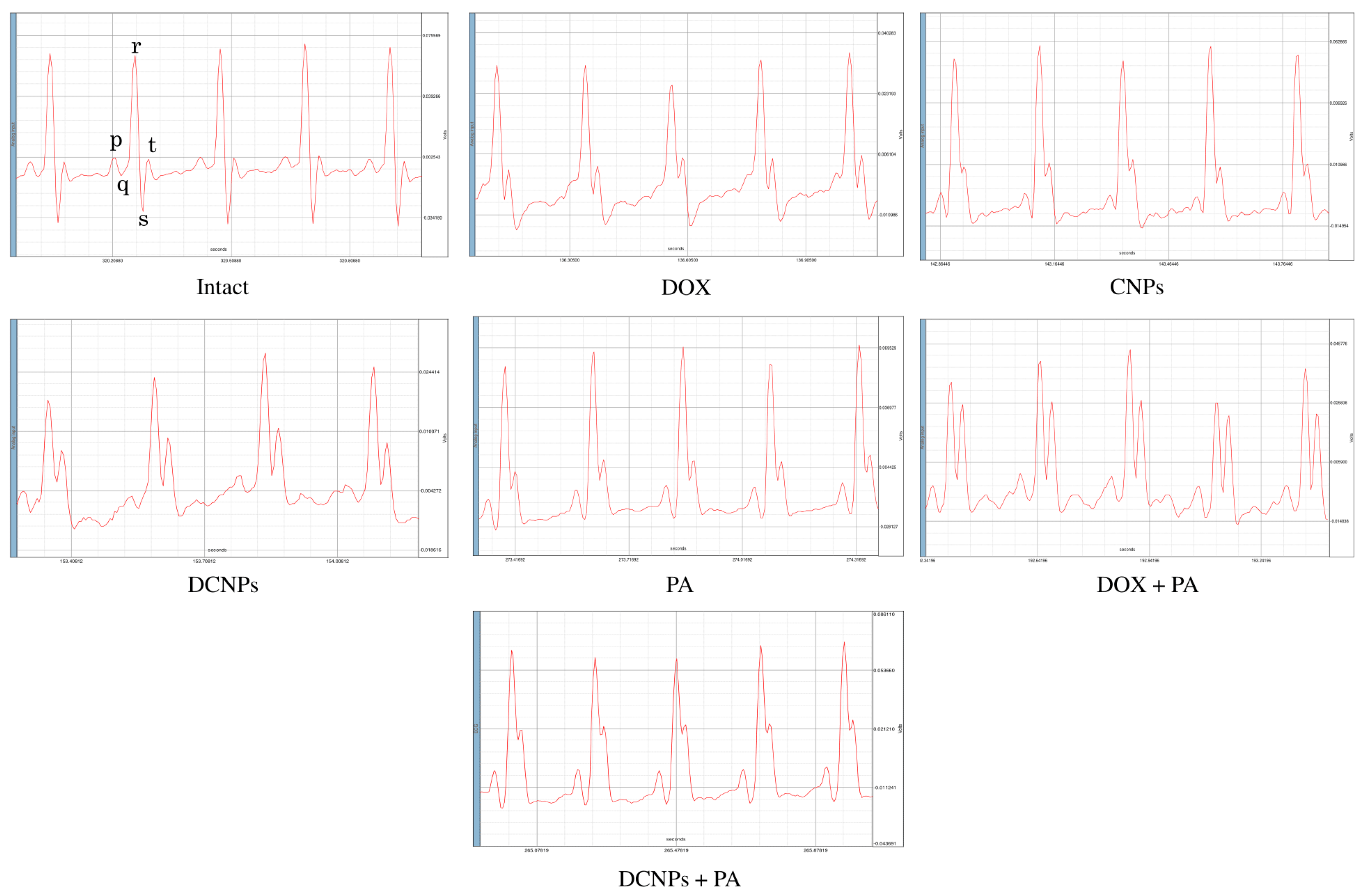
3.3. Electrocardiography
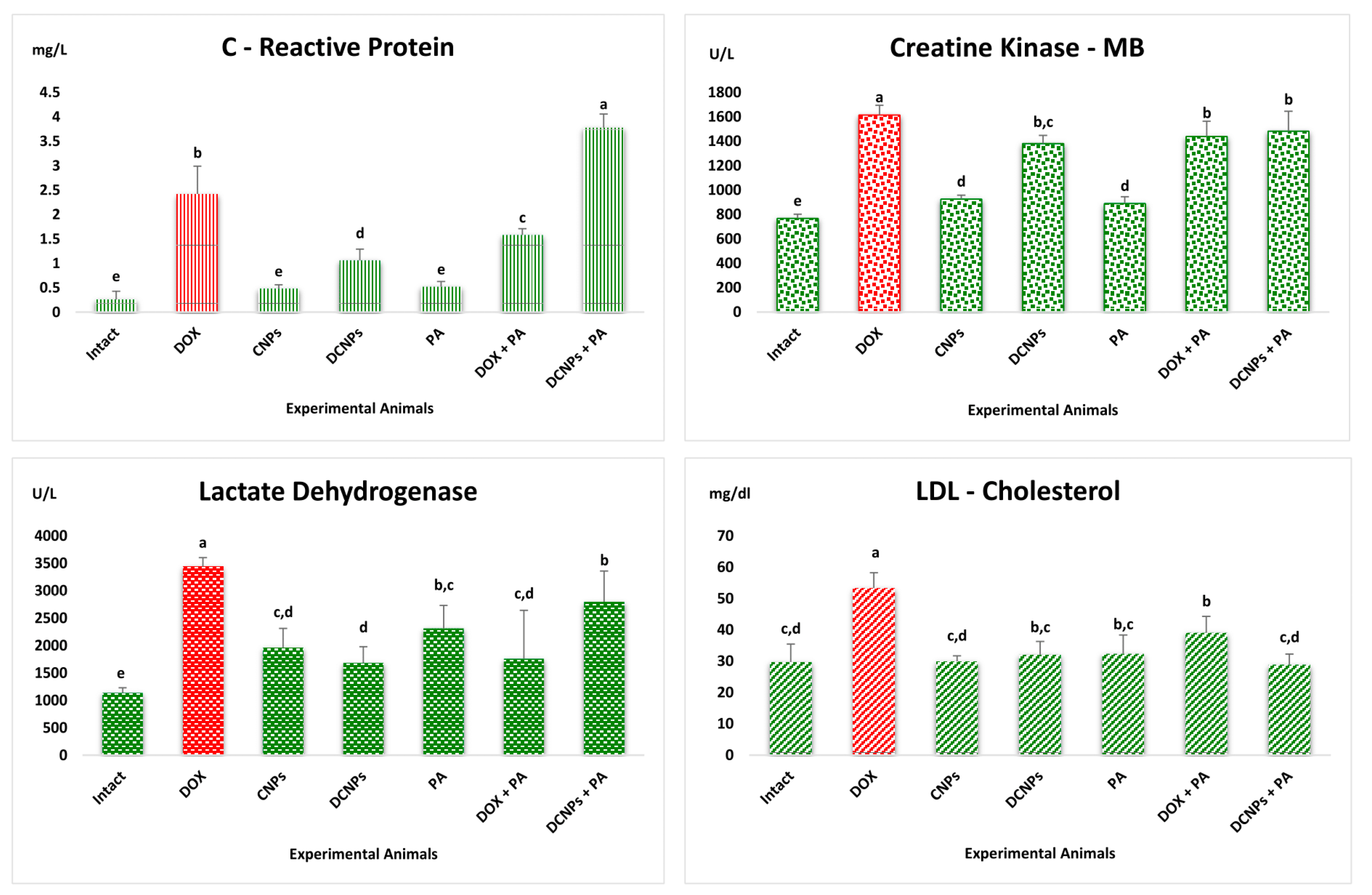
3.4. Analysis of Cardiac Biomarkers
3.5. Antioxidants Assays
3.6. Histological Observation
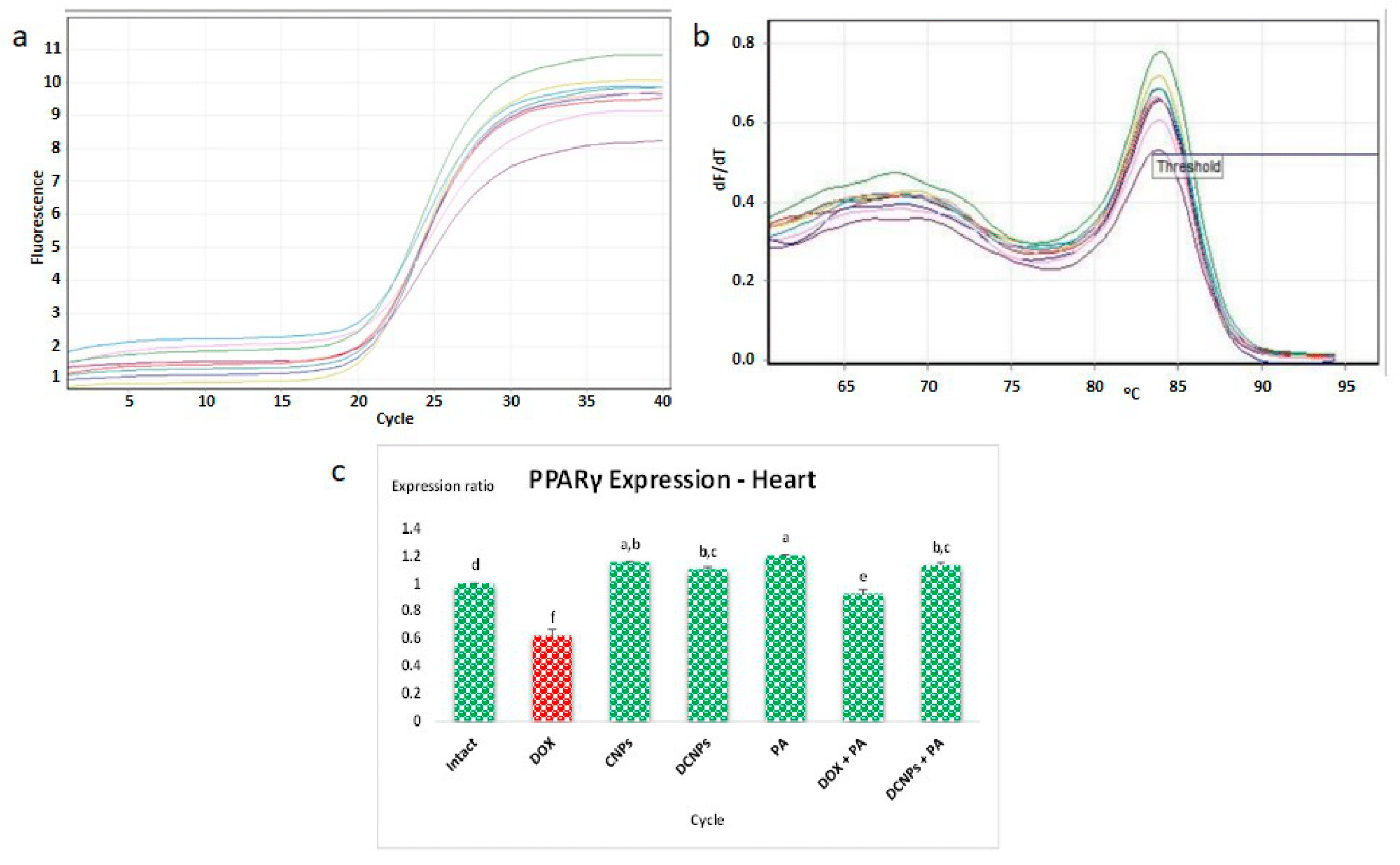
3.7. qPCR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassinelli, G. The roots of modern oncology: From discovery of new antitumor anthracyclines to their clinical use. Tumori 2016, 102, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Iliskovic, N.; Li, T.; Kumar, D. Adriamycin cardiomyopathy: Pathophysiology and prevention. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1997, 11, 931–936. [Google Scholar] [CrossRef]
- Wu, J.; Hu, X.; Liu, R.; Zhang, J.; Song, A.; Luan, Y. pH-responsive and self-targeting assembly from hyaluronic acid-based conjugate toward all-in-one chemo-photodynamic therapy. J. Colloid Interface Sci. 2019, 547, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-induced heart failure: Mechanism and modulation. Mol. Cell. Biochem. 2000, 207, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, S.; Tirupathi Pichiah, P.B.; Achiraman, S. Doxorubicin treatment inhibits PPARγ and may induce lipotoxicity by mimicking a type 2 diabetes-like condition in rodent models. FEBS Lett. 2013, 587, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phielix, E.; Szendroedi, J.; Roden, M. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol. Sci. 2011, 32, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, J.; Zheng, Y.; Guo, R.; Wang, S.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Shi, X. Doxorubicin-conjugated PAMAM dendrimers for pH-responsive drug release and folic acid-targeted cancer therapy. Pharmaceutics 2018, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Kang, K.H.; Je, J.Y.; Pham, H.N.D.; Byun, H.G.; Kim, S.K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- López-León, T.; Carvalho, E.L.S.