Preparation, Characterization, and Evaluation of Pyraclostrobin Nanocapsules by In Situ Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
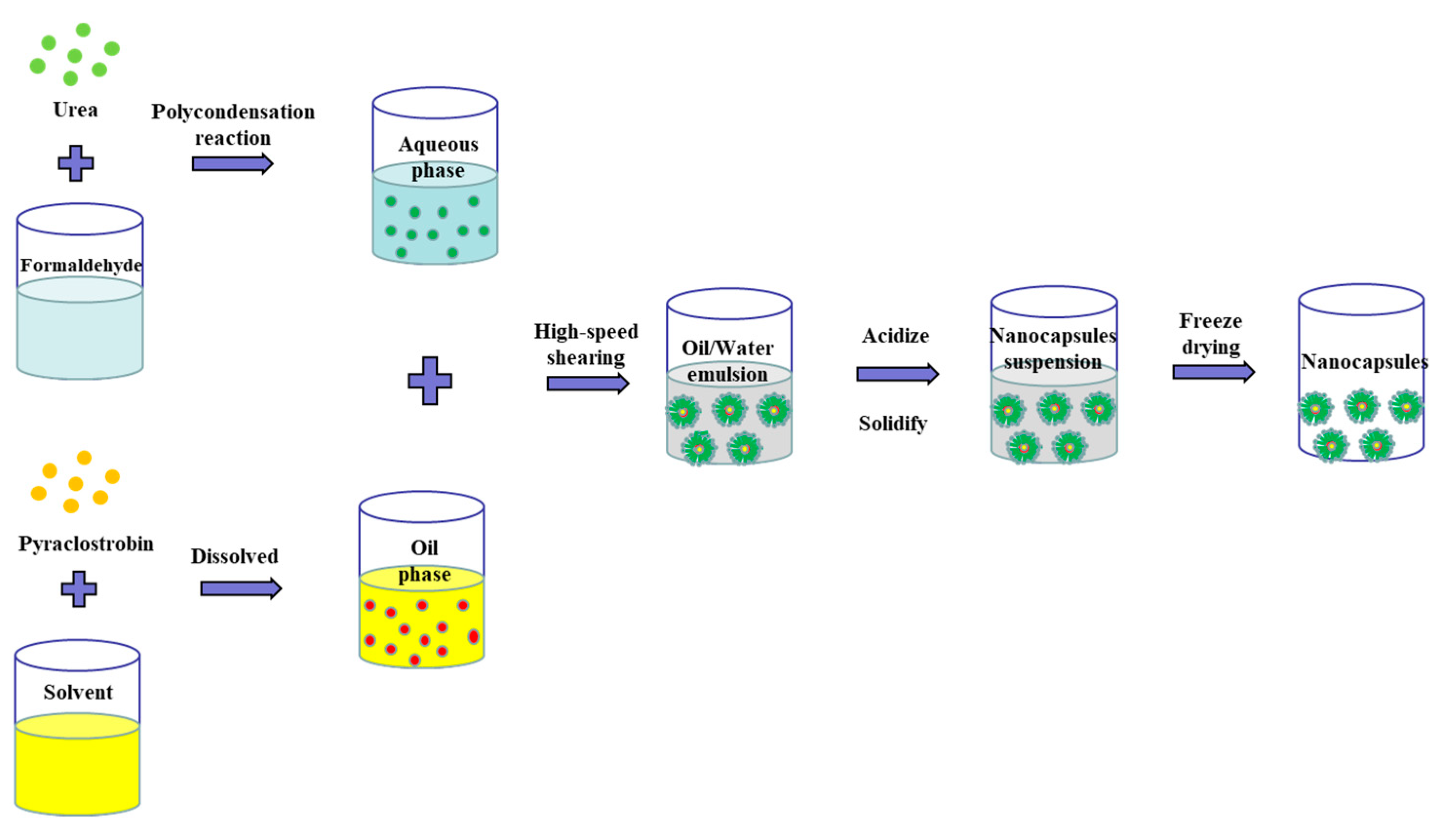
2.2.1. Preparation of the Pyraclostrobin Nanocapsules
2.2.2. Preparation of the Pyraclostrobin Nanocapsules Using Different Emulsifiers
2.2.3. Preparation of the Pyraclostrobin Nanocapsules Using Different Solvents
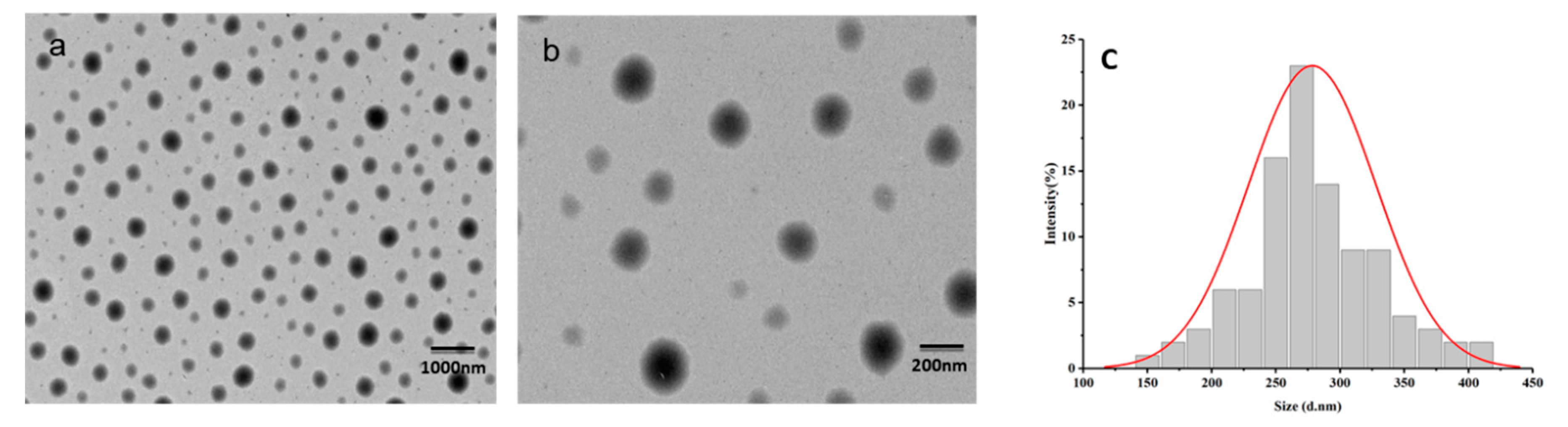
2.2.4. Characterization of Morphology and Particle sizes of the Pyraclostrobin Nanocapsules
2.2.5. Loading Content Determination of the Pyraclostrobin Nanocapsules
2.2.6. Investigation of In Vitro Release Behaviors of the Pyraclostrobin Nanocapsules
3. Results and Discussion
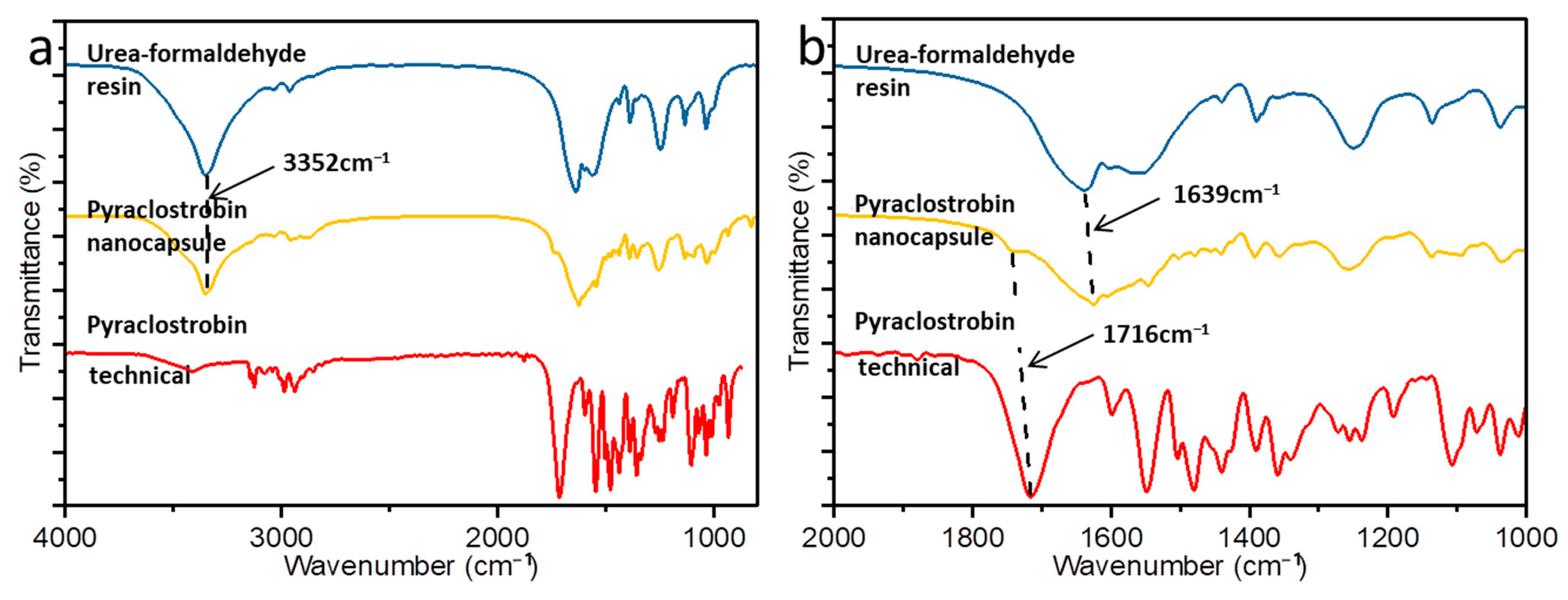
3.1. Preparation of the Pyraclostrobin Nanocapsules
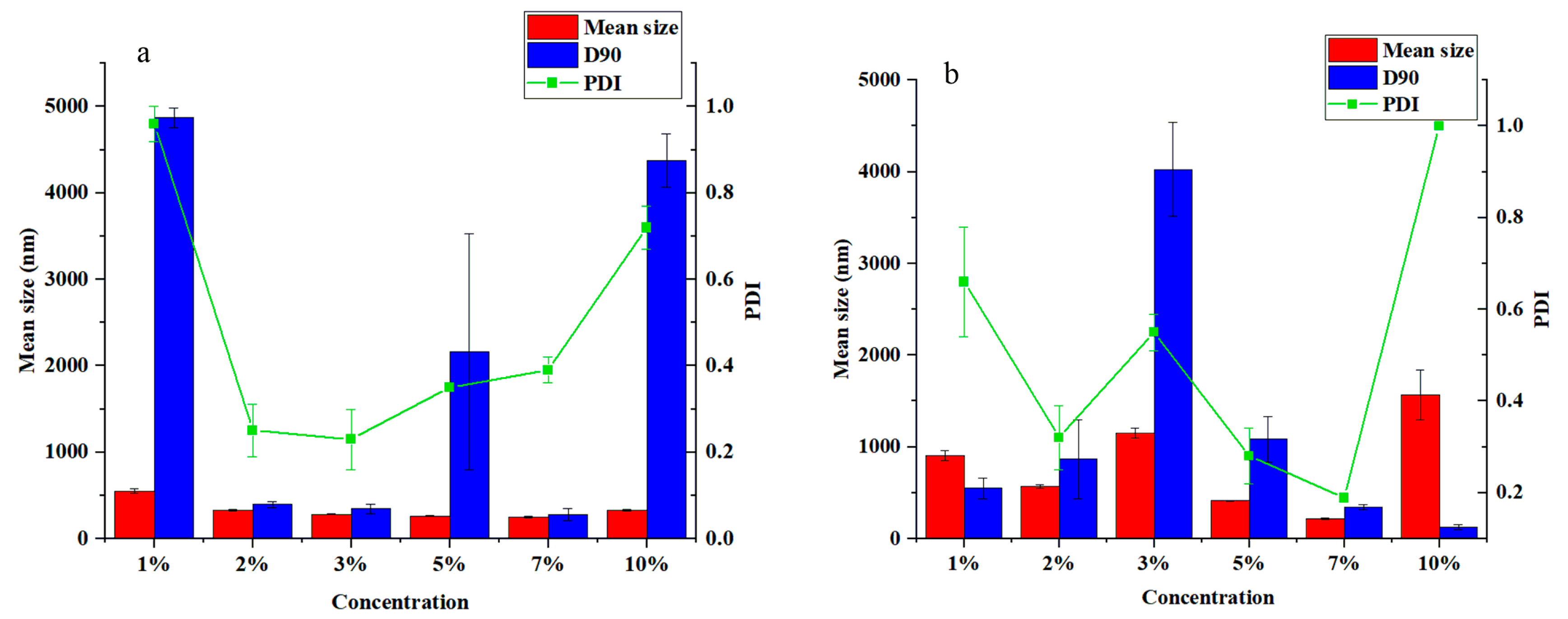
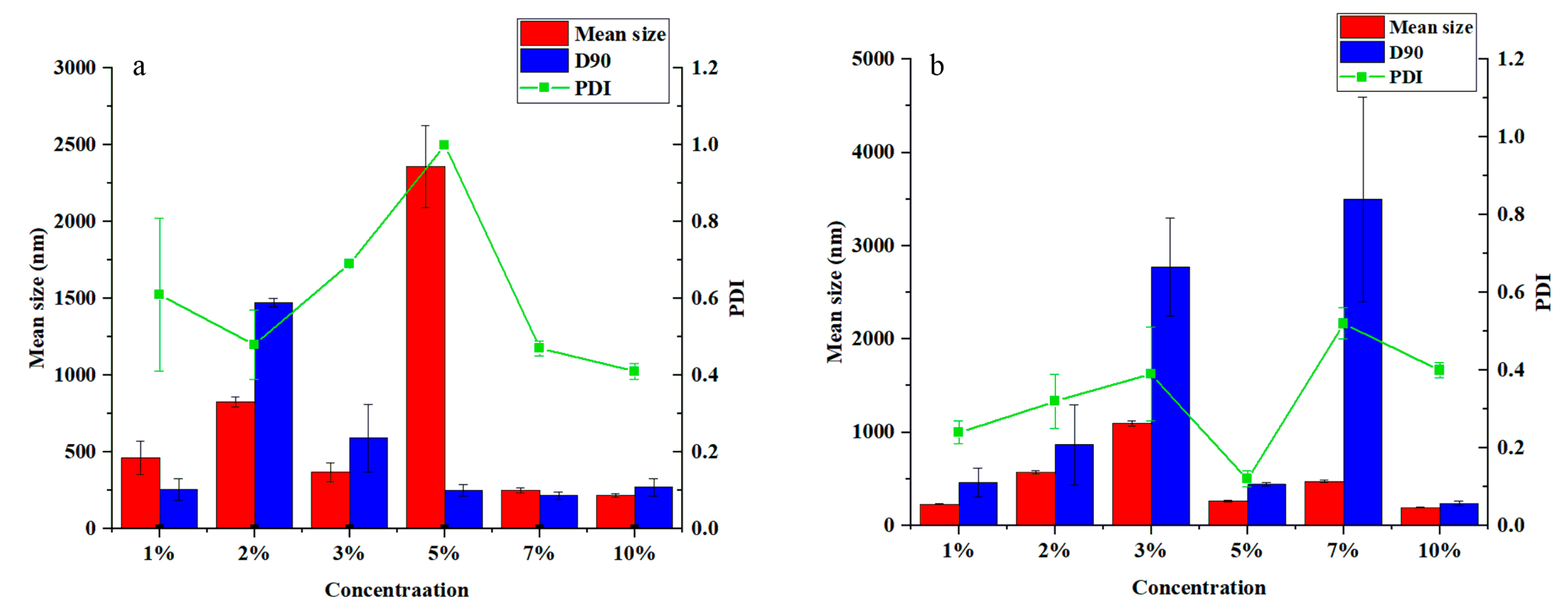
3.2. Effect of Emulsifier Types on the Particle Size and Dispersibility of the Pyraclostrobin Nanocapsules
3.3. Effect of Emulsifier Concentrations on the Particle Size and Dispersibility of the Pyraclostrobin Nanocapsules
3.4. Effect of Solvent on Morphology and Particle Size of the Pyraclostrobin Nanocapsules
3.5. Loading Content of the Pyraclostrobin Nanocapsules
3.6. In Vitro Release Properties of the Pyraclostrobin Nanocapsules
