Extended Line Defect Graphene Modified by the Adsorption of Mn Atoms and Its Properties of Adsorbing CH4
Abstract
:1. Introduction
2. Calculation Methods and Models
3. Results and Discussion
3.1. CH4 Adsorption in the ELD-42C Graphene Configuration
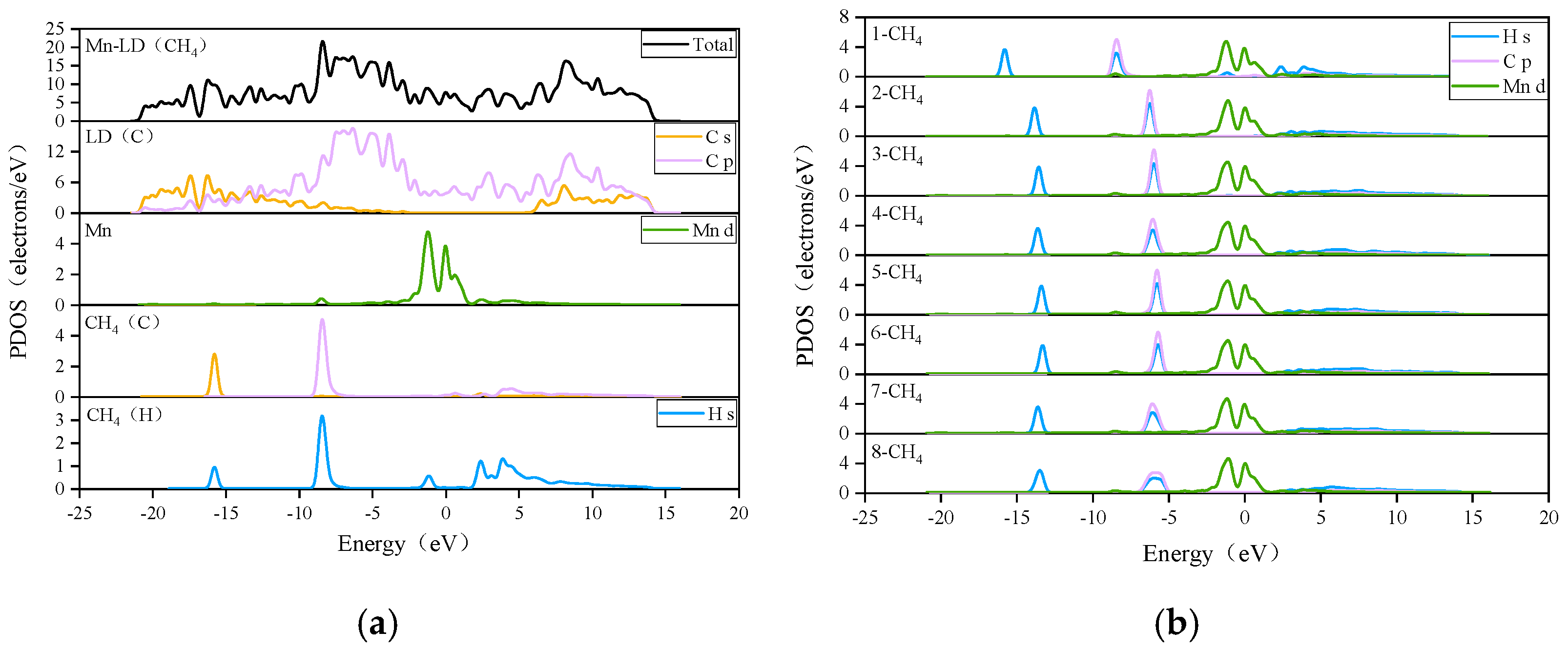
3.2. CH4 Adsorption in the Mn-ELD-42C Graphene Configuration
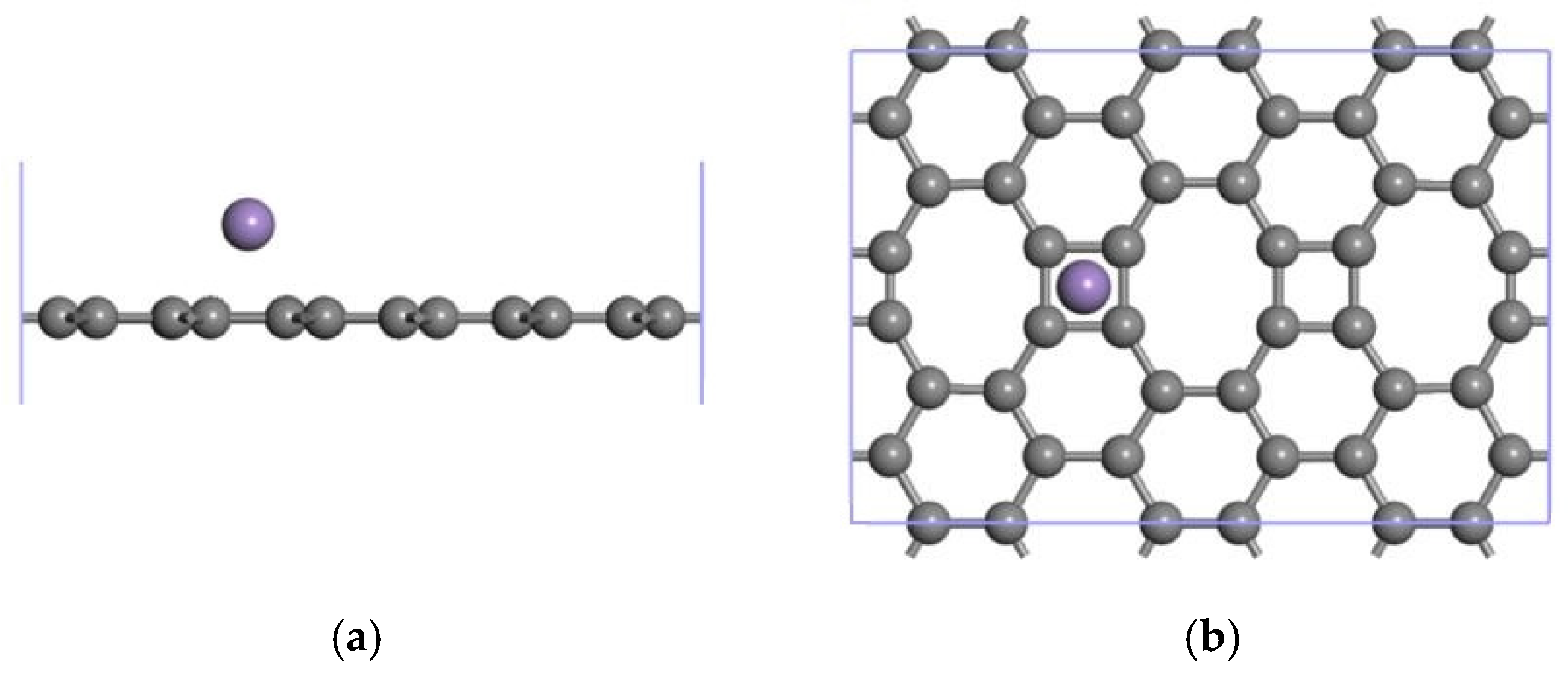
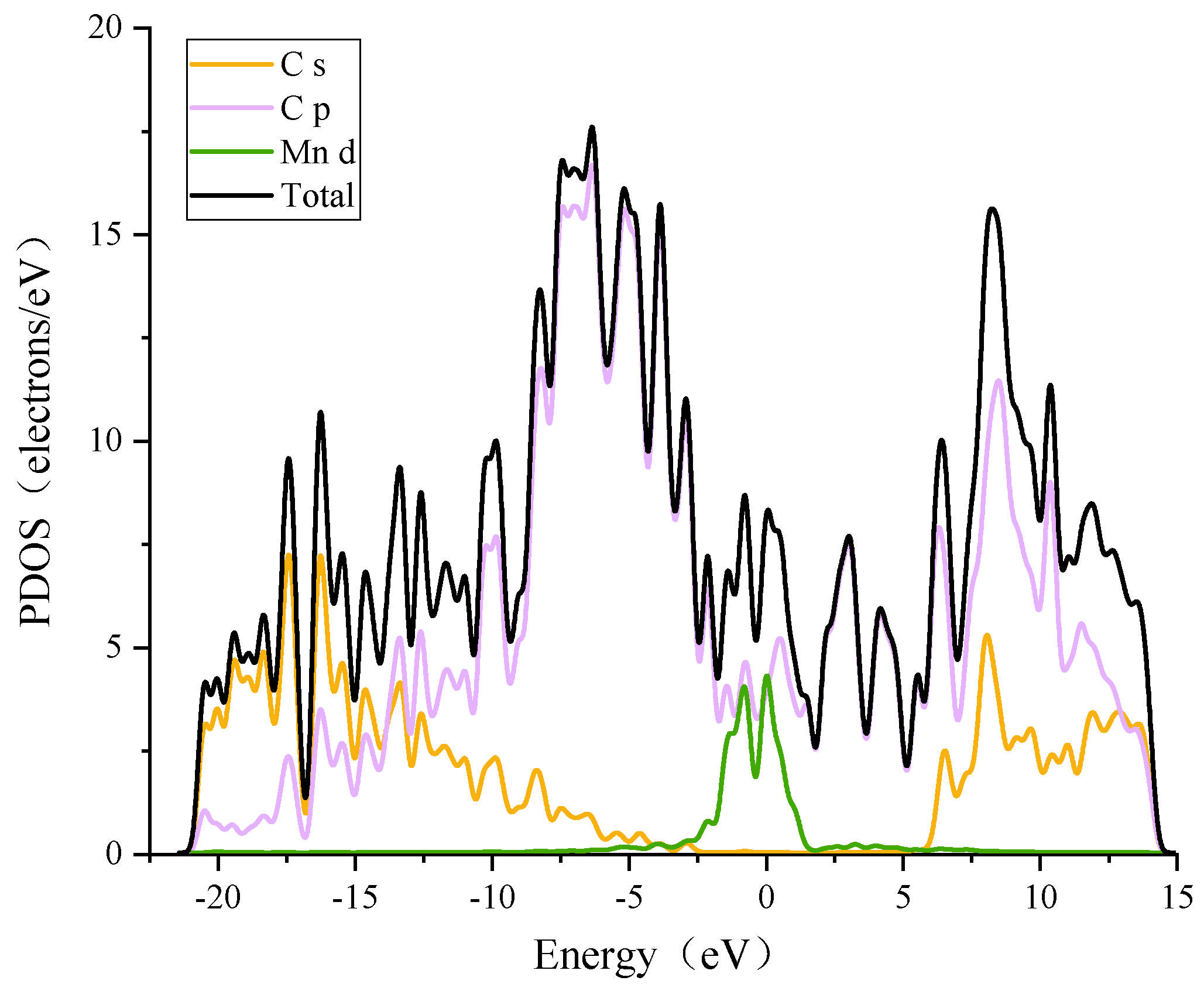
3.2.1. Modification of the ELD-42C Graphene Configuration by a Single Mn Atom
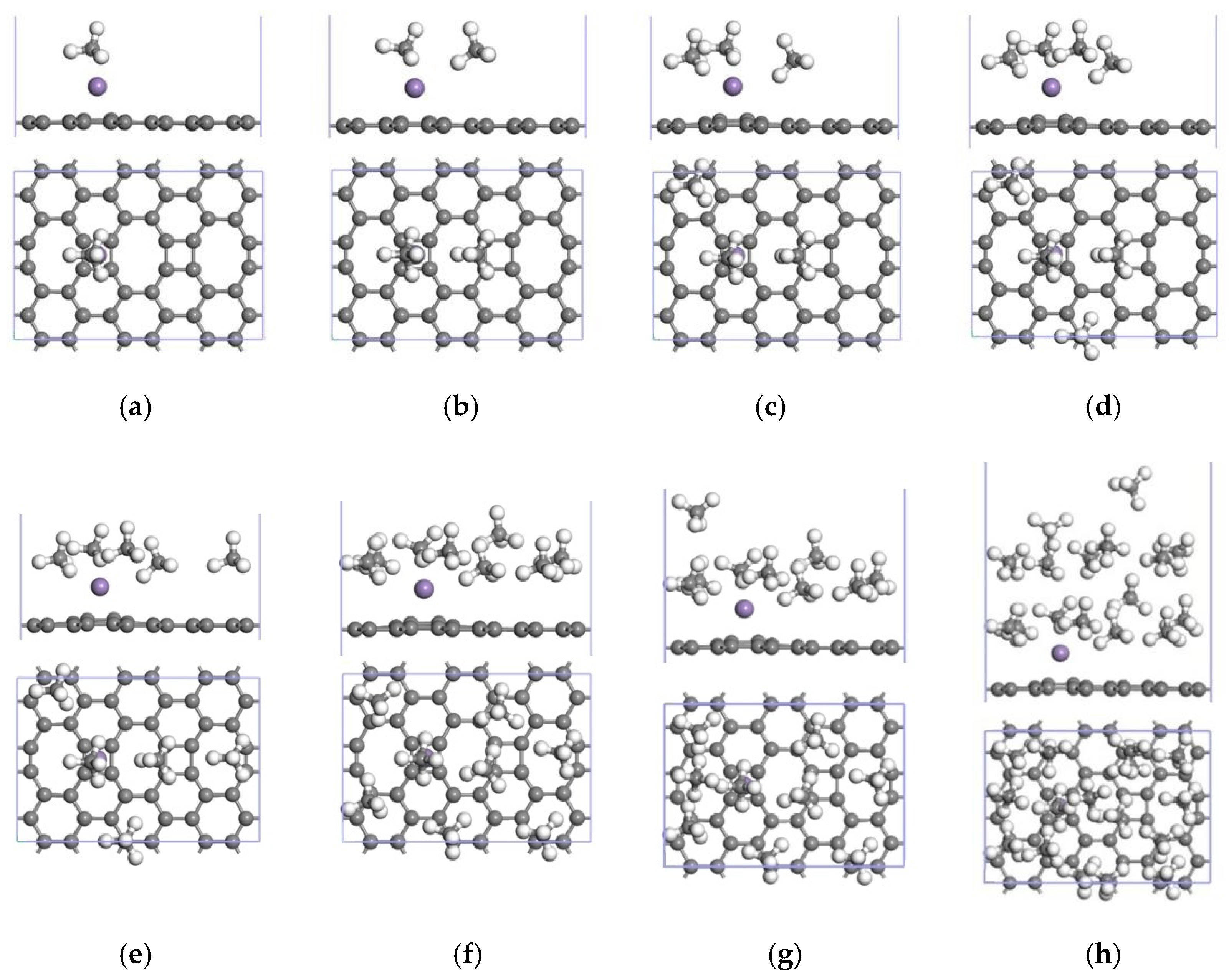
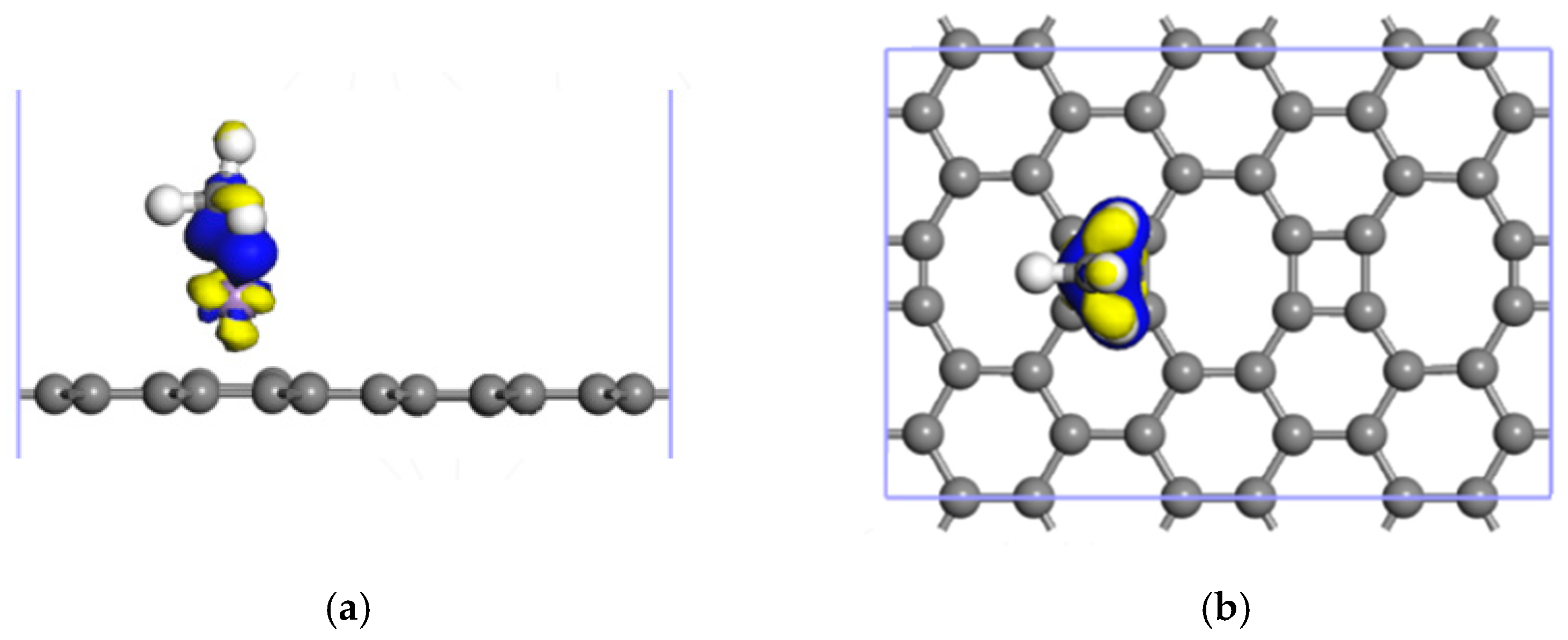
3.2.2. Adsorption of CH4 by the Mn-ELD-42C Graphene Configuration
3.3. CH4 Adsorption in the 2Mn-ELD-42C Graphene Configuration
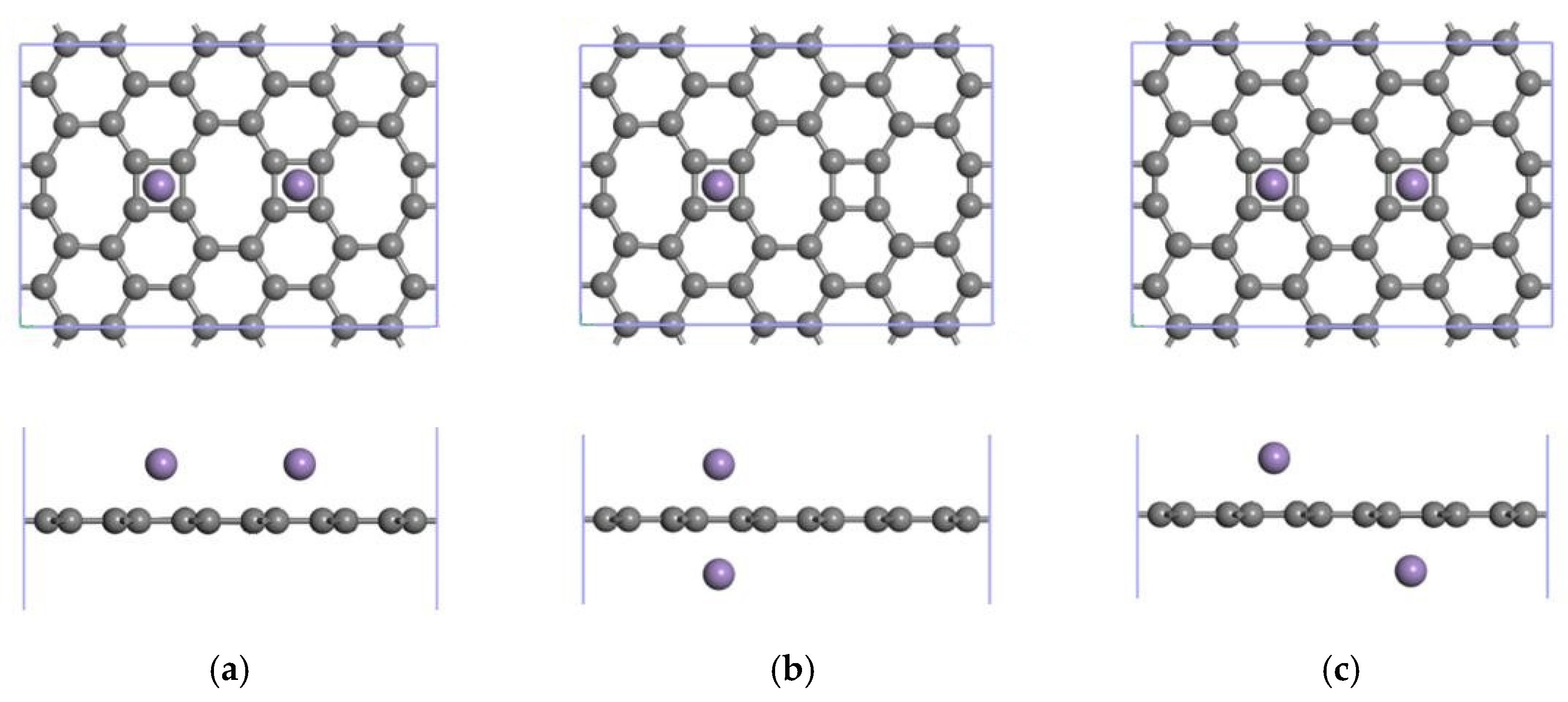
3.3.1. Modification of the ELD-42C Graphene Configuration by Two Mn Atoms
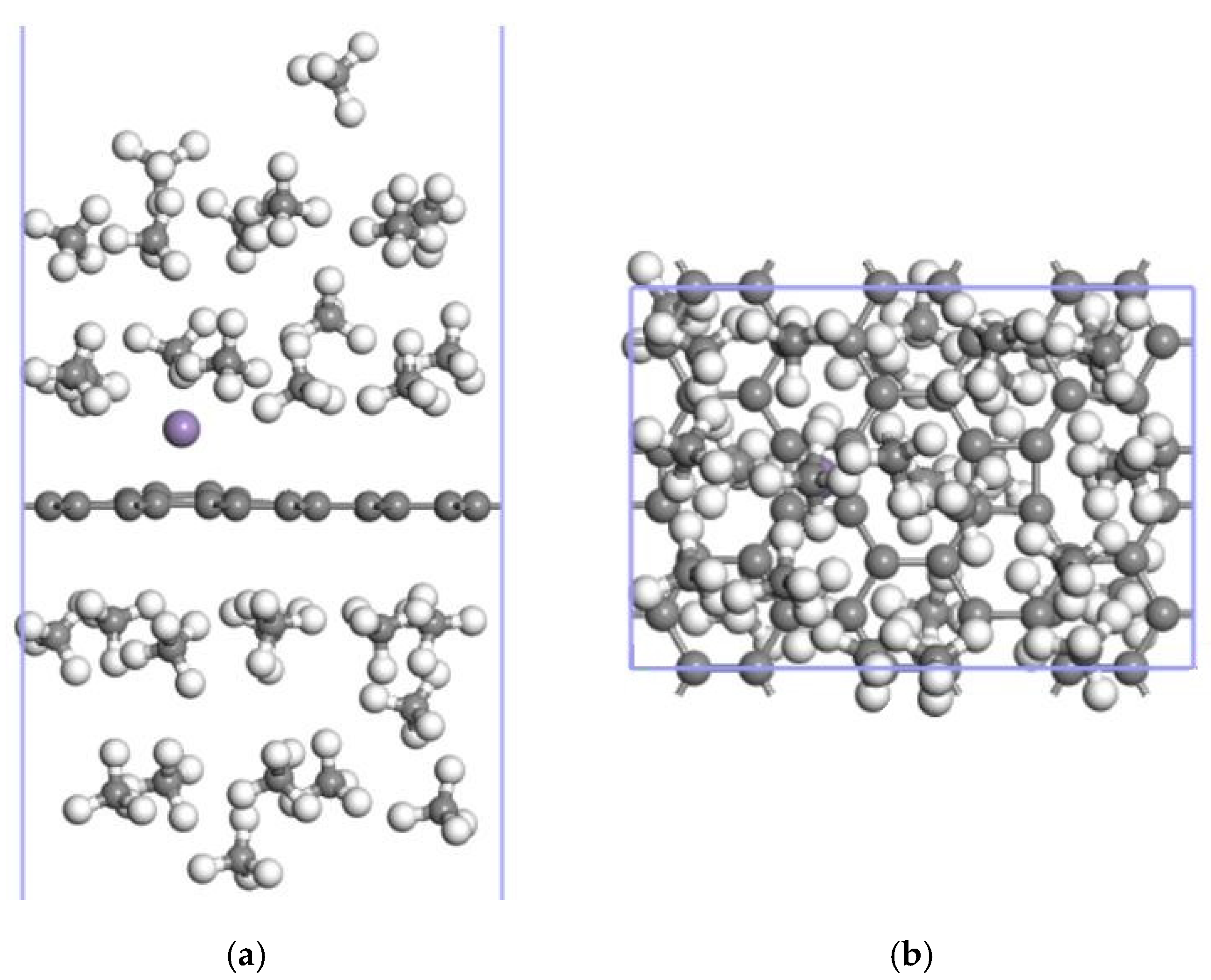
