Cost-Effective Fabrication of Modified Palygorskite-Reinforced Rigid Polyurethane Foam Nanocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Modified Pal
2.3. Synthesis of the Pal/RPUFNs
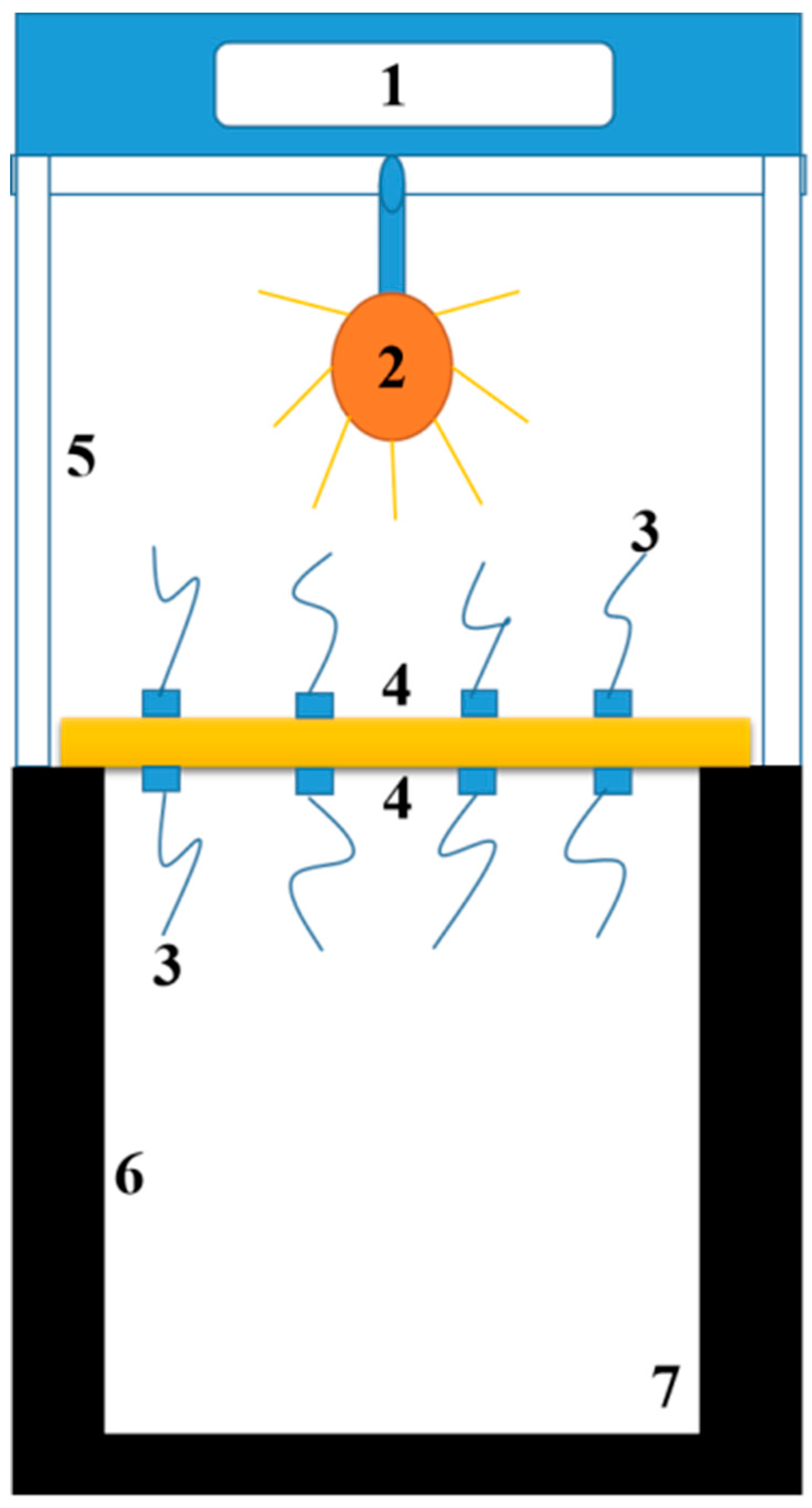
2.4. Characterizations
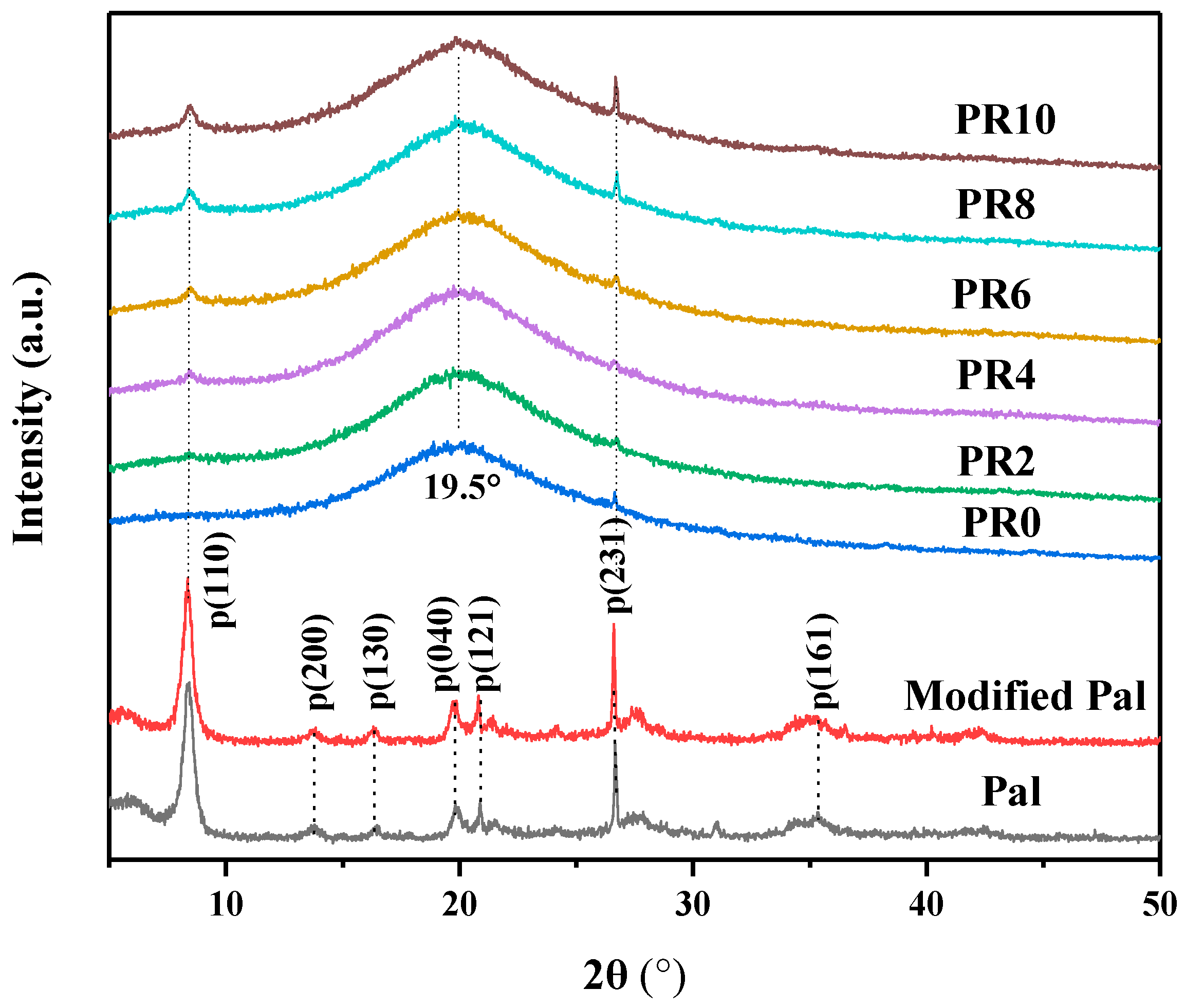
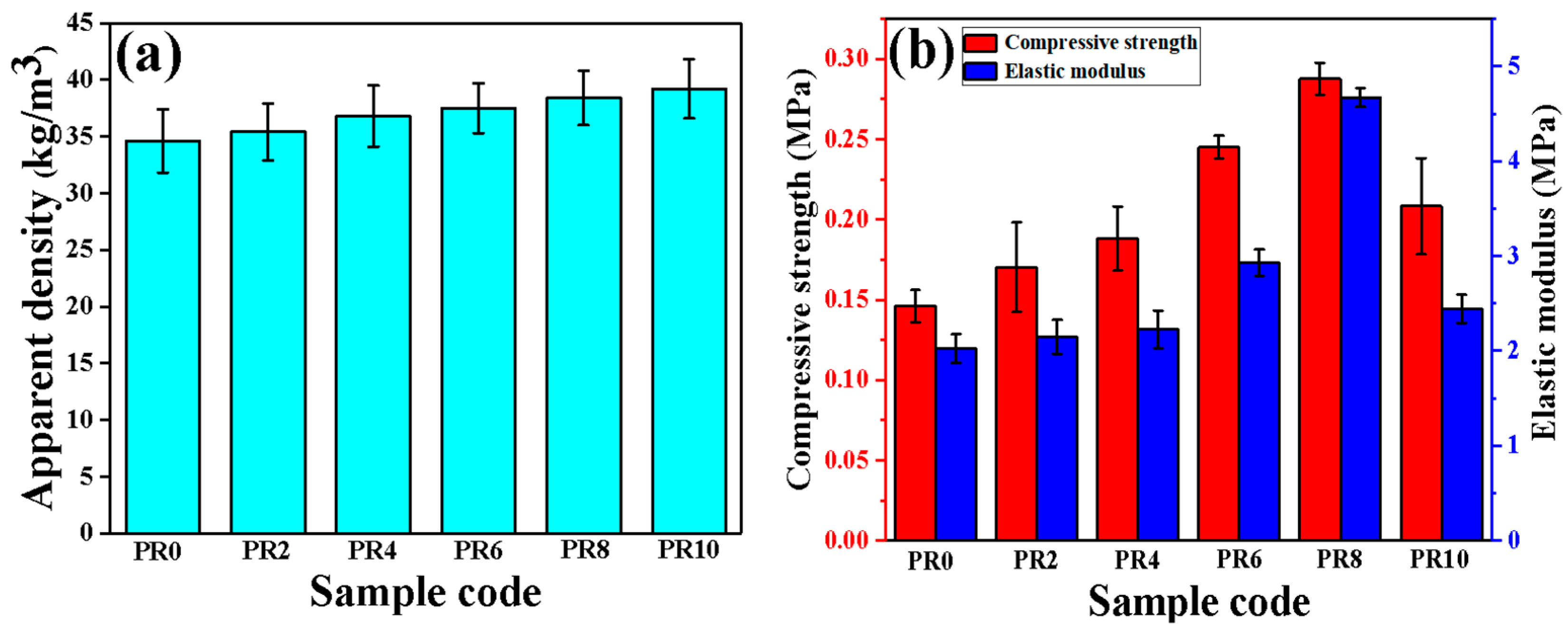
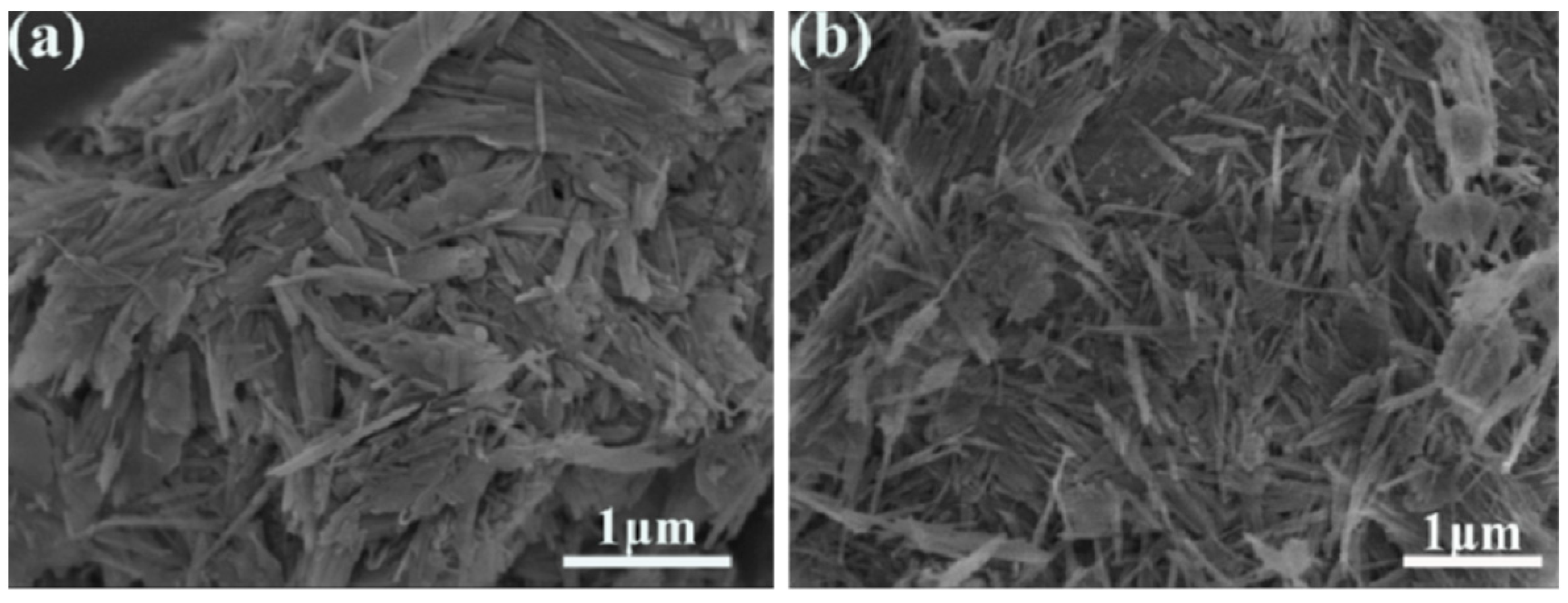
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, D.; Meng, Y.; Jiang, Y.; Qian, S.; Liu, J.; Xu, Y.; Xiong, D.; Cao, Y. Silver/polypyrrole-functionalized polyurethane foam embedded phase change materials for thermal energy harvesting. Nanomaterials 2021, 11, 3011. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, C.; Chen, X.; Li, A.; Zhang, Y. Thermal, mechanical and electrical properties of carbon fiber fabric and graphene reinforced segmented polyurethane composites. Nanomaterials 2021, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Yin, H.; Jang, S. Toward multi-functional road surface design with the nanocomposite coating of carbon nanotube modified polyurethane: Lab-scale experiments. Nanomaterials 2020, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-X.; Zhao, H.-B.; Rao, W.-H.; Huang, S.-C.; Wang, T.; Liao, W.; Wang, Y.-Z. Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer 2018, 153, 616–625. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Yuan, Y.; Liu, L.; Ma, S.; Wang, W.; Hu, Y.; Hu, W.; Gui, Z. Nanosized bimetal-organic frameworks as robust coating for multi-functional flexible polyurethane foam: Rapid oil-absorption and excellent fire safety. Compos. Sci. Technol. 2019, 177, 66–72. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; El-Newehy, M.H.; Alghamdi, T.; Moydeen Abdulhameed, M.; Khil, M.-S. Biocompatibility computation of muscle cells on polyhedral oligomeric silsesquioxane-grafted polyurethane nanomatrix. Nanomaterials 2021, 11, 2966. [Google Scholar] [CrossRef] [PubMed]
- Czlonka, S.; Fischer Kerche, E.; Motta Neves, R.; Strakowska, A.; Strzelec, K. Bio-based rigid polyurethane foam composites reinforced with bleached curaua fiber. Int. J. Mol. Sci. 2021, 22, 11203. [Google Scholar] [CrossRef]
- Huang, M.; Li, W.; Liu, X.; Feng, M.; Yang, J. The effects of cauliflower-like short carbon fibers on the mechanical properties of rigid polyurethane matrix composites. Polym. Test. 2020, 89, 106718. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K. Rigid polyurethane foams reinforced with industrial potato protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, X.; Chen, W.; Zhang, H.; Han, D. Modification of rigid polyurethane foams with the addition of nano-SiO2 or lignocellulosic biomass. Polymer 2020, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Gu, K.; Wang, Y.; Li, Z.; Shen, Z.; Liu, S.; Wei, J. Development of high-performance thermoplastic composites based on polyurethane and ground tire rubber by in-situ synthesis. Resour. Conserv. Recyc. 2021, 173, 105713. [Google Scholar] [CrossRef]
