Hybrid Polyethylene Glycol/Sodium Metaphosphate Composites Prepared via Coacervation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coacervate Formation
2.2. Dried Coacervate Sample Preparation
2.3. Sample Characterization
3. Results
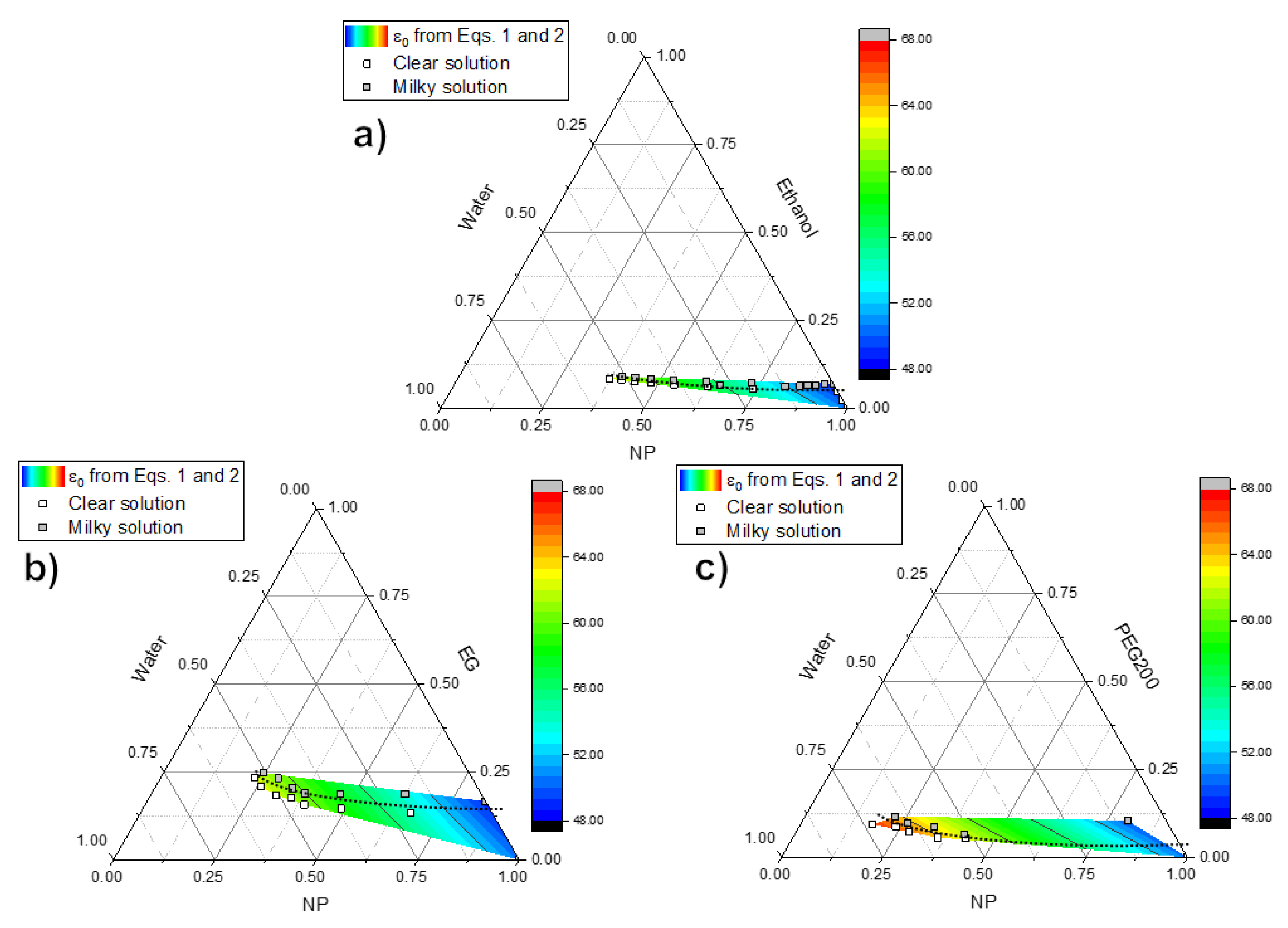
3.1. Phase Separation and Coacervate Formation
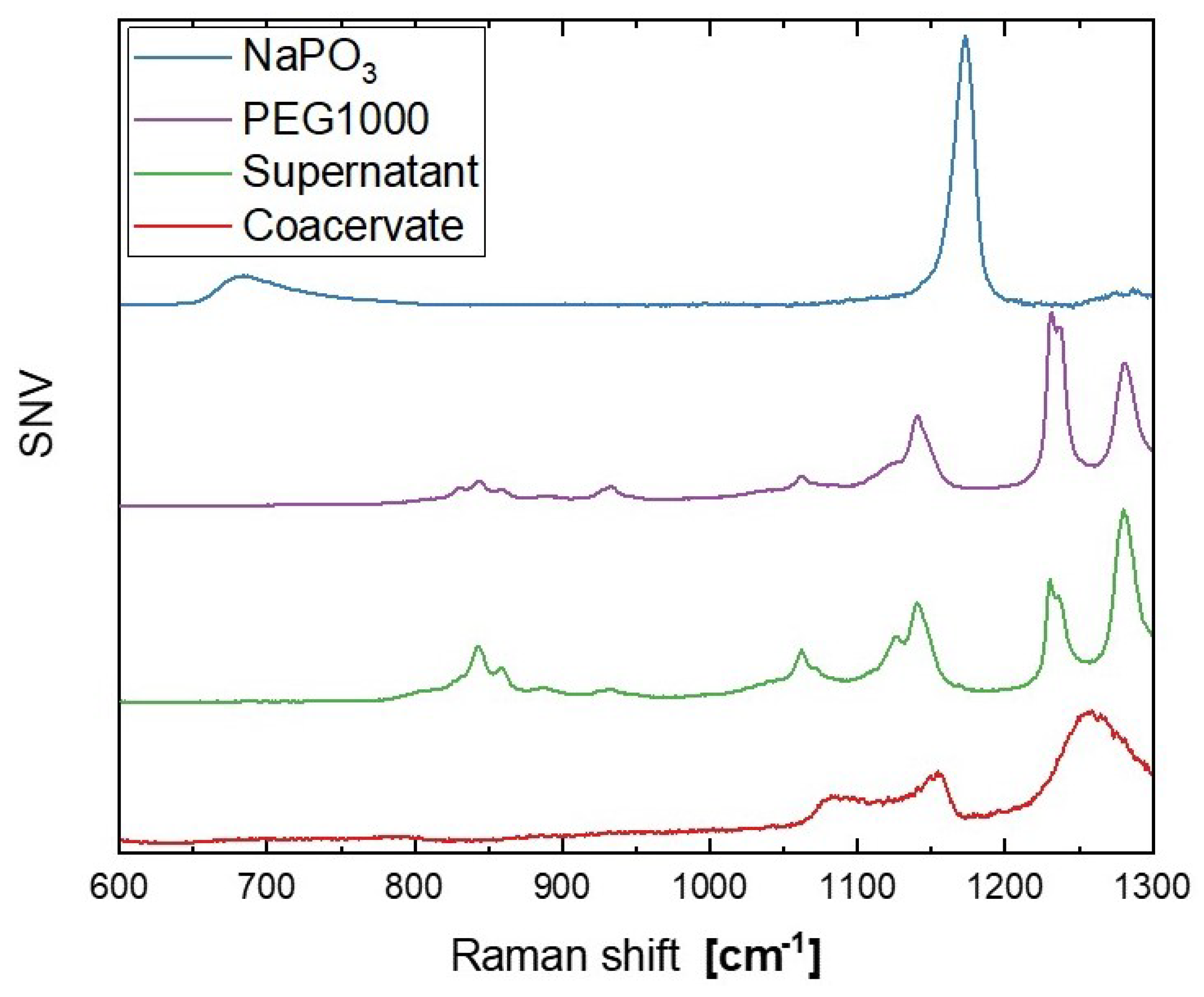
3.2. Raman Spectra
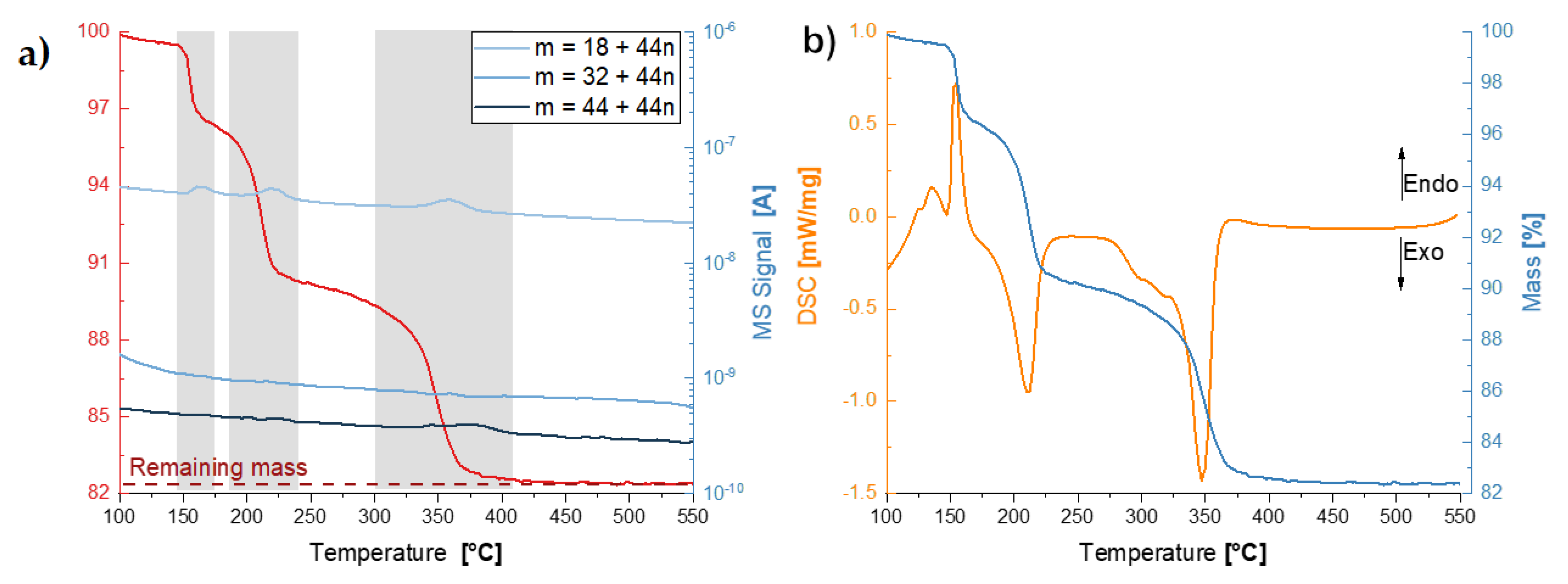
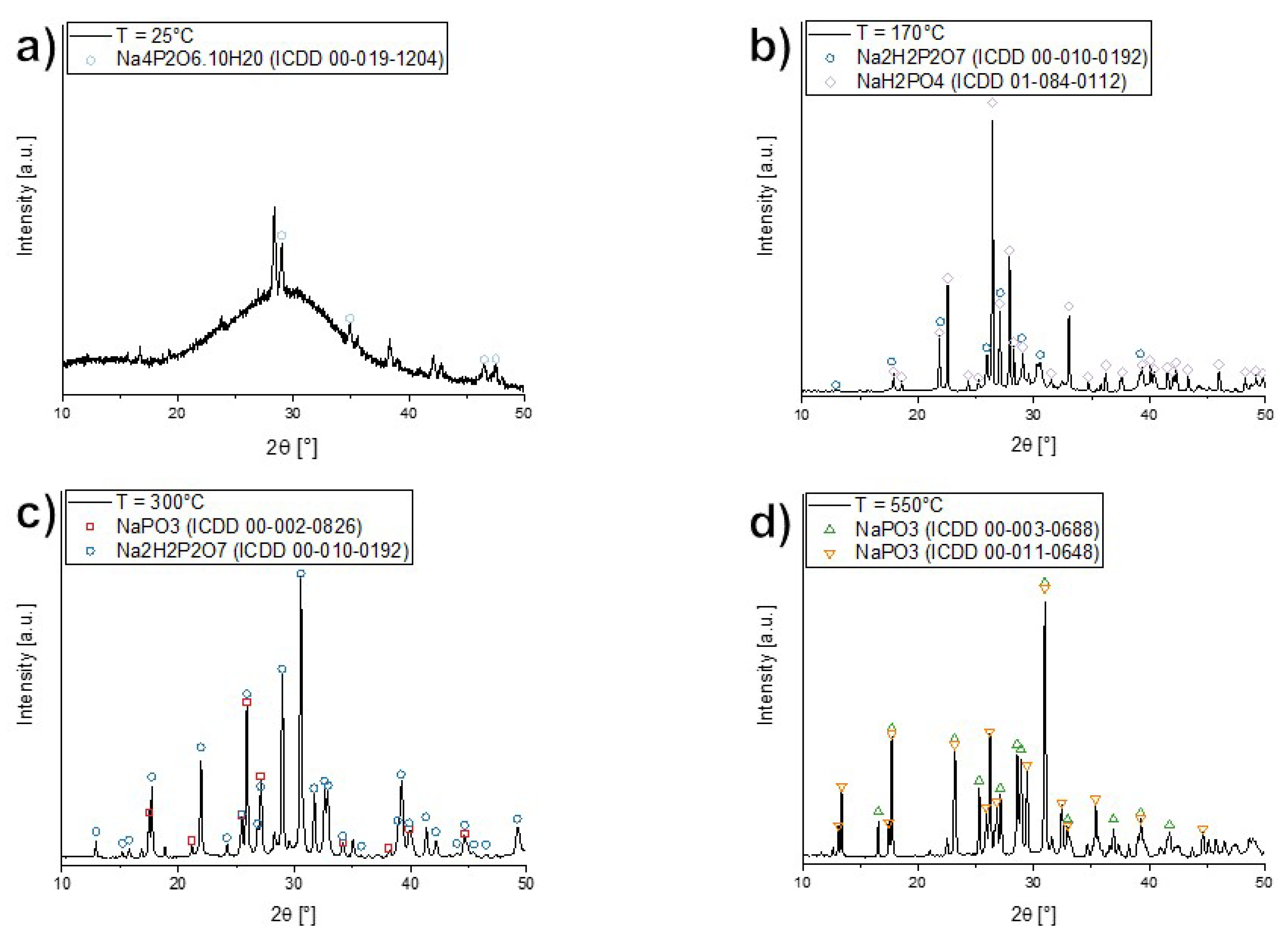
3.3. Thermal Analyses
3.4. Complex Impedance Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bu, J.; Leung, P.; Huang, C.; Lee, S.H.; Grant, P.S. Co-spray printing of LiFePO4 and PEO-Li1.5Al0.5Ge1.5(PO4)3 hybrid electrodes for all-solid-state Li-ion battery applications. J. Mater. Chem. A 2019, 7, 19094–19103. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, S.; Zheng, Y.; Li, C.Y. Morphology control in semicrystalline solid polymer electrolytes for lithium batteries. Mol. Syst. Des. Eng. 2019, 4, 793–803. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A Long Cycle Life, All-Solid-State Lithium Battery with a Ceramic–Polymer Composite Electrolyte. ACS Appl. Energy Mater. 2020, 3, 2916–2924. [Google Scholar] [CrossRef]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Yu, H.; Lu, J. Solid electrolytes and interfaces in all-solid-state sodium batteries: Progress and perspective. Nano Energy 2018, 52, 279–291. [Google Scholar] [CrossRef]
- Yu, X.; Xue, L.; Goodenough, J.B.; Manthiram, A. All-Solid-State Sodium Batteries with a Polyethylene Glycol Diacrylate–Na3Zr2Si2PO12 Composite Electrolyte. Adv. Energy Sustain. Res. 2020, 2, 2000061. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yang, J.; Wang, J.; Hirano, S.I. Polymer electrolytes for rechargeable lithium metal batteries. Sustain. Energy Fuels 2020, 4, 5469–5487. [Google Scholar] [CrossRef]
