CoO Nanozymes with Multiple Catalytic Activities Regulate Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Preparation
2.4. In Vitro and In Vivo Experiment
3. Results and Discussion
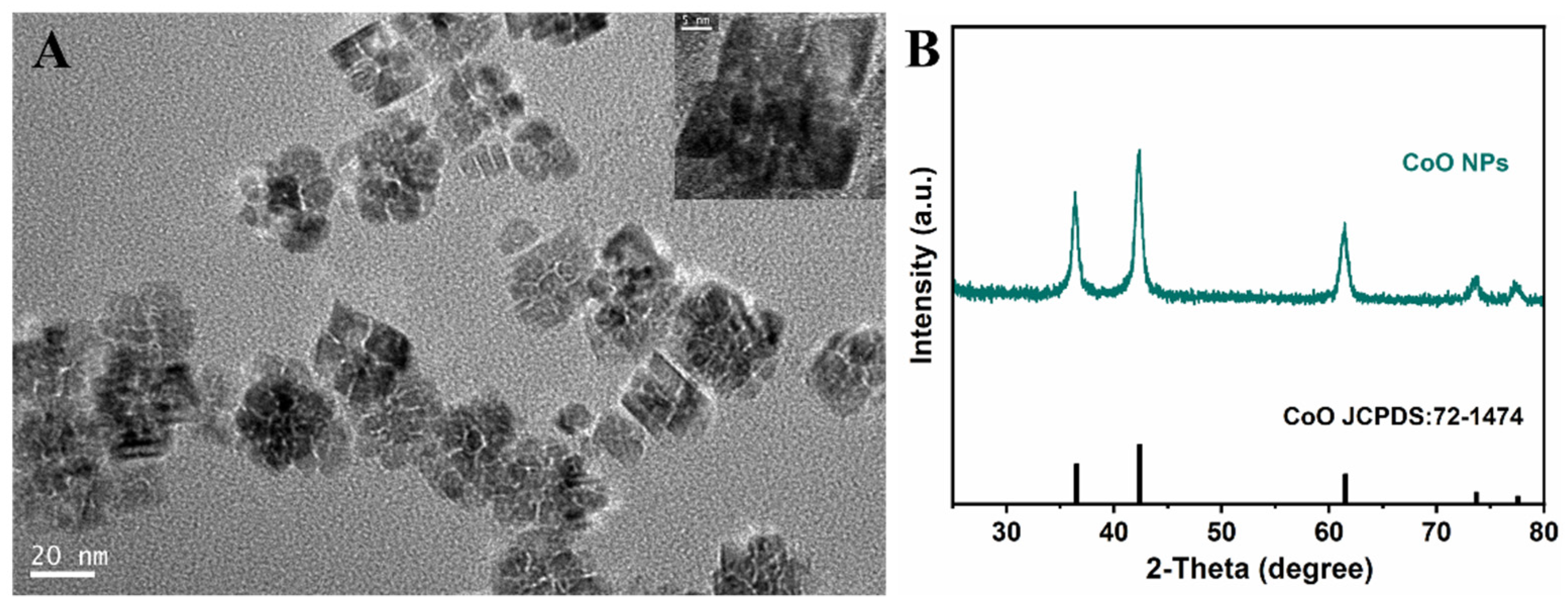
3.1. Properties of CoO Nanoparticles
3.2. Properties of Carboxyl-Modified Hydrophilic CoO Nanoparticles
3.3. Enzyme-like Activity Determination of CoO Nanozymes
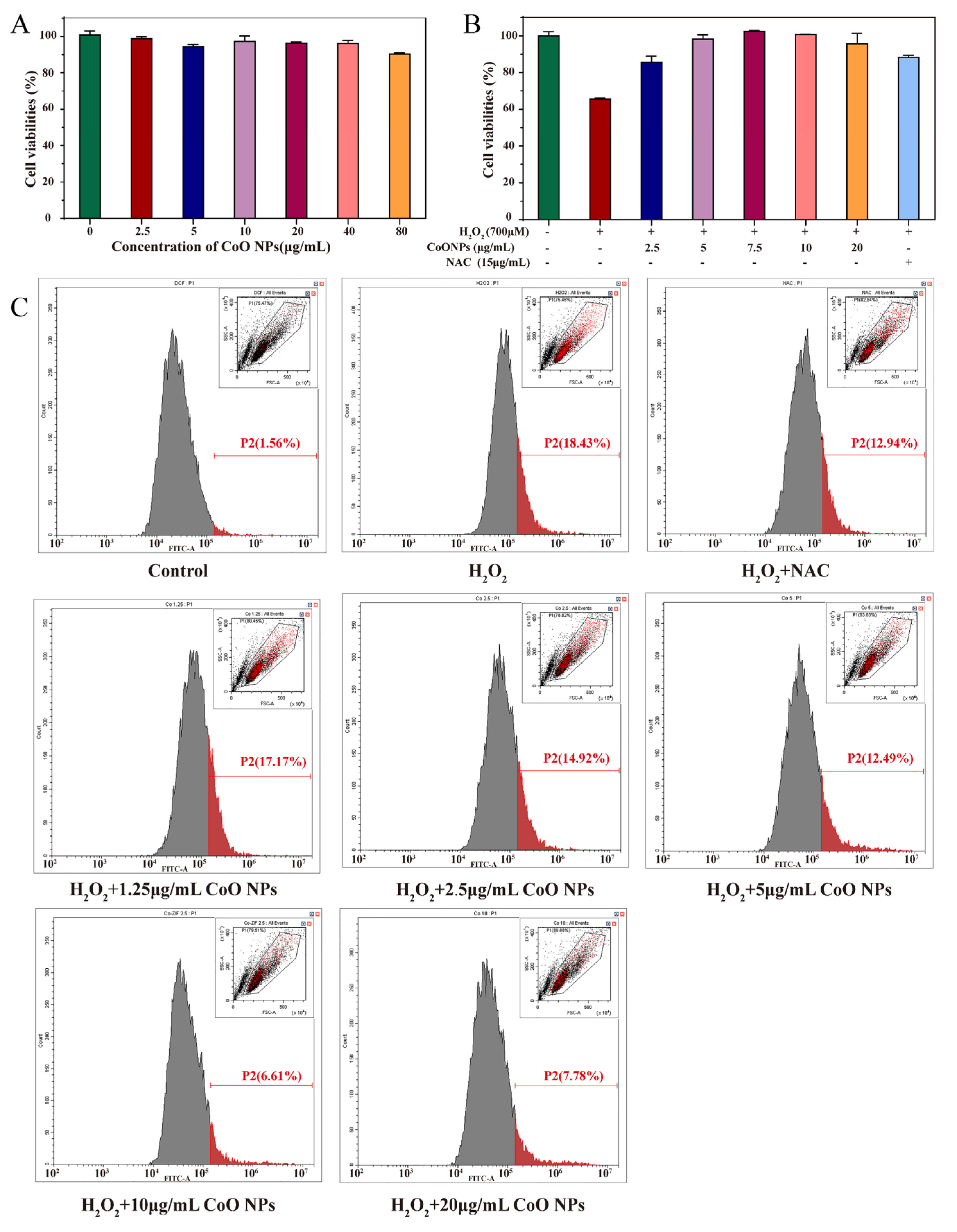
3.4. Cytotoxicity of CoO Nanozymes
3.5. Inhibitory Effect of CoO Nanozymes on Cytotoxicity Induced by H2O2
3.6. Inhibitory Effect of CoO Nanozymes on ROS Production Induced by H2O2
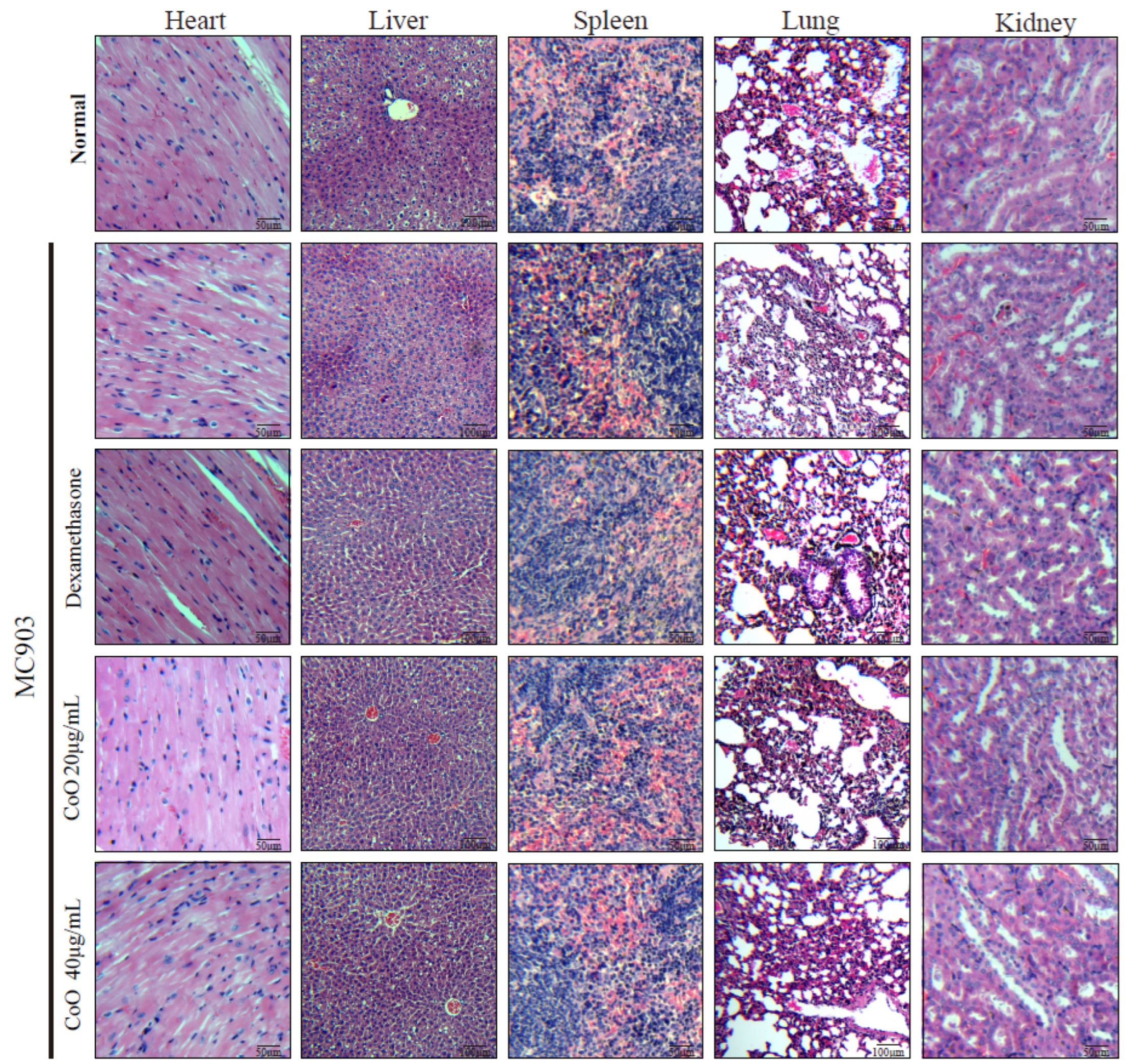
3.7. In Vivo Cytotoxicity of CoO Nanozymes
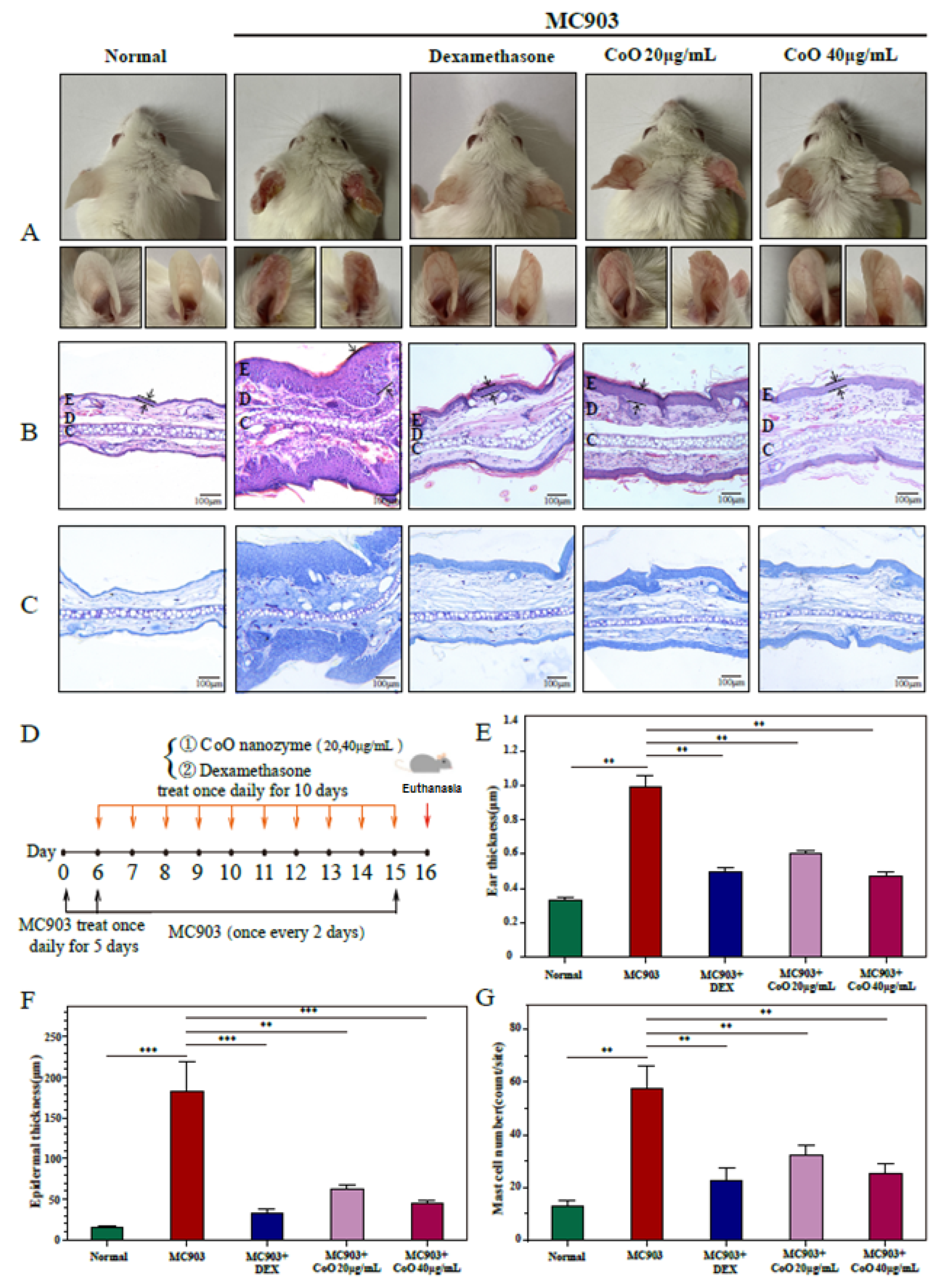
3.8. Relieve the Pathological Symptoms of the Ear Skin of AD Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragg, R.; Tahir, M.N.; Tremel, W. Solids Go Bio: Inorganic Nanoparticles as Enzyme Mimics. Eur. J. Inorg. C 2016, 2016, 1906–1915. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, B.; Yang, R.; Liu, J. Filling in the gaps between nanozymes and enzymes: Challenges and opportunities. Bioconjug. Chem. 2017, 28, 2903–2909. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Multifunctional nanozymes: Enzyme-like catalytic activity combined with magnetism and surface plasmon resonance. Nanoscale Horiz. 2018, 3, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Wei, H. Integrated nanozymes: Facile preparation and biomedical applications. Chem. Commun. 2018, 54, 6520–6530. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron oxide nanozyme: A multifunctional enzyme mimetics for biomedical application. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.J.; Qin, L.; Wang, C.; Song, L.; Zhou, Y.N.; Zhu, G.; Cao, W.; Lin, S.; Zhou, L. eg occupancy as an effective descriptor for the catalytic activity of perovskite oxide-based peroxidase mimics. Nat. Commun. 2019, 10, 704. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Ribeiro, A.; Prieto, T.; Nantes, I.L. Peroxidases in nanostructures. Front. Mol. Biosci. 