- Helland, A.; Wick, P.; Koehler, A.; Schmid, K.; Som, C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ. Health Perspect. 2007, 115, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strakowska, A.; Czlonka, S.; Kairyte, A.; Strzelec, K. Effects of physical and chemical modification of sunflower cake on polyurethane composite foam properties. Materials 2021, 14, 1414. [Google Scholar] [CrossRef] [PubMed]
- Allami, T.; Alamiery, A.; Nassir, M.H.; Kadhum, A.H. Investigating physio-thermo-mechanical properties of polyurethane and thermoplastics nanocomposite in various applications. Polymers 2021, 13, 2467. [Google Scholar] [CrossRef]
- Czlonka, S.; Strakowska, A.; Kairyte, A. Coir fibers treated with henna as a potential reinforcing filler in the synthesis of polyurethane composites. Materials 2021, 14, 1128. [Google Scholar] [CrossRef]
- Wang, C.; Shi, J.; He, M.; Ding, L.; Li, S.; Wang, Z.; Wei, J. High strength cellulose/ATT composite films with good oxygen barrier property for sustainable packaging applications. Cellulose 2018, 25, 4145–4154. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y. Surface-bound humic acid increased propranolol sorption on Fe3O4/attapulgite magnetic nanoparticles. Nanomaterials 2020, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Wang, F.; Liu, X.; Tang, M.; Zeng, Z.; Liang, J.; Guan, X.; Wang, J.; Mu, X. Surface modified palygorskite nanofibers and their applications as reinforcement phase in cis-polybutadiene rubber nanocomposites. Appl. Clay Sci. 2016, 132-133, 175–181. [Google Scholar] [CrossRef]
- Shi, J.; Li, M. Synthesis and characterization of polyethylene glycol/modified attapulgite form-stable composite phase change material for thermal energy storage. Sol. Energy 2020, 205, 62–73. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, F.; Guo, H.; Yang, Y.; Du, Y.; Liang, J.; Zhang, F. Effect of coupling agent on surface free energy of organic modified attapulgite (OAT) powders and tensile strength of OAT/ethyle-ne-propylene-diene monomer rubber nanocomposites. Powder Technol. 2015, 270, 92–97. [Google Scholar] [CrossRef]
- Piao, Y.; Jiang, Q.; Li, H.; Matsumoto, H.; Liang, J.; Liu, W.; Pham-Huu, C.; Liu, Y.; Wang, F. Identify Zr promotion effects in atomic scale for Co-based catalysts in Fischer-Tropsch synthesis. ACS Catal. 2020, 10, 7894–7906. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Liang, J.; Tang, Q.; Fang, B.; Piao, Y.; Hao, M.; Wang, Z. Tourmaline-modified FeMnTiOx catalysts for improved low-temperature NH3-SCR performance. Environ. Sci. Technol. 