Boosting CdS Photocatalytic Activity for Hydrogen Evolution in Formic Acid Solution by P Doping and MoS2 Photodeposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the CdS Nanorods
2.2. Phosphating of CdS Nanorods
2.3. Preparation of CdS/P/MoS2 and CdS/MoS2
2.4. Performance Test of Photocatalytic Hydrogen Evolution
2.5. Characterizations
2.6. Photoelectrochemical Measurements
3. Results and Discussion
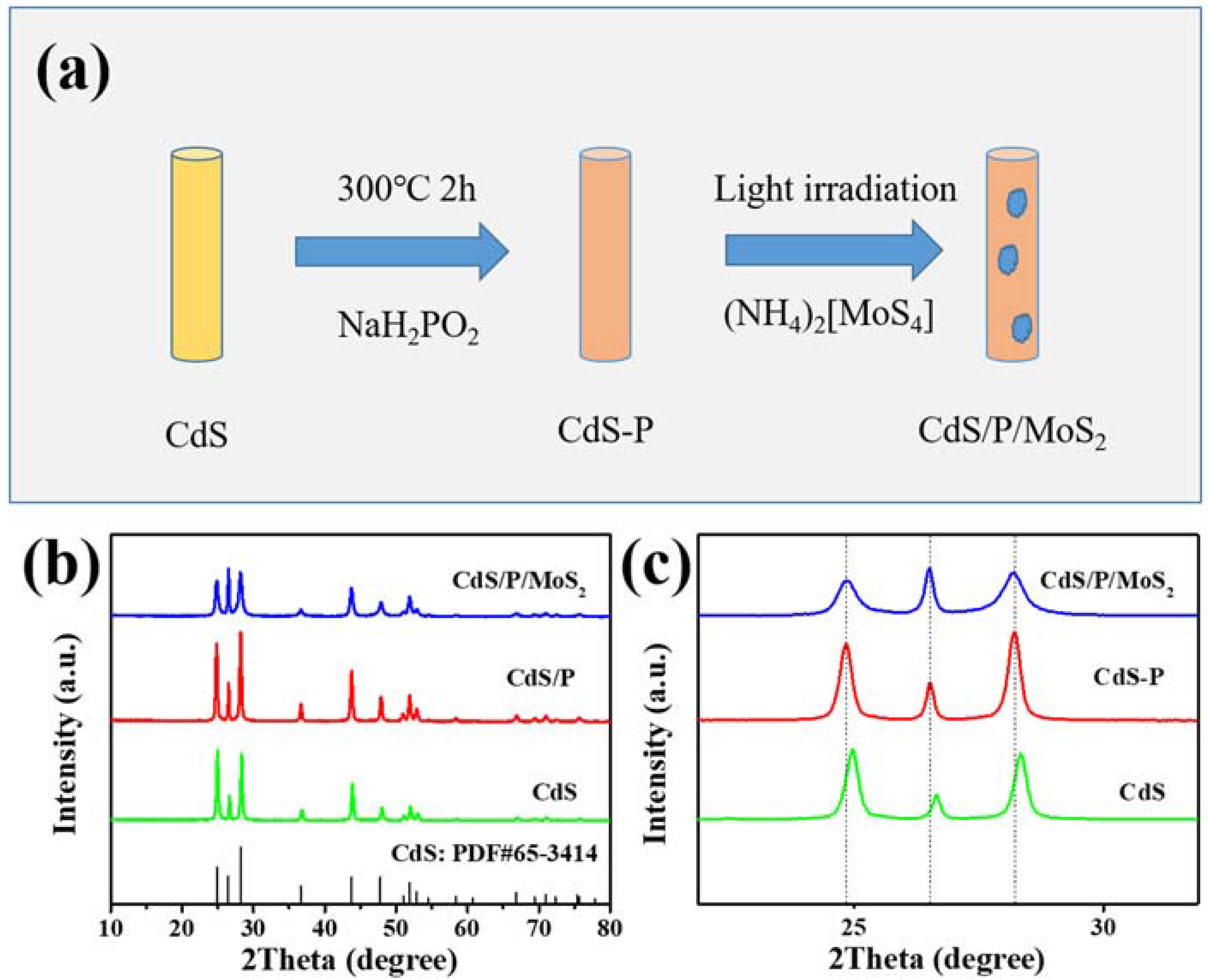
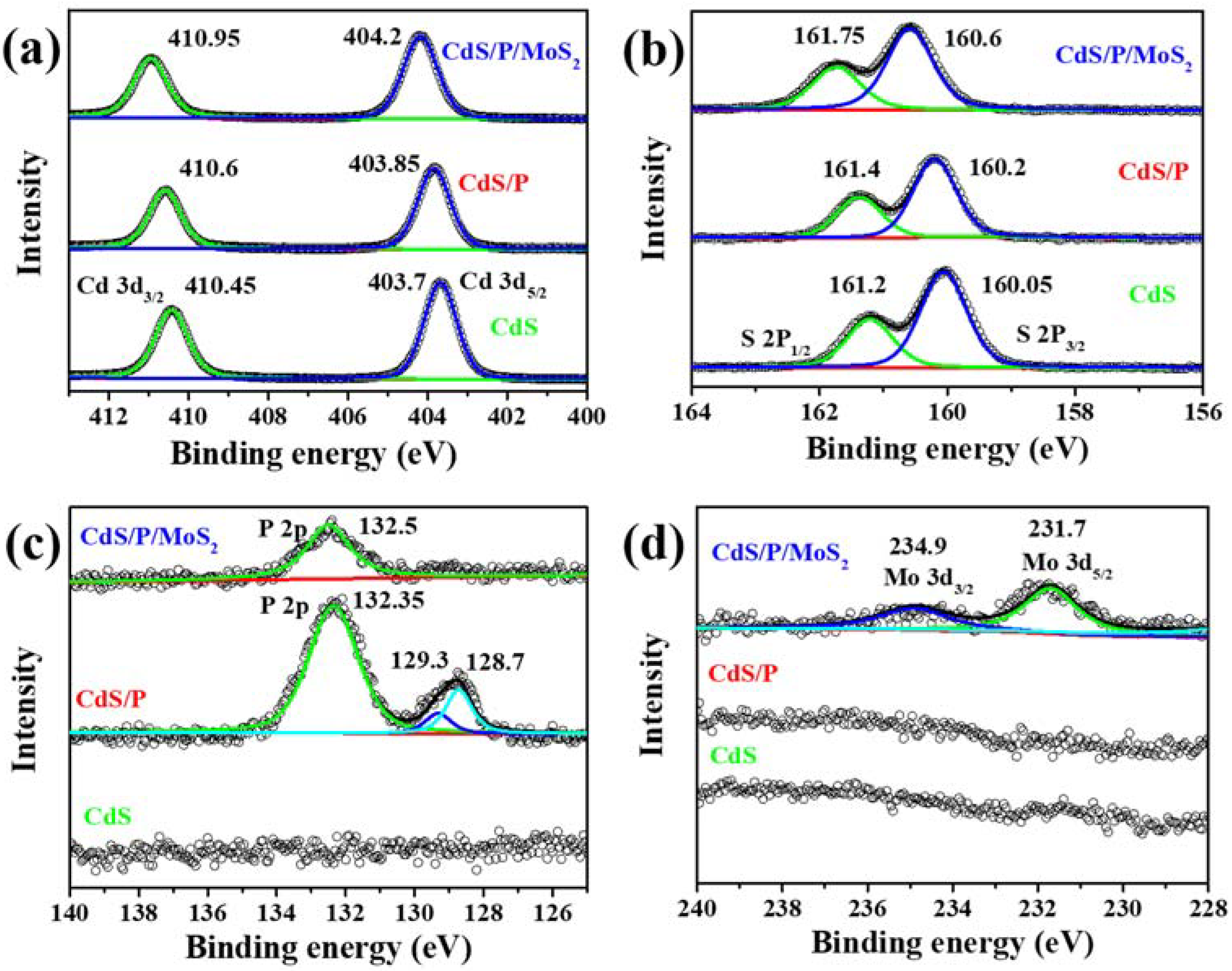
3.1. Characterization of the Composition, Structure and Morphology of the Photocatalysts
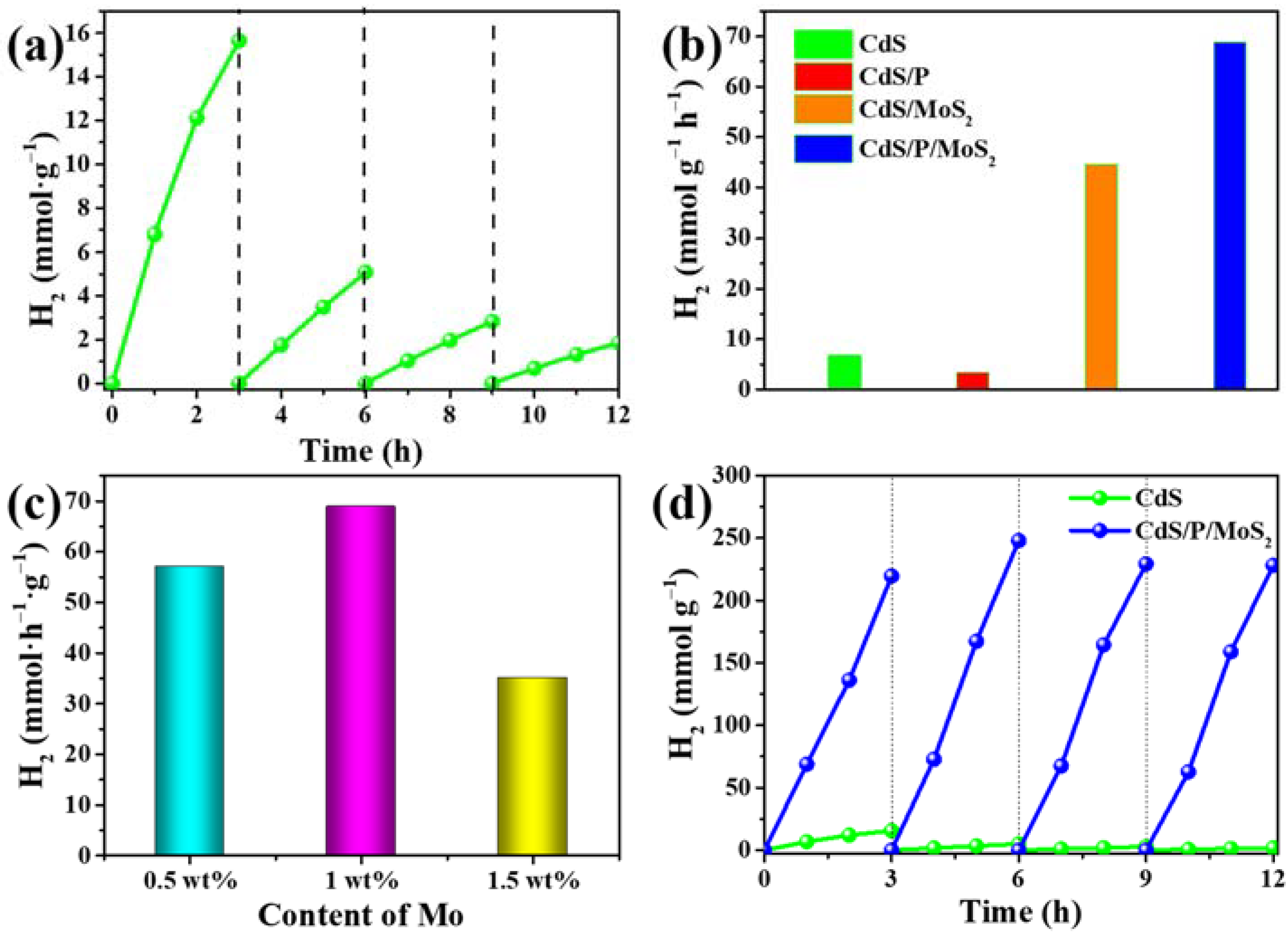
3.2. The Performances of the Photocatalysts for Photocatalytic H2 Evolution
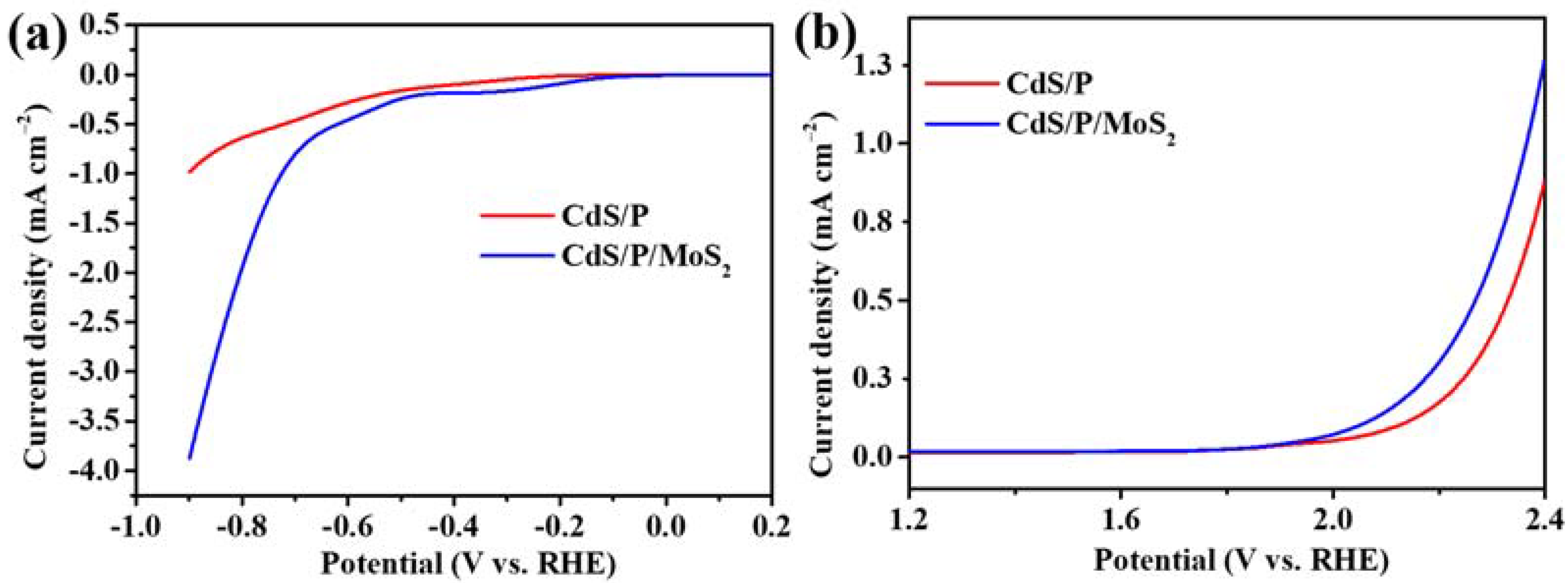
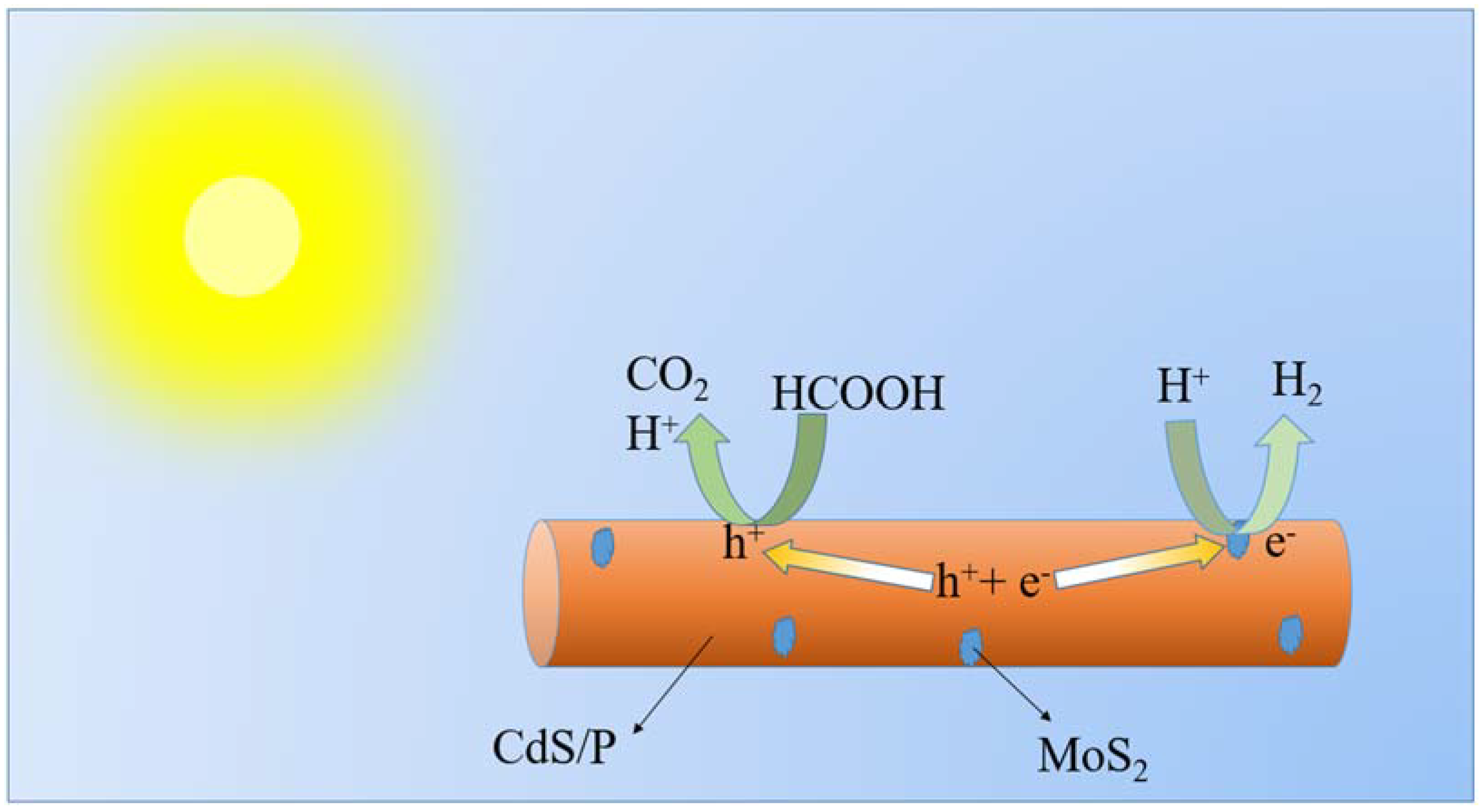
3.3. The Mechanism of Photocatalytic H2 Evolution in Formic Acid Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuehnel, M.F.; Wakerley, D.W.; Orchard, K.L.; Reisner, E. Photocatalytic formic acid conversion on CdS nanocrystals with controllable selectivity for H2 or CO. Angew. Chem. Int. Ed. 2015, 54, 9627–9631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Chen, Y.; Wang, H.; Chen, J.; Shi, X.; Li, H.; Cheng, P.; Liu, X.; Liu, M.; Piao, L. Ultrasmall CoP nanoparticles as efficient cocatalysts for photocatalytic formic acid dehydrogenation. Joule 2018, 2, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Lu, Z.-H.; Jiang, H.-L.; Akita, T.; Xu, Q. Synergistic catalysis of metal–organic framework-immobilized Au–Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J. Am. Chem. Soc. 2011, 133, 11822–11825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Metin, Ö.; Su, D.; Sun, S. Monodisperse AgPd alloy nanoparticles and their superior catalysis for the dehydrogenation of formic acid. Angew. Chem. Int. Ed. 2013, 52, 3681–3684. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W.-B. B-doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid–formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, Q.; Xu, Z.; Shang, Q.; Wang, L.; Chao, Y.; Li, Y.; Chen, H.; Lv, F.; Zhang, Q.; et al. Atomically dispersed Co-P3 on CdS nanorods with electron-rich feature boosts photocatalysis. Adv. Mater. 