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Lei, J.; Yang, L.; Zhan, Y.; Wang, Y.; Ye, T.; Li, Y.; Deng, H.; Li, B. Plasma treated polyethylene terephthalate/polypropylene films assembled with chitosan and various preservatives for antimicrobial food packaging. Colloids Surf. B Biointerfaces 2014, 114, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, J.; Motion, R.; Kulonen, H.; Rättö, M.; Skyttä, E.; Ahvenainen, R. Chitosan-coated paper: Effects of nisin and different acids on the antimicrobial activity. J. Appl. Polym. Sci. 2004, 94, 986–993. [Google Scholar] [CrossRef]
- Zhang, H.L.; Tao, Y.; Guo, J.; Hu, Y.M.; Su, Z.Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011, 11, 457–461. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; He, W.; Zhao, D.; Song, A.; Luan, Y. Disulfide-Linked Amphiphilic Polymer-Docetaxel Conjugates Assembled Redox-Sensitive Micelles for Efficient Antitumor Drug Delivery. Biomacromolecules 2016, 17, 1621–1632. [Google Scholar] [CrossRef]
- Mattaveewong, T.; Wongkrasant, P.; Chanchai, S.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-κB and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef]
- Sugano, M.; Watanabe, S.; Kishi, A.; Izume, M.; Ohtakara, A. Hypocholesterolemic action of chitosans with different viscosity in rats. Lipids 1988, 23, 187–191. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shan, K.; Liu, J.; Tao, X.; Periyasamy, S.; Durairaj, S.; Jiang, Z.; Jacob, J.A. Synthesis, optimization and characterization of silver nanoparticles using the catkin extract of Piper longum for bactericidal effect against food-borne pathogens via conventional and mathematical approaches. Bioorg. Chem. 2020, 103, 104230. [Google Scholar] [CrossRef]