2015, 2, 50. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Fang, L.; Wu, K.; Yan, X.; Fan, K. Ferritins as natural and artificial nanozymes for theranostics. Theranostics 2020, 10, 687–706. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Hong, J.; Yan, X.; Liang, M. Nanozymes: A New Disease Imaging Strategy. Front. Bioeng. Biotechnol. 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 5, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Tonial, C.H.; Rodrigues, M.F.; Bosse, M.A.; Sousa, I.M.; de Lima, J.D.; da Cunha, M.A.A.; Foglio, M.A.; Marques, M.O.M.; Marchese, J.A. Technical and economic evaluation of cultivation and obtaining of Varronia curassavica Jacq. essential oil. Ind. Crops Prod. 2020, 154, 112650. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Ju, E.G.; Guan, Y.J.; Ren, J.S.; Qu, X.G. Lightmediated reversible modulation of ROS level in living cells by using an activity-controllable nanozyme. Small 2017, 13, 6. [Google Scholar]
- Bhagat, S.; Vallabani, N.V.S.; Shutthanandan, V.; Bowden, M.; Karakoti, A.S.; Singh, S. Gold core/ceria shell-based redox active nanozyme mimicking the biological multienzyme complex phenomenon. J. Colloids Interface Sci. 2018, 513, 831–842. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A redox modulatory Mn3O4 nanozyme with multienzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. Int. Ed. 2017, 56, 14267–14271. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Qin, L.; Zhou, M.; Lou, Z.P.; Wei, H. Nanozyme sensor arrays for detecting versatile analytes from small molecules to proteins and cells. Anal. Chem. 2018, 90, 11696–11702. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.; Winther, A.K.; Fruergaard, A.S.; van der Akker, W.; Sørensen, L.; Nielsen, S.M.; Olesen, M.T.J.; Dai, Y.; Jeppesen, H.S.; Lamagni, P.; et al. Identification and directed development of non-organic catalysts with apparent pan-enzymatic mimicry into nanozymes for efficient prodrug conversion. Angew. Chem. Int. Ed. 2019, 58, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Lu, X. Conducting polymer-based peroxidase mimics: Synthesis, synergistic enhanced properties and applications. Sci. China Mater. 