2020, 32, 1904249. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Yang, W.; Shen, M.; Geng, S.; Yu, C.; Shen, B.; Yu, Y. Photocatalytic dehydrogenation of formic acid promoted by a superior PdAg≅g-C3N4 Mott–Schottky heterojunction. J. Mater. Chem. A 2019, 7, 2022–2026. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Q.; Wu, M.; Lv, T.; Jia, L. Novel reduced graphene oxide wrapped-SrTiO3 flower-like nanostructure with Ti–C bond for free noble metal decomposition of formic acid to hydrogen. Chem. Eng. J. 2018, 334, 1886–1896. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Beloshapkin, S.; Guo, Y.; Yuranov, I. Catalytic properties of PdZn/ZnO in formic acid decomposition for hydrogen production. Appl. Catal. A Gen. 2018, 561, 96–103. [Google Scholar] [CrossRef]
- Villa, K.; Domènech, X.; Malato, S.; Maldonado, M.I.; Peral, J. Heterogeneous photocatalytic hydrogen generation in a solar pilot plant. Int. J. Hydrogen Energy 2013, 38, 12718–12724. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Jiang, D.; Cao, H.; Minja, A.C.; Du, P. CdS nanorods anchored with crystalline FeP nanoparticles for efficient photocatalytic formic acid dehydrogenation. ACS Appl. Mater. Interfaces 2021, 13, 23751–23759. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. CdS/graphene nanocomposite photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, X.; Yu, L.; Wang, Y.; Ning, J.; Xu, S.; Lou, X.W. Carbon-coated CdS petalous nanostructures with enhanced photostability and photocatalytic activity. Angew. Chem. Int. Ed. 2013, 52, 5636–5639. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Li, Y.; Zhang, L.; Wang, A.; Xiao, N.; Gao, Y.; Li, N.; Song, W.; Ge, L.; et al. Novel photocatalyst incorporating Ni-Co layered double hydroxides with P-doped CdS for enhancing photocatalytic activity towards hydrogen evolution. Appl. Catal. B Environ. 2019, 254, 145–155. [Google Scholar] [CrossRef]