- RathnaKumari, P.; Kolanchinathan, P.; Siva, D.; Abirami, B.; Masilamani, V.; John, G.; Achiraman, S.; Balasundaram, A. Antibacterial efficacy of seagrass Cymodocea serrulata-engineered silver nanoparticles against prawn pathogen Vibrio parahaemolyticus and its combative effect on the marine shrimp Penaeus monodon. Aquaculture 2018, 493, 158–164. [Google Scholar] [CrossRef]
- Siva, D.; Srivethi, G.; Vasan, P.T.; Rajesh, D.; Alfarhan, A.; Rajagopal, R. Enhanced cellulase enzyme production by Aspergillus niger using cellulase/iron oxide magnetic nano-composites. J. King Saud Univ.-Sci. 2022, 34, 101695. [Google Scholar] [CrossRef]
- Renu, K.; Abilash, V.G.; Tirupathi, T.P.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef] [PubMed]
- MARKLUND, S.; MARKLUND, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Pulicharla, R.; Marques, C.; Das, R.K.; Rouissi, T.; Brar, S.K. Encapsulation and release studies of strawberry polyphenols in biodegradable chitosan nanoformulation. Int. J. Biol. Macromol. 2016, 88, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.J.; Sivalingam, P.; Siva, D.; Kamalakkannan, S.; Anbarasu, K.; Sukirtha, R.; Krishnan, M.; Achiraman, S. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B Biointerfaces 2011, 88, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.; Bikiaris, D.; Avgoustakis, K.; Karavas, E.; Georgarakis, M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr. Polym. 2008, 73, 44–54. [Google Scholar] [CrossRef]
- Burnett, C.J.; Li, C.; Webber, E.; Tsaousidou, E.; Xue, S.Y.; Brüning, J.C.; Krashes, M.J. Hunger-Driven Motivational State Competition. Neuron 2016, 92, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugan, K.; Lam, H.R.; Knudsen, C.B.; Petersen, J.S. Atrial fibrillation in rats induced by rapid transesophageal atrial pacing during brief episodes of asphyxia: A new in vivo model. J. Cardiovasc. Pharmacol. 2004, 44, 125–135. [Google Scholar] [CrossRef]