- Sing, C.E.; Perry, S.L. Recent progress in the science of complex coacervation. Soft Matter 2020, 16, 2885–2914. [Google Scholar] [CrossRef] [Green Version]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Arshady, R. Microspheres and microcapsules, a survey of manufactuing techniques Part II: Coacervation. Polym. Eng. Sci. 1990, 30, 905–914. [Google Scholar] [CrossRef]
- Umegaki, T.; Nakayama, Y.; Kanazawa, T. Thermal Change of Magnesium Highpolyphosphate Coacervates. Bull. Chem. Soc. Jpn. 1976, 49, 2105–2107. [Google Scholar] [CrossRef] [Green Version]
- Umegaki, T.; Kanazawa, T. Degradation of Magnesium and Calcium Highpolyphosphate Coacervates. Bull. Chem. Soc. Jpn. 1979, 52, 2124–2126. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, L.F.C.; Silva, M.A.P.; Brandão, A.R.; Stephani, R.; de Oliveira, C.I.R.; Gonçalves, R.R.; Barbosa, A.J.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J.L. Amorphous manganese polyphosphates: Preparation, characterization and incorporation of azo dyes. J. Sol-Gel Sci. Technol. 2009, 50, 158–163. [Google Scholar] [CrossRef]
- de Oliveira, C.I.R.; de Oliveira, L.F.C.; Dias Filho, F.A.; Messaddeq, Y.; Ribeiro, S.J.L. Spectroscopic investigation of a new hybrid glass formed by the interaction between croconate ion and calcium polyphosphate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.P.; Franco, D.F.; de Oliveira, L.F.C. New Insight on the Structural Trends of Polyphosphate Coacervation Processes. J. Phys. Chem. A 2008, 112, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Cini, N.; Ball, V. Polyphosphates as inorganic polyelectrolytes interacting with oppositely charged ions, polymers and deposited on surfaces: Fundamentals and applications. Adv. Colloid Interface Sci. 2014, 209, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Filiaggi, M.J. Comprehensive Study of the Chelation and Coacervation of Alkaline Earth Metals in the Presence of Sodium Polyphosphate Solution. Langmuir 2014, 30, 5256–5266. [Google Scholar] [CrossRef]