2018, 61, 653–670. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.-Q.; Liu, C.-Y.; Zeng, X.-Y.; Chen, J.; Lü, J.; Lin, R.-G.; Cao, R.; Lin, Z.-J.; Su, J.-W. MOF-808: A metal–organic framework with intrinsic peroxidase-like catalytic activity at neutral pH for colorimetric biosensing. Inorg. Chem. 2018, 57, 9096–9104. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T. Carbon nanodots as peroxidase nanozymes for biosensing. Molecules 2016, 21, 1653. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Chen, G.; Wu, D.; Zhou, X.; Wu, Y. Single-atom nanozymes: A rising star for biosensing and biomedicine. Coord. Chem. Rev. 2020, 418, 213376. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Y.; Cui, H.; Yan, X.; Fan, K. Nanozyme-based catalytic Theranostics. RSC Adv. 2020, 10, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Bayr, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Wu, G.; Berka, V.; Derry, P.J.; Mendoza, K.; Kakadiaris, E.; Roy, T.; Kent, T.A.; Tour, J.M.; Tsai, A.-L. Critical comparison of the superoxide dismutase-like activity of carbon antioxidant nanozymes by direct superoxide consumption kinetic measurements. ACS Nano 2019, 13, 11203–11213. [Google Scholar] [CrossRef]
- Singh, N.; Geethika, M.; Eswarappa, S.M.; Mugesh, G. Manganese-based nanozymes: Multienzyme redox activity and effect on the nitric oxide produced by endothelial nitric oxide synthase. Chem.-Eur. J. 2018, 24, 8393–8403. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Hu, X.; Wu, X.; Zheng, Z.; Boudreau, M.D.; Yin, J.-J. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2013, 34, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; Liu, C.; Ju, E.; Zhang, Y.; Ren, J.; Qu, X. Selfassembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. Int. Ed. 2016, 55, 6646–6650. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 10, 1056–1058. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Najda, A.; Kaur, P.; Shah, M.; Singh, H.; Kaur, P.; Cavalu, S.; Jaroszuk-Sierocińska, M.; Rahman, M.H. Potentiality of Nanoenzymes for Cancer Treatment and Other Diseases: Current Status and Future Challenges. Materials 2021, 14, 5965. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticlesvia Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 295–328. [Google Scholar]
- Luo, L.; Zhang, Y.; Li, F.; Si, X.; Ding, Y.; Deng, D.; Wang, T. Enzyme mimics of spinel-type CoxNi1−xFe2O4 magnetic nanomaterial for eletroctrocatalytic oxidation of hydrogen peroxide. Anal. Chim. Acta 2013, 788, 46–51. [Google Scholar] [CrossRef]
- Wu, Q.; He, L.; Jiang, Z.W.; Li, Y.; Zheng, M.C.; Cheng, Z.H.; Li, Y.F. CuO Nanoparticles Derived from Metal-Organic Gelwith Excellent Electrocatalytic and Peroxidase-Mimicking Activities for Glucose and Cholesterol Detection. Biosens. Bioelectron. 2019, 145, 111704. [Google Scholar] [CrossRef]
- Zheng, S.; Li, B.; Tang, Y.; Li, Q.; Xue, H.; Pang, H. Ultrathin Nanosheet Assembled [Ni3(OH)2(PTA)2(H2O)4]2H2O Hierarchical Flowers for High Performance Electrocatalysis of Glucose Oxidation Reactions. Nanoscale 2018, 10, 13270–13276. [Google Scholar] [CrossRef]
- Chou, K.-S.; Ren, C.-Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. [Google Scholar] [CrossRef]
- DAS, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef]
- Han, R.; Lu, Y.; Mingjun, L.; Yanbo, W.; Xuan, L.; Chongyang, L.; Kun, L.; Lingxing, Z.; Aihua, L. Green tide biomass templated synthesis of molybdenumoxide nanorods supported on carbon as efficient nanozyme for sensitive glucose colorimetric assay. Sens. Actuators B Chem. 2019, 296, 126517. [Google Scholar]
- Han, L.; Zhang, H.; Li, F. Bioinspired Nanozymes with pH Independent and MetalIons-Controllable Activity: Field Programmable Logic Conversion of Sole Logic Gate System. Part. Part. Syst. Charact. 2018, 35, 1800207. [Google Scholar] [CrossRef]