- Nattel, S.; Shiroshita-Takeshita, A.; Brundel, B.J.J.M.; Rivard, L. Mechanisms of atrial fibrillation: Lessons from animal models. Prog. Cardiovasc. Dis. 2005, 48, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Approach to asymptomatic creatine kinase elevation. Clevel. Clin. J. Med. 2016, 83, 37–42. [Google Scholar] [CrossRef]
- Cota, D.; Rasal, V.; Mishra, S.; Shengule, S. Cardioprotective effect of oregano oil against doxorubicin-induced myocardial infarction in rats. Pharmacogn. Mag. 2018, 14, 363–368. [Google Scholar] [CrossRef]
- Walsh, A.M.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Multi-Functional Roles of Chitosan as a Potential Protective Agent against Obesity. PLoS ONE 2013, 8, e53828. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jung, J.S.; Lee, S.H.; Kim, Y.W. Effects of antioxidants and Ca2+ in cisplatin-induced cell injury in rabbit renal cortical slices. Toxicol. Appl. Pharmacol. 1997, 146, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, S.E.; Alarabi, O.M.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Khan, L.M.; Osman, A.M.M. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014, 10, 1455–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifinasab, Z.; Banaee, M.; Mohiseni, M.; Noori, A. The protective role of vitamin C and Chitosan against paraquatinduced oxidative stress in muscles of common carp (Cyprinus carpio). Ribar. Croat. J. Fish. 2016, 74, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Iliskovic, N.; Singal, P.K. Lipid lowering: An important factor in preventing adriamycin-induced heart failure. Am. J. Pathol. 1997, 150, 727–734. [Google Scholar]