- Zubair, M.; Vanhaecke, E.M.M.; Svenum, I.-H.; Rønning, M.; Yang, J. Core-shell particles of C-doped CdS and graphene: A noble metal-free approach for efficient photocatalytic H2 generation. Green Energy Environ. 2020, 5, 461–472. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Z.; Fan, X.; Leung, D.Y.C.; Long, J.; Zhang, Z.; Miao, T.; Meng, S.; Chen, S.; Fu, X. Intimately contacted Ni2P on CdS nanorods for highly efficient photocatalytic H2 evolution: New phosphidation route and the interfacial separation mechanism of charge carriers. Appl. Catal. B Environ. 2021, 281, 119443. [Google Scholar] [CrossRef]
- Shen, L.; Luo, M.; Liu, Y.; Liang, R.; Jing, F.; Wu, L. Noble-metal-free MoS2 co-catalyst decorated UiO-66/CdS hybrids for efficient photocatalytic H2 production. Appl. Catal. B Environ. 2015, 166–167, 445–453. [Google Scholar] [CrossRef]
- Wei, R.-B.; Huang, Z.-L.; Gu, G.-H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.-Q. Dual-cocatalysts decorated rimous CdS spheres advancing highly-efficient visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Li, Y.-L.; Wang, X.-J.; Hao, Y.-J.; Zhao, J.; Liu, Y.; Mu, H.-Y.; Li, F.-T. Rational design of stratified material with spatially separated catalytic sites as an efficient overall water-splitting photocatalyst. Chin. J. Catal. 2021, 42, 1040–1050. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Fan, J.C.; Xu, Q.J.; Min, Y.L. BiVO4 nanowires decorated with CdS nanoparticles as Z-scheme photocatalyst with enhanced H2 generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.; Cui, Z.; Zhou, J.; Chu, S.; Wang, Y.; Zou, Z. Enhanced photocarrier separation in conjugated polymer engineered CdS for direct Z-scheme photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 260, 118131. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Huang, H.; Dai, B.; Wang, W.; Lu, C.; Kou, J.; Ni, Y.; Wang, L.; Xu, Z. Oriented built-in electric field introduced by surface gradient diffusion doping for enhanced photocatalytic H2 evolution in CdS nanorods. Nano Lett. 2017, 17, 3803–3808. [Google Scholar] [CrossRef]