- Golchin, J.; Golchin, K.; Alidadian, N.; Ghaderi, S.; Eslamkhah, S.; Eslamkhah, M.; Akbarzadeh, A. Nanozyme applications in biology and medicine: An overview. Artif. Cell. Nanomed. B 2017, 45, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trend. Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.S.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent advances in the synthesis of Fe3O4@AU core/shell nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178. [Google Scholar] [CrossRef]
- Likhitkar, S.; Bajpai, A.K. Magnetically controlled release of cisplatin from superparamagnetic starch nanoparticles. Carbohydr. Polym. 2012, 87, 300–308. [Google Scholar] [CrossRef]
- Dong, J.; Song, L.; Yin, J.J.; He, W.W.; Wu, Y.H.; Gu, N.; Zhang, Y. Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl. Mater. Interfaces 2014, 6, 1959–1970. [Google Scholar] [CrossRef]
- Bedair, T.M.; Min, I.J.; Park, W.; Joung, Y.K.; Han, D.K. Sustained drug release using cobalt oxide nanowires for the preparation of polymer-free drug-eluting stents. J. Biomater. Appl. 2018, 33, 352–362. [Google Scholar] [CrossRef]
- Jarestan, M.; Khalatbari, K.; Pouraei, A.; Shandiz, S.A.S.; Beigi, S.; Hedayati, M.; Majlesi, A.; Akbari, F.; Salehzadeh, A. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech. 2020, 10, 230. [Google Scholar] [CrossRef]
- Yun, T.; Liu, Y.; Yi, S.; Qi, J.; Liu, Y.; Zhou, J. Artificially controlled degradable nanoparticles for contrast switch MRI and programmed cancer therapy. Int. J. Nanomed. 2018, 13, 6647–6659. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yan, L.; Zhang, R.; Ren, H.; Liu, A.H. CoO-supported ordered mesoporous carbon nanocomposite based nanozyme with peroxidase-like activity for colorimetric detection of glucose. Process Biochem. 2019, 81, 92–98. [Google Scholar] [CrossRef]
- Lian, J.; He, Y.; Li, N.; Liu, P.; Liu, Z.X.; Liu, Q.Y. Magnetic flower-like Fe-doped CoO nanocomposites with dual enzyme-like activities for facile and sensitive determination of H2O2 and dopamine. Inorg. Chem. 2021, 60, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, S.; Hai, J.; Liang, K.; Sun, S.H.; Meng, G.P.; Wang, B.D. Nitrogen-doped