- Willot, G.; Gomez, F.; Vast, P.; Andries, V.; Martines, M.; Messaddeq, Y.; Poulain, M. Preparation of zinc sodium polyphosphates glasses from coacervates precursors. Characterisation of the obtained glasses, and their applications. C. R. Chim. 2002, 5, 899–906. [Google Scholar] [CrossRef]
- Franco, D.F.; Barud, H.S.; Santagneli, S.; Lamarca, R.S.; Santos, B.F.; Silva, M.A.P.; de Oliveira, L.F.C.; Ribeiro, S.J.L.; Nalin, M. Preparation and structural characterization of sodium polyphosphate coacervate as a precursor for optical materials. Mater. Chem. Phys. 2016, 180, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, R.; Jahani, F. Salting-In and Salting-Out of Water-Soluble Polymers in Aqueous Salt Solutions. J. Phys. Chem. B 2012, 116, 5234–5241. [Google Scholar] [CrossRef]
- Pickup, D.M.; Newport, R.J.; Barney, E.R.; Kim, J.Y.; Valappil, S.P.; Knowles, J.C. Characterisation of phosphate coacervates for potential biomedical applications. J. Biomater. Appl. 2014, 28, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Kyffin, B.A.; Foroutan, F.; Raja, F.N.S.; Martin, R.A.; Pickup, D.M.; Taylor, S.E.; Carta, D. Antibacterial silver-doped phosphate-based glasses prepared by coacervation. J. Mater. Chem. B 2019, 7, 7744–7755. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.F.; Barud, H.G.O.; Barud, H.S.; Oliveira Junior, O.B.; Meneguin, A.B.; de Oliveira, L.F.C.; Silva, M.A.P.; Ribeiro, S.J.L.; Nalin, M. A review on polyphosphate coacervates—Structural properties and bioapplications. J. Sol-Gel Sci. Technol. 2020, 94, 531–543. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–88. [Google Scholar] [CrossRef]