2019, 53, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Li, H.; Cui, L.; Liu, W.; Fang, B.; Liang, J.; Xie, X.; Wang, D.; Wang, F. Higher photocatalytic removal of organic pollutants using pangolin-like composites made of 3-4 atomic layers of MoS2 nanosheets deposited on tourmaline. Environ. Chem. Lett. 2021, 19, 3573–3582. [Google Scholar] [CrossRef]
- GB/T 6343-2009-Cellular Plastics and Rubbers-Determination of Apparent (Bulk) Density. Available online: http://std.samr.gov.cn/ (accessed on 4 May 2009).
- GB/T 8813-2008-Rigid Cellular Plastics-Determination of Compression Properties. Available online: http://std.samr.gov.cn/ (accessed on 4 January 2008).
- Wang, C.; Dai, L.; Yang, Z.; Ge, C.; Li, S.; He, M.; Ding, L.; Xie, H. Reinforcement of castor oil-based polyurethane with surface modification of attapulgite. Polymers 2018, 10, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurańska, M.; Polaczek, K.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell rigid polyurethane bio-foams based on modified used cooking oil. Polymer 2020, 190, 122164. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Mielewczyk-Gryn, A.; Piszczyk, L. Bio-based polyurethane composites and hybrid composites containing a new type of bio-polyol and addition of natural and synthetic fibers. Materials 2020, 13, 2028. [Google Scholar] [CrossRef]