- Shi, R.; Ye, H.-F.; Liang, F.; Wang, Z.; Li, K.; Weng, Y.; Lin, Z.; Fu, W.-F.; Che, C.-M.; Chen, Y. Interstitial P-doped CdS with long-lived photogenerated electrons for photocatalytic water splitting without sacrificial agents. Adv. Mater. 2018, 30, 1705941. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Yang, J.; Yan, H.; Wang, X.; Wen, F.; Wang, Z.; Fan, D.; Shi, J.; Li, C. Roles of cocatalysts in Pt–PdS/CdS with exceptionally high quantum efficiency for photocatalytic hydrogen production. J. Catal. 2012, 290, 151–157. [Google Scholar] [CrossRef]
- Wang, P.; Sheng, Y.; Wang, F.; Yu, H. Synergistic effect of electron-transfer mediator and interfacial catalytic active-site for the enhanced H2-evolution performance: A case study of CdS-Au photocatalyst. Appl. Catal. B Environ. 2018, 220, 561–569. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, B.; Li, W.; Liu, X.; Wang, K.; Deng, Y.; Guo, Z.; Lv, Z. A well-designed honeycomb Co3O4≅CdS photocatalyst derived from cobalt foam for high-efficiency visible-light H2 evolution. J. Mater. Chem. A 2021, 9, 11665–11673. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, J.; Gao, Q.; Chen, S.; Li, J.; Li, X.; Fang, Y. Amorphous Co3O4 modified CdS nanorods with enhanced visible-light photocatalytic H2-production activity. Dalton Trans. 2015, 44, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Jiang, Z.; Shi, P.; Fan, J.; Xu, Q.; Min, Y. Noble-metal-free heterostructure for efficient hydrogen evolution in visible region: Molybdenum nitride/ultrathin graphitic carbon nitride. Appl. Catal. B Environ. 2018, 238, 318–327. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Liu, M.; Miao, T.; Ye, X.; Meng, S.; Chen, S.; Fu, X. A new phosphidation route for the synthesis of NiPx and their cocatalytic performances for photocatalytic hydrogen evolution over g-C3N4. J. Energy Chem. 2020, 48, 241–249. [Google Scholar] [CrossRef]