- Rizzo, M.; Giglio, R.V.; Nikolic, D.; Patti, A.M.; Campanella, C.; Cocchi, M.; Katsiki, N.; Montalto, G. Effects of chitosan on plasma lipids and lipoproteins: A 4-month prospective pilot study. Angiology 2013, 65, 538–542. [Google Scholar] [CrossRef] [Green Version]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-Chain Fatty Acids Suppress Cholesterol Synthesis in Rat Liver and Intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef] [Green Version]
- Antony, J.J.; Sithika, M.A.A.; Joseph, T.A.; Suriyakalaa, U.; Sankarganesh, A.; Siva, D.; Kalaiselvi, S.; Achiraman, S. In vivo antitumor activity of biosynthesized silver nanoparticles using Ficus religiosa as a nanofactory in DAL induced mice model. Colloids Surf. B Biointerfaces 2013, 108, 185–190. [Google Scholar] [CrossRef]
- Mohammed Asik, R.; Manikkaraja, C.; Tamil Surya, K.; Suganthy, N.; Priya Aarthy, A.; Mathe, D.; Sivakumar, M.; Archunan, G.; Padmanabhan, P.; Gulyas, B. Anticancer Potential of L-Histidine-Capped Silver Nanoparticles against Human Cervical Cancer Cells (SiHA). Nanomaterials 2021, 11, 3154. [Google Scholar] [CrossRef]
- Omóbòwálé, T.O.; Oyagbemi, A.A.; Folasire, A.M.; Ajibade, T.O.; Asenuga, E.R.; Adejumobi, O.A.; Ola-Davies, O.E.; Oyetola, O.; James, G.; Adedapo, A.A.; et al. Ameliorative effect of gallic acid on doxorubicin-induced cardiac dysfunction in rats. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 19–27. [Google Scholar] [CrossRef]


| Intact | DOX | CNPs | DCNPs | PA | DOX + PA | DCNPs + PA | |
|---|---|---|---|---|---|---|---|
| Body Weight changes | |||||||
| Initial (g) | 161 ± 11 c | 162 ± 13 b,c | 178 ± 17 b | 173 ± 15 b,c | 160 ± 12 c | 160 ± 12 c | 176 ± 10 b,c |
| Final (g) | 254 ± 21 b,c | 227 ± 21 c,d | 250 ± 34 b,c,d | 290 ± 16 a | 265 ± 13 a,b | 224 ± 27 d | 245 ± 15 b,c,d |