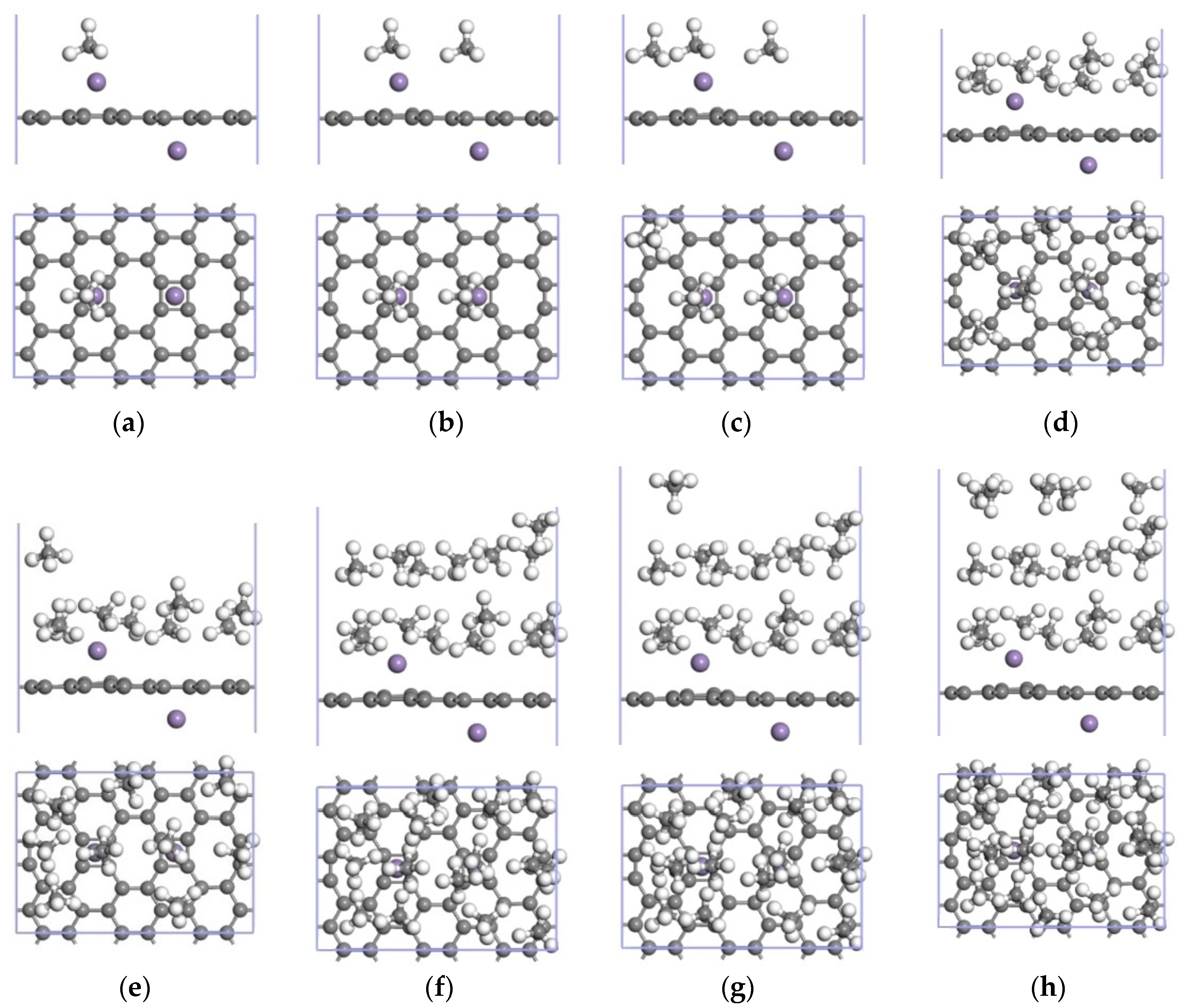
3.3.2. Adsorption of CH4 by the 2Mn-ELD-42C Graphene Configuration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantha, N.; Ulman, K.; Narasimhan, S. Adsorption of methane on single metal atoms supported on graphene: Role of electron back-donation in binding and activation. J. Chem. Phys. 2020, 153, 244701. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, H.; Zhang, Z.; An, L. First-principles investigation of vacancy-defected graphene and Mn-doped graphene towards adsorption of H2S. Superlattices Microstruct. 2019, 134, 106235. [Google Scholar] [CrossRef]
- Vekeman, J.; Cuesta, I.G.; Faginas-Lago, N.; Wilson, J.; Sánchez-Marín, J.; de Merás, A.S. Potential models for the simulation of methane adsorption on graphene: Development and CCSD(T) benchmarks. Phys. Chem. Chem. Phys. 2018, 20, 25518–25530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L. Recent