- Yang, F.; Liu, D.; Li, Y.; Ning, S.; Cheng, L.; Ye, J. Solid-state synthesis of ultra-small freestanding amorphous MoP quantum dots for highly efficient photocatalytic H2 production. Chem. Eng. J. 2021, 406, 126838. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Iqbal, S.; Nadeem, M.; Bashir, T.; Maqsood, M.; Zhao, J.; Gao, L. Co3C as a promising cocatalyst for superior photocatalytic H2 production based on swift electron transfer processes. J. Mater. Chem. C 2021, 9, 3145–3154. [Google Scholar] [CrossRef]
- Shen, R.; Ding, Y.; Li, S.; Zhang, P.; Xiang, Q.; Ng, Y.H.; Li, X. Constructing low-cost Ni3C/twin-crystal Zn0.5Cd0.5S heterojunction/homojunction nanohybrids for efficient photocatalytic H2 evolution. Chin. J. Catal. 2021, 42, 25–36. [Google Scholar] [CrossRef]
- Lallimathi, M.; Kalisamy, P.; Suryamathi, M.; Alshahrani, T.; Shkir, M.; Venkatachalam, M.; Palanivel, B. Carbon dot loaded integrative CoFe2O4/g-C3N4 P-N heterojunction: Direct solar light-driven photocatalytic H2 evolution and organic pollutant degradation. ChemistrySelect 2020, 5, 10607–10617. [Google Scholar] [CrossRef]
- Palanivel, B.; Shkir, M.; Alshahrani, T.; Mani, A. Novel NiFe2O4 deposited S-doped g-C3N4 nanorod: Visible-light-driven heterojunction for photo-Fenton like tetracycline degradation. Diam. Relat. Mater. 2021, 112, 108148. [Google Scholar] [CrossRef]
- Palanivel, B.; Hu, C.; Shkir, M.; AlFaify, S.; Ibrahim, F.A.; Hamdy, M.S.; Mani, A. Fluorine doped g-C3N4 coupled NiFe2O4 heterojunction: Consumption of H2O2 for production of hydroxyl radicals towards paracetamol degradation. Colloid Interface Sci. Commun. 2021, 42, 100410. [Google Scholar] [CrossRef]
- Ma, S.; Xie, J.; Wen, J.; He, K.; Li, X.; Liu, W.; Zhang, X. Constructing 2D layered hybrid CdS nanosheets/MoS2 heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2017, 391, 580–591. [Google Scholar] [CrossRef]
- Yang, X.; Yang, H.; Zhang, T.; Lou, Y.; Chen, J. P-Doped CdS integrated with multiphasic MoSe2 nanosheets accomplish prominent photocatalytic activity for hydrogen evolution. Catal. Sci. Technol. 2021, 11, 5849–5858. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Sun, R.; Li, X.; Sun, D. An efficient hydrogen evolution catalyst composed of palladium phosphorous sulphide (PdP∼0.33S∼1.67) and twin nanocrystal Zn0.5Cd0.5S solid solution with both homo- and hetero-junctions. Energy Environ. Sci. 2017, 10, 225–235. [Google Scholar] [CrossRef]