| Antioxidants | |||||||
| Superoxide Dismutase (U/mg Protein) | 14.7 ± 0.8 a | 5.5 ± 0.6 d | 10.9 ± 1.8 b | 8.0 ± 0.8 c | 11.9 ± 0.7 b | 7.9 ± 0.0 c | 6.1 ± 0.07 d |
| Glutathione S Transferase (U/mg Protein) | 0.043 ± 0.0 a | 0.011 ± 0.0 d | 0.032 ± 0.0 b | 0.035 ± 0.0 b | 0.031 ± 0.0 b | 0.024 ± 0.0 c | 0.033 ± 0.0 b |
| Malondialdehyde (n mol/mg Protein) | 0.86 ± 0.13 e | 3.02 ± 0.68 a | 0.62 ± 0.09 e | 1.56 ± 0.20 d | 0.56 ± 0.09 e | 2.03 ± 0.14 c | 0.54 ± 0.23 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siva, D.; Abinaya, S.; Rajesh, D.; Archunan, G.; Padmanabhan, P.; Gulyás, B.; Achiraman, S. Mollification of Doxorubicin (DOX)-Mediated Cardiotoxicity Using Conjugated Chitosan Nanoparticles with Supplementation of Propionic Acid. Nanomaterials 2022, 12, 502. https://doi.org/10.3390/nano12030502
Siva D, Abinaya S, Rajesh D, Archunan G, Padmanabhan P, Gulyás B, Achiraman S. Mollification of Doxorubicin (DOX)-Mediated Cardiotoxicity Using Conjugated Chitosan Nanoparticles with Supplementation of Propionic Acid. Nanomaterials. 2022; 12(3):502. https://doi.org/10.3390/nano12030502
Chicago/Turabian StyleSiva, Durairaj, Subramanian Abinaya, Durairaj Rajesh, Govindaraju Archunan, Parasuraman Padmanabhan, Balázs Gulyás, and Shanmugam Achiraman. 2022. "Mollification of Doxorubicin (DOX)-Mediated Cardiotoxicity Using Conjugated Chitosan Nanoparticles with Supplementation of Propionic Acid" Nanomaterials 12, no. 3: 502. https://doi.org/10.3390/nano12030502
APA StyleSiva, D., Abinaya, S., Rajesh, D., Archunan, G., Padmanabhan, P., Gulyás, B., & Achiraman, S. (2022). Mollification of Doxorubicin (DOX)-Mediated Cardiotoxicity Using Conjugated Chitosan Nanoparticles with Supplementation of Propionic Acid. Nanomaterials, 12(3), 502. https://doi.org/10.3390/nano12030502