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Cui, B.; Zeng, Z.; Wang, Y.; Sun, C.; Zhao, X.; Feng, L.; Liu, G.; Cui, H. Research Progress on Pesticide Solid Nanodispersion and Its Preparation Methods. J. Agric. Sci. Technol. 2017, 19, 108–114. [Google Scholar]
- Hu, D. The Research on the Water Floating and Effervescent Dispersible Granules of the New Pesticide Formulation. Zhejiang University of Technology: Hangzhou, China, 2009. [Google Scholar]
- Yu, M.; Sun, C.; Xue, Y.; Liu, C.; Qiu, D.; Cui, B.; Zhang, Y.; Cui, H.; Zeng, Z. Tannic acid-based nanopesticides coating with highly improved foliage adhesion to enhance foliar retention. RSC Adv. 2019, 9, 27096–27104. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Cui, B.; Wang, Y.; Wang, M.; Zeng, Z.; Gao, F.; Sun, C.; Guo, L.; Zhao, X.; Cui, H. Preparation and Size Control of Efficient and Safe Nanopesticides by Anodic Aluminum Oxide Templates-Assisted Method. Int. J. Mol. Sci. 2021, 22, 8348. [Google Scholar] [CrossRef]
- Cui, B.; Gao, F.; Zeng, Z.; Wang, C.; Wang, Y.; Sun, C.; Zhao, X.; Guo, L.; Shen, Y.; Liu, G.; et al. Construction and characterization of avermectin B2 solid nanodispersion. Sci. Rep. 2020, 10, 9096. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.; Yang, F.; Wang, X.; Shen, H.; Cui, H.; Wu, D. Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloids Surf. B. 2016, 144, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.; Zhao, X.; Cui, B.; Zeng, Z.; Cui, H. Research on Preparation Technology of Polylactic Acid Nano-microsphere. J. Agric. Sci. Tech. 2018, 20, 148–153. [Google Scholar]
- Sun, C.; Cui, H.; Wang, Y.; Zeng, Z.; Zhao, X.; Cui, B. Studies on Applications of Nanomaterial and Nanotechnology in Agriculture. J. Agric. Sci. Tech. 2018, 18, 18–25. [Google Scholar]
- Lin, Z. Fundamentals and Applications of Nano Materials; Peking University Press: Beijing, China, 2010. [Google Scholar]
- Guo, W.; Cui, R.; Zhuang, Z.; Zuo, W.; Zhu, Y.; Gao, J. Research Situation and Prospect of Pesticide Microcapsule. Mod. Agrochem. 2017, 16, 1–6+13. [Google Scholar]
- Huang, B.; Chen, F.; Yue, S.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in Targeted Pesticides with Environmentally Responsive Controlled Release by Nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Li, C. Preparation of hierarchically nanofibrous membrane and its high adaptability in hexavalent chromium removal from water. Chem. Eng. J. 2012, 198–199, 310–317. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara, M.; Campos, E.; Monteiro, A.; Pereira, A.; Fraceto, L. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobio-Technol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, B.; Feng, L.; Pan, Z.; Yu, M.; Zeng, Z.; Sun, C.; Zhao, X.; Wang, Y.; Cui, H. Evaluation of Stability and Biological Activity of Solid Nanodispersion of Lambda-Cyhalothrin. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Cui, B.; Zhao, X.; Zeng, Z.; Wang, Y.; Sun, C.; Liu, G.; Cui, H. Comparative Study on Characterization and Field Efficacy Evaluation of New Pesticide Nanodispersion. J. Agric. Sci. Technol. 2018, 20, 103–112. [Google Scholar]
- Wang, A.; Wang, Y.; Sun, C.; Wang, C.; Cui, B.; Zhao, X.; Zeng, Z.; Yao, J.; Yang, D.; Liu, G.; et al. Fabrication, Characterization, and Biological Activity of Avermectin Nano-delivery Systems with Different Particle Sizes. Nanoscale Res. Lett. 2018, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Zuo, W.; Zhu, Y.; Zhuang, Z.; Cui, W.; Guo, W.; Liu, Y.; Fan, J. Research Status and aparospects of Pyraclostrobin. World Pestic. 2017, 39, 22–25. [Google Scholar]
- Zhang, Y. A novel methoxy acrylic acid vinegar bactericide-limonide vinegar. World Pestic. 2007, 3, 47–48. [Google Scholar]
- Hao, H. Preparation and Characterization of the Pyraclostrobin Microcapsule Suspension Formulation; Jilin Agricultural University: Jilin, China, 2015. [Google Scholar]