developments in methane decomposition over heterogeneous catalysts: An overview. Mater. Renew. Sustain. Energy 2020, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hassani, A.; Mosavian, M.T.H.; Ahmadpour, A.; Farhadian, N. Improvement of methane uptake inside graphene sheets using nitrogen, boron and lithium-doped structures: A hybrid molecular simulation. Korean J. Chem. Eng. 2017, 34, 876–884. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Song, M.; Liu, X.; Xu, W.; Zhang, M.; Zhang, C. Methane Adsorption Properties of Mn-Modified Graphene: A First-Principles Study. Adv. Theory Simul. 2020, 3, 2000035. [Google Scholar] [CrossRef]
- Ghanbari, R.; Safaiee, R.; Golshan, M. A dispersion-corrected DFT investigation of CH4 adsorption by silver-decorated monolayer graphene in the presence of ambient oxygen molecules. Appl. Surf. Sci. 2018, 457, 303–314. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.; Zhao, Y.; Zhang, M.; Tian, R.; Zhang, C. First-principles study on the methane adsorption properties by Ti-modified graphyne. Int. J. Quantum Chem. 2021, 121, e26811. [Google Scholar] [CrossRef]
- Mahmoud, E. Recent advances in the design of metal–organic frameworks for methane storage and delivery. J. Porous Mater. 2020, 28, 213–230. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S. Interfacial Strengthening of Graphene/Aluminum Composites through Point Defects: A First-Principles Study. Nanomaterials 2021, 11, 738. [Google Scholar] [CrossRef]
- Feicht, P.; Eigler, S. Defects in Graphene Oxide as Structural Motifs. ChemNanoMat 2018, 4, 244–252. [Google Scholar] [CrossRef]
- Xiong, L.; Gong, B.; Peng, Z.; Yu, Z. Spin-Seebeck effect and thermoelectric properties of one-dimensional graphene-like nanoribbons periodically embedded