chiral CuO/CoO nanofibers: An enhanced electrochemiluminescence sensing strategy for detection of 3,4-dihydroxy-phenylalanine enantiomers. Anal. Chem. 2021, 93, 11470–11478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, S.; Li, S.; Yao, X.X.; Zhu, X.X.; Chen, W.; Fan, G.C.; Liu, Z.X.; Liu, Q.Y.; Yue, K. CoO nanotubes loaded on graphene and modified with porphyrin moieties for colorimetric sensing of dopamine. ACS Appl. Nano Mater. 2021, 4, 8706–8715. [Google Scholar] [CrossRef]
- Ling, T.; Yan, D.; Jiao, Y.; Wang, H.; Zheng, Y.; Zheng, X.; Mao, J.; Du, X.; Hu, Z.; Jaroniec, M.; et al. Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis. Nat. Commun. 2016, 7, 12876. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Jiao, L.; Liu, Y.; Liu, H.; Wang, Y.; Yuan, H. Ultra-high capacity lithium-ion batteries with hierarchical CoO nanowire clusters as binder free electrodes. Adv. Funct. Mater. 2015, 25, 1082–1089. [Google Scholar] [CrossRef]
- Jin, W.; Guo, X.; Zhang, J.; Zheng, L.; Liu, F.; Hu, Y.; Mao, J.; Liu, H.; Xue, Y.; Tang, C. Ultrathin carbon coated CoO nanosheet arrays as efficient electrocatalysts for the hydrogen evolution reaction. Catal. Sci. Technol. 2019, 9, 6957–6964. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Wang, H.; Han, M.; Li, H.; Yuan, S.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots decorated the exposing highreactive (111) facets CoO octahedrons with enhanced photocatalytic activity and stability for tetracycline degradation under visible light irradiation. Appl. Catal. B 2017, 219, 36–44. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, D.; Zhan, J.; Xie, M.; Tang, J.; Fang, G.; Zhang, M.; Kong, L.; Zhu, F. Biomass Schiff base polymer-derived N-doped porous carbon embedded with CoO nanodots for adsorption and catalytic degradation of chlorophenol by peroxymonosulfate. J. Hazard. Mater. 2020, 384, 121345. [Google Scholar]