- Zhang, X.; An, W.; Li, Y.; Hu, J.; Gao, H.; Cui, W. Efficient photo-catalytic hydrogen production performance and stability of a three-dimensional porous CdS NPs-graphene hydrogel. Int. J. Hydrogen Energy 2018, 43, 9902–9913. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Ke, J.; Zhang, J.; Tian, W.; Xu, X.; Duan, X.; Sun, H.; O Tade, M.; Wang, S. 0D (MoS2)/2D (g-C3N4) heterojunctions in Z-scheme for enhanced photocatalytic and electrochemical hydrogen evolution. Appl. Catal. B Environ. 2018, 228, 64–74. [Google Scholar] [CrossRef]
- Shkir, M.; Palanivel, B.; Khan, A.; Kumar, M.; Chang, J.-H.; Mani, A.; AlFaify, S. Enhanced photocatalytic activities of facile auto-combustion synthesized ZnO nanoparticles for wastewater treatment: An impact of Ni doping. Chemosphere 2021, 291, 132687. [Google Scholar] [CrossRef]
- Shkir, M.; Chandekar, K.V.; Hossain, M.M.; Palanivel, B.; Ahmad, N.; Ashraf, I.M.; Somaily, H.H.; Algarni, H.; AlFaify, S. Enhanced dielectric and electrical properties of PbS nanostructures facilely synthesized by low-cost chemical route: An effect of Ce doping concentrations. Mater. Chem. Phys. 2022, 278, 125626. [Google Scholar] [CrossRef]
- Han, L.-L.; Kulinich, S.A.; Zhang, Y.-Y.; Zou, J.; Liu, H.; Wang, W.-H.; Liu, H.; Li, H.-B.; Yang, J.; Xin, H.L.; et al. Synergistic synthesis of quasi-monocrystal CdS nanoboxes with high-energy facets. J. Mater. Chem. A 2015, 3, 23106–23112. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Luo, H.; Bao, Y.; Guo, S.; Lei, D.; Chen, Y. Construction of hierarchical BiOI/MoS2/CdS heterostructured microspheres for boosting photocatalytic CO2 reduction under visible light. Sol. RRL 2021, 5, 2100051. [Google Scholar] [CrossRef]
- Zhuge, K.; Chen, Z.; Yang, Y.; Wang, J.; Shi, Y.; Li, Z. In-suit photodeposition of MoS2 onto CdS quantum dots for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2021, 539, 148234. [Google Scholar] [CrossRef]