- Mizera, K.; Ryszkowska, J.; Kurańska, M.; Prociak, A. The effect of rapeseed oil-based polyols on the thermal and mechanical properties of ureaurethane elastomers. Polym. Bull. 2019, 77, 823–846. [Google Scholar] [CrossRef] [Green Version]
- Kurańska, M.; Barczewski, M.; Uram, K.; Lewandowski, K.; Prociak, A.; Michałowski, S. Basalt waste management in the production of highly effective porous polyurethane composites for thermal insulating applications. Polym. Test. 2019, 76, 90–100. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Drying of the natural fibers as a solvent-free way to improve the cellulose-filled polymer composite performance. Polymer 2020, 12, 484. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Xu, Y.; Li, X.; Wang, L.; He, X.; Ma, Y.; Zou, D. The immobilization effect of natural mineral materials on Cr(VI) remediation in water and soil. Int. J. Environ. Res. Public Health 2020, 17, 2832. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.M.; Wang, H.Y.; Fang, S.Y.; Yang, W.D. Influence of fluorine-containing monomer content on the hydrophobic and transparent properties of nanohybrid silica polyacrylate coating materials. Materials 2021, 14, 4261. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Wu, Y.; Nie, C.; Wu, C.; Wang, G. Improvement of the tribological properties and corrosion resistance of epoxy–PTFE composite coating by nanoparticle modification. Coatings 2020, 11, 10. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Ryszkowska, J.; Szczepkowski, L.; Kurańska, M.; Prociak, A.; Leszczyński, M.K.; Gloc, M.; Antos-Bielska, M.; Mizera, K. Cooperative effect of rapeseed oil-based polyol and egg shells on the structure and properties of rigid polyurethane foams. Polym Test. 2020, 90, 106696. [Google Scholar] [CrossRef]
- Bo, G.; Xu, X.; Tian, X.; Wu, J.; Yan, Y. Enhancing the fire safety and smoke safety of bio-based rigid polyurethane foam via inserting a reactive flame retardant containing P@N and blending silica aerogel powder. Polymers 2021, 13, 2140. [Google Scholar] [CrossRef]
- Aranberri, I.; Montes, S.; Wesolowska, E.; Rekondo, A.; Wrzesniewska-Tosik, K.; Grande, H.J. Improved thermal insulating properties of renewable polyol based polyurethane foams reinforced with chicken feathers. Polymers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Augaitis, N.; Vaitkus, S.; Czlonka, S.; Kairyte, A. Research of wood waste as a potential filler for loose-fill building insulation: Appropriate selection and incorporation into polyurethane biocomposite foams. Materials 2020, 13, 5336. [Google Scholar] [CrossRef]
- Czlonka, S.; Strakowska, A.; Kairyte, A. The impact of hemp shives impregnated with selected plant oils on mechanical, thermal, and insulating properties of polyurethane composite foams. Materials 2020, 13, 4709. [Google Scholar] [CrossRef]