- Jin, C.; Lian, J.; Gao, Y.; Guo, K.; Wu, K.; Gao, L.; Zhang, X.; Zhang, X.; Liu, Q. Si doped CoO nanorods as peroxidase mimics for colorimetric sensing of reduced glutathione. ACS Sustain. Chem. Eng. 2019, 7, 13989–13998. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.K. Oxidative stress in atopic dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Xu, J.; Luo, Q.; Hou, C.X.; Liu, J.Q. Rational design and biological application of antioxidant nanozymes. Front. Chem. 2020, 8, 831. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.H.; Lee, E.; Lee, B.C.; Choi, M.J.; Kwon, T.R.; Hong, J.; Mun, S.K.; Lee, K.; Kim, S.; Na, J.; et al. Therapeutic potential of topically administered γ-AlOOH on 2,4-dinitrochlorobenzene-induced atopic dermatitis-like lesions in Balb/c mice. Exp. Dermatol. 2019, 28, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Mu, Z.L.; Zhao, Y.; Zhang, J.Z. Topical tetracycline improves MC903-induced atopic dermatitis in mice through inhibition of inflammatory cytokines and thymic stromal lymphopoietin expression. Chin. Med. J. 2016, 129, 1483–1490. [Google Scholar] [CrossRef]
- Lee, H.; Yeom, M.; Shin, S.; Jeon, K.; Park, D.; Jung, E. Protective effects of aqueous extract of mentha suaveolens against oxidative Stress-Induced damages in human keratinocyte HaCaT cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 5045491. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, M.; Guan, X.; Wu, F.; Ma, L. CoO Nanozymes with Multiple Catalytic Activities Regulate Atopic Dermatitis. Nanomaterials 2022, 12, 638. https://doi.org/10.3390/nano12040638
Mao M, Guan X, Wu F, Ma L. CoO Nanozymes with Multiple Catalytic Activities Regulate Atopic Dermatitis. Nanomaterials. 2022; 12(4):638. https://doi.org/10.3390/nano12040638
Chicago/Turabian StyleMao, Mao, Xuejiao Guan, Feng Wu, and Lan Ma. 2022. "CoO Nanozymes with Multiple Catalytic Activities Regulate Atopic Dermatitis" Nanomaterials 12, no. 4: 638. https://doi.org/10.3390/nano12040638
APA StyleMao, M., Guan, X., Wu, F., & Ma, L. (2022). CoO Nanozymes with Multiple Catalytic Activities Regulate Atopic Dermatitis. Nanomaterials, 12(4), 638. https://doi.org/10.3390/nano12040638