- Yuan, M.; Zhou, W.; Kou, D.; Zhou, Z.; Meng, Y.; Wu, S. Cu2ZnSnS4 decorated CdS nanorods for enhanced visible-light-driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2018, 43, 20408–20416. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Peng, S.; Lu, G.; Li, S. Synthesis of CdS nanorods by an ethylenediamine assisted hydrothermal method for photocatalytic hydrogen evolution. J. Phys. Chem. C 2009, 113, 9352–9358. [Google Scholar] [CrossRef]
- Yeh, H.M.; Lo, S.L.; Chen, M.J.; Chen, H.Y. Hydrogen production from formic acid solution by modified TiO2 and titanate nanotubes in a two-step system under visible light irradiation. Water Sci. Technol. 2014, 69, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, W.-C.; Li, X.-Y. Photocatalytic hydrogen generation with simultaneous organic degradation by composite CdS–ZnS nanoparticles under visible light. Int. J. Hydrogen Energy 2014, 39, 13454–13461. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.-Y.; Li, X.-H.; Zhang, Y.-N.; Wei, X.; Wang, K.-X.; Chen, J.-S. Highly efficient dehydrogenation of formic acid over a palladium-nanoparticle-based Mott–Schottky photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 11822–11825. [Google Scholar] [CrossRef]
- Nasir, J.A.; Hafeez, M.; Arshad, M.; Ali, N.Z.; Teixeira, I.F.; McPherson, I.; Zia-ur-Rehman; Khan, M.A. Photocatalytic dehydrogenation of formic acid on CdS nanorods through Ni and Co redox mediation under mild conditions. ChemSusChem 2018, 11, 2587–2592. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, S.-W.; Liao, Y.; Xue, C. Selective photocatalytic decomposition of formic acid over AuPd nanoparticle-decorated TiO2 nanofibers toward high-yield hydrogen production. Appl. Catal. B Environ. 2015, 162, 204–209. [Google Scholar] [CrossRef]
- Cao, H.; Wang, T.; Minja, A.C.; Jiang, D.; Du, P. NiCoP nanoparticles anchored on CdS nanorods for enhanced hydrogen production by visible light-driven formic acid dehydrogenation. Int. J. Hydrogen Energy 2021, 46, 32435–32444. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Zhang, L.; Jin, Z.; Zhao, T. Hydroxides Ni(OH)2&Ce(OH)3 as a novel hole storage layer for enhanced photocatalytic hydrogen evolution. Dalton Trans. 2019, 48, 17660–17672. [Google Scholar] [CrossRef]