- Kim, C.W.; Someren, J.T.; Kirshen, M.; Rha, C. Steric Exclusion of Salts by Polyethylene Glycol. Phys. Chem. Liq. 1988, 18, 11–20. [Google Scholar] [CrossRef]
- Kim, C.W.; Rha, C. Phase Separation of Polyethylene Glycol/Salt Aqueous Two-Phase Systems. Phys. Chem. Liq. 2000, 38, 181–191. [Google Scholar] [CrossRef]
- Willauer, H.D.; Hddleston, J.G.; Rogers, R.D. Solute Partitioning in Aqueous Biphasic Systems Composed of Polyethylene Glycol and Salt: The Partitioning of Small Neutral Organic Species. Ind. Eng. Chem. Res. 2002, 41, 1892–1904. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; Hussain, H.I.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQueen, L.; Lai, D. Ionic Liquid Aqueous Two-Phase Systems From a Pharmaceutical Perspective. Front. Chem. 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, B.P.; Limbach, R.; de Souza, G.B.; Ebendorff-Heidepriem, H.; Wondraczek, L. Correlation between ionic mobility and plastic flow events in NaPO3-NaCl-Na2SO4 glasses. Front. Mater. 2019, 6, 128. [Google Scholar] [CrossRef]
- Wang, P.; Anderko, A. Computation of dielectric constants of solvent mixtures and electrolyte solutions. Fluid Phase Equilibria 2001, 186, 103–122. [Google Scholar] [CrossRef]
- Christensen, J.H.; Smith, A.J.; Reed, R.B.; Elmore, K.L. Dielectric properties of phosphoric acid solutions at 25 °C. J. Chem. Eng. Data 1966, 11, 60–63. [Google Scholar] [CrossRef]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z.G. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar] [CrossRef]
- Adam, O.E.A.; Al-Dujaili, A.H.; Awwad, A.M. Volumetric properites of aqueous solutions of ethylene glycols in the temperature range of 293.15 K–318.15 K. Int. Sch. Res. Not. 2014, 2014, 639813. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Kaur, K.; Chaudhary, R. Dielectric properties of low molecular weight poly(ethylene glycol)s. Polym. Int. 2000, 2000, 599–608. [Google Scholar] [CrossRef]