- Yang, L.; Bai, Y. Strobilurin Fungicide—Pyraclostrobin. Mod. Agrochem. 2012, 11, 46–50+56. [Google Scholar]
- Zhi, Y.; Wang, G.; Chen, L. Preparation Technology and Development Situation of Pesticide Microcapsule. Synth. Mater Aging Appl. 2015, 44, 97–100+110. [Google Scholar]
- Zhang, Y.; Zhu, L.; Tan, H. Adhesive and Bonding Technology; Chemical Industry Press: Beijing, China, 2018. [Google Scholar]
- Yang, B.; Du, F.; Li, Z. Adhesives—Formulation, Process and Equipment; Chemical Industry Press: Beijing, China, 2018. [Google Scholar]
- Li, J. Study on Gel, Morphology and Crystallization Characteristics of Urea-formaldehyde Resin; Guangxi University: Nanning, China, 2021. [Google Scholar]
- Wang, H.; Liu, X.; Jin, C.; Zhou, Y.; Ou, X. Research Progress of Micro-capsule Suspension of Pesticide. Guangdong Chem. Ind. 2017, 44, 146–147+137. [Google Scholar]
- Gong, S.; Shen, Z.; Zhou, X.; Chen, H.; Xu, H. Sustained-release Kinetics and Preparation Process Optimization of Chlorpyrifos/Urea-formaldehyde Resin Microcapsules. Mater. Rep. 2018, 32, 1241–1246. [Google Scholar]
- Li, J.; Dai, L.; Gu, A.; Bi, Y. Preparation of 30% chlorpyrifos microcapsule suspension. JiangSu Agric. Sci. 2020, 48, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L. The Development of Wood Polymer Penetrant and In Situ Polymerization with Electron Beam and x-radiaton; State University of New York College of Environmental Science and Forestry: New York, NY, USA, 2006. [Google Scholar]