- Guo, C.; Li, L.; Chen, F.; Ning, J.; Zhong, Y.; Hu, Y. One-step phosphorization preparation of gradient-P-doped CdS/CoP hybrid nanorods having multiple channel charge separation for photocatalytic reduction of water. J. Colloid Interface Sci. 2021, 596, 431–441. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Li, D.-C.; Li, Z.-J.; Wang, Y.-X.; Kong, X.-J.; Zhao, J.-S.; Jiang, J.-H.; Qian, J.-C.; Pang, D.-H.; et al. Noble-metal-free CdS≅MoS2 core-shell nanoheterostructures for efficient and stabilized visible-light-driven H2 generation. Int. J. Hydrogen Energy 2019, 44, 16657–16666. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Yan, X.; Yang, X.; Li, J.; Gao, S.; Cao, R. Visible-light-driven photocatalytic hydrogen production coupled with selective oxidation of benzyl alcohol over CdS≅MoS2 heterostructures. Sci. China Mater. 2020, 63, 2239–2250. [Google Scholar] [CrossRef]
- Pan, J.; Wang, P.; Wang, P.; Yu, Q.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The photocatalytic overall water splitting hydrogen production of g-C3N4/CdS hollow core–shell heterojunction via the HER/OER matching of Pt/MnOx. Chem. Eng. J. 2021, 405, 126622. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Huang, H.; Ge, C.; Wang, Z.; Zhou, X.; Fang, Y. Boosting CdS Photocatalytic Activity for Hydrogen Evolution in Formic Acid Solution by P Doping and MoS2 Photodeposition. Nanomaterials 2022, 12, 561. https://doi.org/10.3390/nano12030561
Liu J, Huang H, Ge C, Wang Z, Zhou X, Fang Y. Boosting CdS Photocatalytic Activity for Hydrogen Evolution in Formic Acid Solution by P Doping and MoS2 Photodeposition. Nanomaterials. 2022; 12(3):561. https://doi.org/10.3390/nano12030561
Chicago/Turabian StyleLiu, Junchen, Haoran Huang, Chunyu Ge, Zhenghui Wang, Xunfu Zhou, and Yueping Fang. 2022. "Boosting CdS Photocatalytic Activity for Hydrogen Evolution in Formic Acid Solution by P Doping and MoS2 Photodeposition" Nanomaterials 12, no. 3: 561. https://doi.org/10.3390/nano12030561