- Chaudhary, N.; Nain, A.K. Densities, speed of sound, refractive indices, excess and partial molar properties of polythylene glycol 200 + benzyl methacrylate binary mixtures at temperatures from 293.15 K to 318.15 K. J. Mol. Liq. 2021, 2021, 117923. [Google Scholar] [CrossRef]
- Shucker, R.; Gammon, R.W. Raman-scattering selection-rule breaking and the density of states in amorphous materials. Phys. Rev. Lett. 1970, 25, 222–225. [Google Scholar] [CrossRef]
- Liland, K.H.; Kohler, A.; Afseth, N.K. Model-based pre-processing in Raman spectroscopy of biological samples. J. Raman Spectrosc. 2016, 47, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Barkusov, E.; Macdonald, J.R. (Eds.) Impedance Spectroscopy: Theory, Experiment and Applications, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Almond, D.P.; Hunter, C.C.; West, A.R. The extraction of ionic conductivities and hopping rates from a.c. conductivity data. J. Mater. Sci. 1984, 19, 3236–3248. [Google Scholar] [CrossRef]
- Hudgens, J.J.; Brow, R.K.; Tallant, D.R.; Martin, S.W. Raman spectroscopy study of the structure of lithium and sodium ultraphosphate glasses. J. Non-Cryst. Solids 1998, 223, 21–31. [Google Scholar] [CrossRef]
- Koenig, J.L.; Angood, A.C. Raman spectra of poly(ethylene glycols) in solution. J. Polym. Sci. Part A-2 Polym. Phys. 1970, 8, 1787–1796. [Google Scholar] [CrossRef]
- Samuel, A.Z.; Umapathy, S. Energy funneling and macromolecular conformational dynamics: A 2D Raman correlation study of PEG melting. Polym. J. 2014, 46, 330–336. [Google Scholar] [CrossRef]
- Lattimer, R.P. Mass spectral analysis of low-temperature pyrolysis products from poly(ethylene glycol). J. Anal. Appl. Pyrolysis 2000, 56, 61–78. [Google Scholar] [CrossRef]