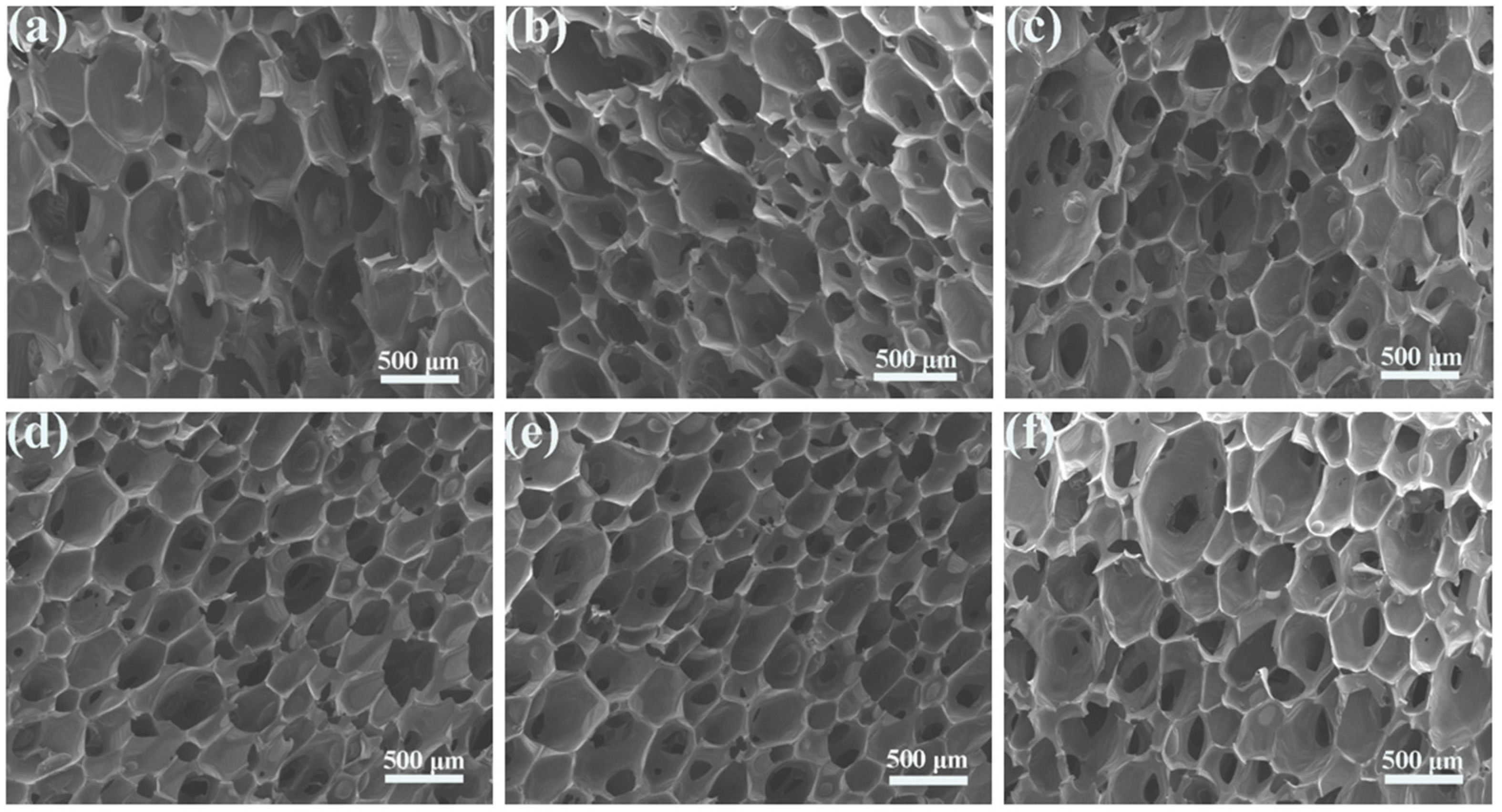

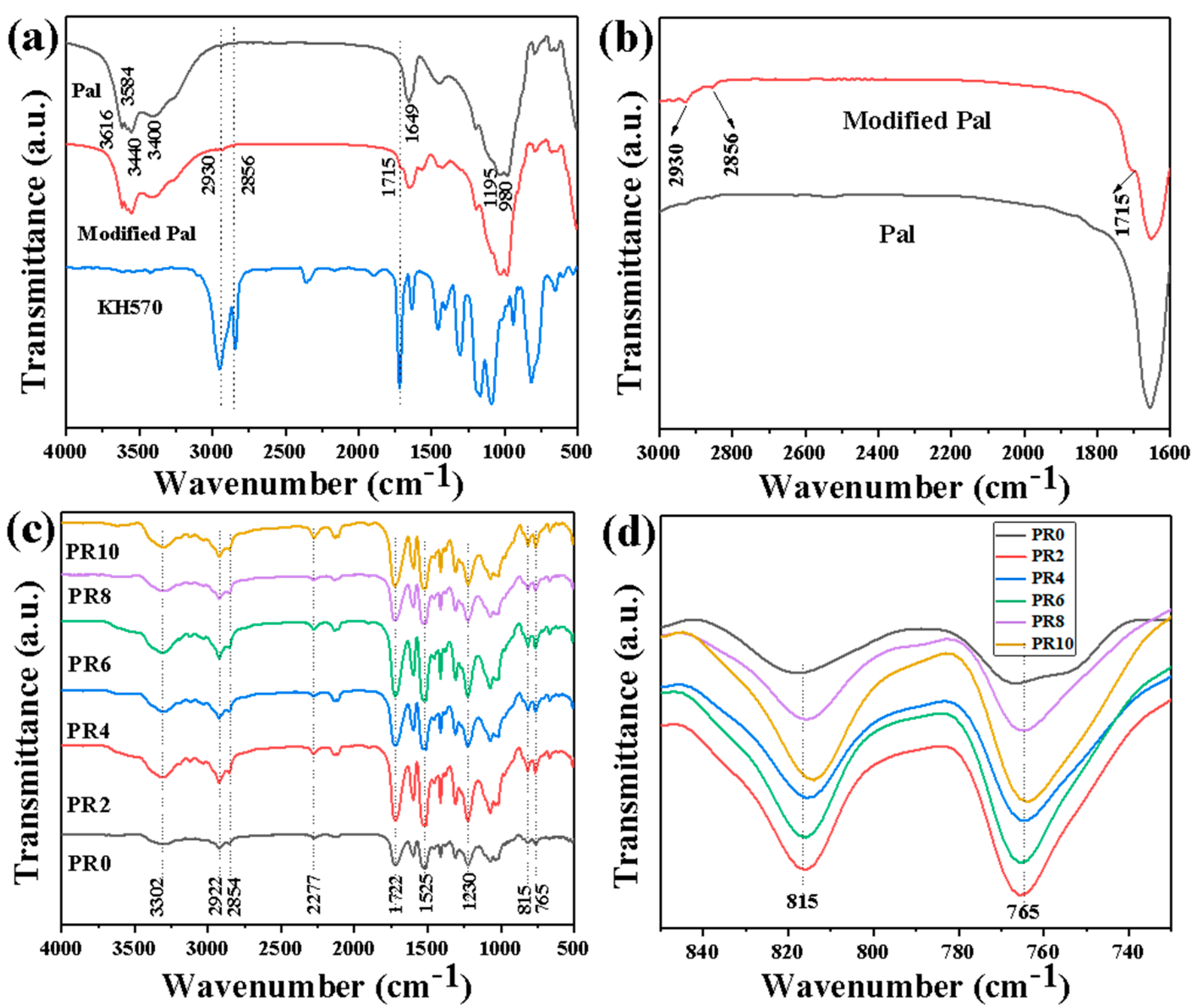
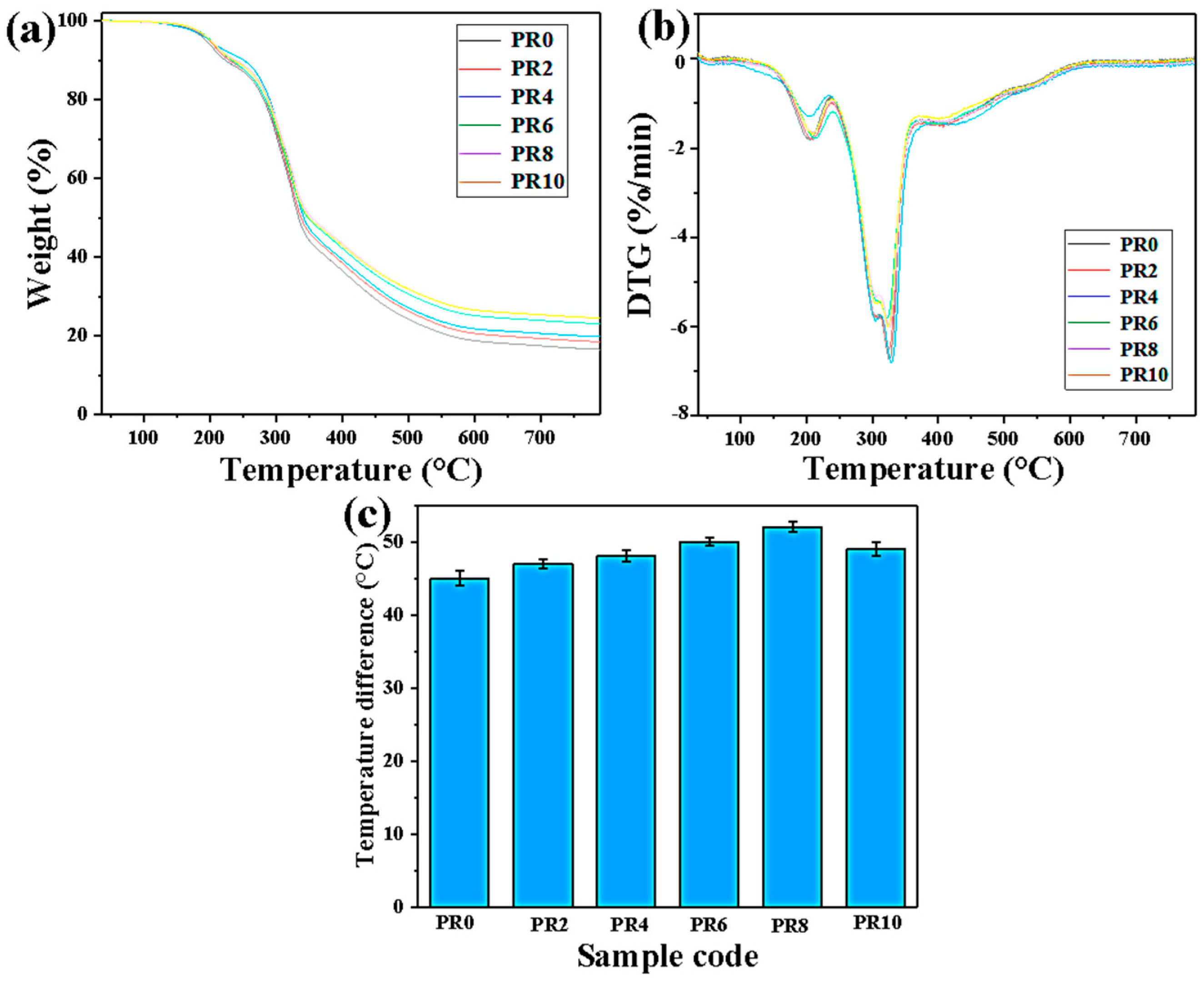
| Sample | T−5% (°C) | T−10% (°C) | T−50% (°C) | Tmax (°C) | Residual Rate (wt%) |
|---|---|---|---|---|---|
| PR0 | 193 | 222 | 333 | 326 | 16 |
| PR2 | 198 | 228 | 337 | 328 | 18 |
| PR4 | 200 | 250 | 341 | 330 | 19 |
| PR6 | 202 | 232 | 347 | 326 | 23 |
| PR8 | 203 | 237 | 353 | 328 | 24 |
| PR10 | 200 | 235 | 349 | 327 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cui, K.; Fang, B.; Wang, F. Cost-Effective Fabrication of Modified Palygorskite-Reinforced Rigid Polyurethane Foam Nanocomposites. Nanomaterials 2022, 12, 609. https://doi.org/10.3390/nano12040609
Wang Y, Cui K, Fang B, Wang F. Cost-Effective Fabrication of Modified Palygorskite-Reinforced Rigid Polyurethane Foam Nanocomposites. Nanomaterials. 2022; 12(4):609. https://doi.org/10.3390/nano12040609
Chicago/Turabian StyleWang, Yulei, Kaibin Cui, Baizeng Fang, and Fei Wang. 2022. "Cost-Effective Fabrication of Modified Palygorskite-Reinforced Rigid Polyurethane Foam Nanocomposites" Nanomaterials 12, no. 4: 609. https://doi.org/10.3390/nano12040609
APA StyleWang, Y., Cui, K., Fang, B., & Wang, F. (2022). Cost-Effective Fabrication of Modified Palygorskite-Reinforced Rigid Polyurethane Foam Nanocomposites. Nanomaterials, 12(4), 609. https://doi.org/10.3390/nano12040609