- Bergman, R.; Ibach, R.; Lapasha, C.; Denig, J. Evaluating physical property changes for small-diameter, plantation-grown southern pine after in situ polymerization of an acrylic monomer. For. Prod. J. 2009, 59, 64–71. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Chen, F.; Shen, Y.; An, C.; Li, N.; Jiang, J.; Wang, C.; Sun, C.; Zhao, X.; Cui, B.; et al. Preparation, Characterization, and Evaluation of Pyraclostrobin Nanocapsules by In Situ Polymerization. Nanomaterials 2022, 12, 549. https://doi.org/10.3390/nano12030549
Huang B, Chen F, Shen Y, An C, Li N, Jiang J, Wang C, Sun C, Zhao X, Cui B, et al. Preparation, Characterization, and Evaluation of Pyraclostrobin Nanocapsules by In Situ Polymerization. Nanomaterials. 2022; 12(3):549. https://doi.org/10.3390/nano12030549
Chicago/Turabian StyleHuang, Bingna, Feifei Chen, Yue Shen, Changcheng An, Ningjun Li, Jiajun Jiang, Chong Wang, Changjiao Sun, Xiang Zhao, Bo Cui, and et al. 2022. "Preparation, Characterization, and Evaluation of Pyraclostrobin Nanocapsules by In Situ Polymerization" Nanomaterials 12, no. 3: 549. https://doi.org/10.3390/nano12030549