- Vrandecic, N.S.; Erceg, M.; Jakic, M.; Klaric, I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim. Acta 2010, 498, 71–80. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer 1997, 38, 317–323. [Google Scholar] [CrossRef]
- de Jager, H.J.; Prinsloo, L.C. The dehydration of phosphates monitored by DSC/TGA and in situ Raman spectroscopy. Thermochim. Acta 2001, 376, 187–196. [Google Scholar] [CrossRef]
- Marple, M.A.T.; Avila-Paredes, H.; Kim, S.; Sen, S. Atomistic interpretation of the ac-dc crossover frequency in crystalline and glassy ionic conductors. J. Chem. Phys. 2018, 148, 204507. [Google Scholar] [CrossRef] [PubMed]
- Noskov, S.Y.; Lamoureux, G.; Roux, B. Molecular dynamics study of hydration in ethanol-water mixtures using a polarizable force field. J. Phys. Chem. B 2005, 109, 6705–6713. [Google Scholar] [CrossRef]
- Bieze, T.W.N.; Barnes, A.C.; Huige, C.J.M.; Enderby, J.E.; Leyte, J.C. Distribution of water around poly(ethylene oxide): A neutron diffraction study. J. Phys. Chem. 1994, 98, 6568–6576. [Google Scholar] [CrossRef]
- Shikata, T.; Takahashi, R.; Sakamoto, A. Hydration of poly(ethylene oxide)s in aqueous solution as studied by dielectric relaxation measurements. J. Phys. Chem. B 2006, 110, 8941–8945. [Google Scholar] [CrossRef]
- Kjellander, R.; Florin, E. Water structure and changes in thermal stability of the system poly(ethylene oxide)-water. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 9, 2053–2077. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, L.; Gao, X.; Cao, Z.; Wang, Q. Accurate quantification of hydration number of polyethylene glycol molecules. Chin. Phys. B 2018, 27, 055101. [Google Scholar] [CrossRef]
- Demos, S.G.; Raman, R.N.; Yang, S.T.; Negres, S.A.; Schaffers, K.I.; Henesian, M.A. Measurement of the Raman scattering cross section of the breathing mode in KDP and DKDP crystals. Opt. Express 2011, 19, 21050–21059. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Maeda, T.E.; Misra, A.K.; Porter, J.N.; Bates, D.E.; Sharma, S.K. Remote Raman Efficiencies and Cross-Sections of Organic and Inorganic Chemicals. Appl. Spectrosc. 2016, 71, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Boschin, A.; Johansson, P. Characterization of NaX (X: TFSI, FSI)—PEO based solid polymer electrolytes for sodium batteries. Electrochim. Acta 2015, 175, 124–133. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Bisquert, J. Entropy factor in the hopping frequency for ionic conduction in oxide glasses induced by energetic clustering. J. Chem. Phys. 2005, 123, 074504. [Google Scholar] [CrossRef]
- Exarhos, G.J.; Miller, P.J.; Risen, W.M., Jr. Interionic vibrations and glass transitions in ionic oxide metaphosphate glasses. J. Chem. Phys. 1974, 60, 4145–4155. [Google Scholar] [CrossRef]
- Palmer, R.G.; Stein, D.L.; Abrahams, E.; Anderson, P.W. Models of Hierarchically Constrained Dynamics for Glassy Relaxation. Phys. Rev. Lett. 1984, 53, 958–961. [Google Scholar] [CrossRef]
- Cruje, C.; Chithrani, D.B. Polyethylene Glycol Density and Length Affects Nanoparticle Uptake by Cancer Cells. J. Nanomed. Res. 2014, 1, 00006. [Google Scholar] [CrossRef] [Green Version]
- Shoar Abouzari, M.R.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements. Solid State Ionics 2009, 180, 922–927. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Fratuer, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films: II. Applications. J. Electrochem. Soc. 2010, 157, C458–C463. [Google Scholar] [CrossRef]
- Anderson, O.L.; Stuart, D.A. Calculation of Activation Energy of Ionic Conductivity in Silica Glasses by Classical Methods. J. Am. Ceram. Soc. 1954, 37, 573–580. [Google Scholar] [CrossRef]
- Nascimento, M.L.F. Test of the Anderson–Stuart model and correlation between free volume and the ‘universal’ conductivity in sodium silicate glasses. J. Mater. Sci. 2007, 42, 3841–3850. [Google Scholar] [CrossRef] [Green Version]


| (g/) | () | p | ||
|---|---|---|---|---|
| NP | 19.72 | 50.4 £ | 11.1 | |
| Water | 0.997 † | 18.02 | 80.1 † | 17.69 |
| Ethanol | 0.789 † | 58.39 | 25.3 † | 5.51 |
| EG | 1.106 ‡ | 56.11 | 41.2 ¶ | 9.04 |
| PEG200 | 1.120 § | 178.57 | 22.1 ¶ | 4.79 |
| Temperature (°C) | R (MΩ) | Q (p) | (μS/m) | (kHz) | |
|---|---|---|---|---|---|
| 25 | 3.4 ± 0.5 | 60 ± 10 | 0.82 ± 0.01 | 11.90 ± 0.05 | 13.8 |
| 30 | 2.4 ± 0.3 | 100 ± 10 | 0.80 ± 0.01 | 16.83 ± 0.06 | 16.8 |
| 35 | 1.8 ± 0.3 | 110 ± 10 | 0.797 ± 0.009 | 22.82 ± 0.09 | 22.0 |
| 40 | 1.4 ± 0.2 | 120 ± 10 | 0.797 ± 0.007 | 29.2 ± 0.1 | 27.1 |
| 45 | 1.1 ± 0.2 | 140 ± 10 | 0.799 ± 0.006 | 36.9 ± 0.1 | 33.1 |
| 50 | 0.9 ± 0.1 | 140 ± 10 | 0.804 ± 0.006 | 46.4 ± 0.1 | 41.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, B.P.; de Macedo, G.N.B.M.; Xia, Y.; Balducci, A.; Wondraczek, L. Hybrid Polyethylene Glycol/Sodium Metaphosphate Composites Prepared via Coacervation. Nanomaterials 2022, 12, 528. https://doi.org/10.3390/nano12030528
Rodrigues BP, de Macedo GNBM, Xia Y, Balducci A, Wondraczek L. Hybrid Polyethylene Glycol/Sodium Metaphosphate Composites Prepared via Coacervation. Nanomaterials. 2022; 12(3):528. https://doi.org/10.3390/nano12030528
Chicago/Turabian StyleRodrigues, Bruno Poletto, Guilherme Nunes Braga Maurício de Macedo, Yang Xia, Andrea Balducci, and Lothar Wondraczek. 2022. "Hybrid Polyethylene Glycol/Sodium Metaphosphate Composites Prepared via Coacervation" Nanomaterials 12, no. 3: 528. https://doi.org/10.3390/nano12030528