with four- and eight-membered rings. Phys. Chem. Chem. Phys. 2021, 23, 23667–23672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, Y.; Chen, Y.; Ju, J.; Xu, W.; Zhang, M.; Sang, C.; Zhang, C. First-principles study on methane storage properties of porous graphene modified with Mn. Appl. Phys. A 2021, 127, 1–12. [Google Scholar] [CrossRef]
- Cao, C.; Wu, M.; Jiang, J.; Cheng, H.-P. Transition metal adatom and dimer adsorbed on graphene: Induced magnetization and electronic structures. Phys. Rev. B 2010, 81, 205424. [Google Scholar] [CrossRef]
- Yu, G.; Zhu, M.; Zheng, Y. First-principles study of 3d transition metal atom adsorption onto graphene: The role of the extended line defect. J. Mater. Chem. C 2014, 2, 9767–9774. [Google Scholar] [CrossRef]
- Guan, Z.; Ni, S.; Hu, S. First-Principles Study of 3d Transition-Metal-Atom Adsorption onto Graphene Embedded with the Extended Line Defect. ACS Omega 2020, 5, 5900–5910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.K. Diversity in the Family of Manganese Oxides at the Nanoscale: From Fundamentals to Applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef] [PubMed]
- Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Aluminothermic reduction of manganese oxide from selected MnO-containing slags. Materials 2021, 14, 356. [Google Scholar] [CrossRef]
- Luo, X.; Peng, C.; Shao, P.; Tang, A.; Huang, A.; Wu, Q.; Sun, L.; Yang, L.; Shi, H.; Luo, X. Enhancing nitrate removal from wastewater by integrating heterotrophic and autotrophic denitrification coupled manganese oxidation process (IHAD-MnO): Internal carbon utilization performance. Environ. Res. 2021, 194, 110744. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Xi, W.; Zhao, Q.; Wang, S.; Qiu, M.; Wang, J.; Wang, X. Removal of Radionuclides from Aqueous Solution by Manganese Dioxide-Based Nanomaterials and Mechanism Research: A Review. ACS ES&T Eng. 2021, 1, 685–705. [Google Scholar] [CrossRef]
- Mansouri, M. Effects of Vacancy-Defected, Dopant and the Adsorption of Water upon Mn2O3 and Mn3O4 (001) Surfaces: A First-Principles Study. Acta Phys. Pol. A 2018, 133, 1178–1185. [Google Scholar] [CrossRef]
- Yan, H.; Hu, W.; Cheng, S.; Xia, H.; Chen, Q.; Zhang, L.; Zhang, Q. Microwave-assisted preparation of manganese dioxide modified activated carbon for adsorption of lead ions. Water Sci. Technol. 2020, 82, 170–184. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Wang, K.; Miao, L.; Lan, Q.; Jiang, K.; Lu, H.; Li, M.; Li, Y.; Shen, B.; et al. Analysis of oxidation degree of graphite oxide and chemical structure of corresponding reduced graphite oxide by selecting different-sized original graphite. RSC Adv. 2018, 8, 17209–17217. [Google Scholar] [CrossRef] [Green Version]
- Jiao, S.; Li, T.; Xiong, C.; Tang, C.; Li, H.; Zhao, T.; Dang, A. A Facile Method to Prepare Silver Doped Graphene Combined with Polyaniline for High Performances of Filter Paper Based Flexible Electrode. Nanomaterials 2019, 9, 1434. [Google Scholar] [CrossRef] [Green Version]
- Ghashghaee, M.; Ghambarian, M. Methane adsorption and hydrogen atom abstraction at diatomic radical cation metal oxo clusters: First-principles calculations. Mol. Simul. 2018, 44, 850–863. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Q.H.; Jiang, J.; Wong, C.K.Y.; Leung, S.Y.; Ye, H.; Yang, D.G.; Ren, T.L. Functionalization-induced changes in the structural and physical properties of amorphous polyaniline: A first-principles and molecular dynamics study. Sci. Rep. 2016, 6, 20621. [Google Scholar] [CrossRef]
- Zhao, S.; Larsson, K. First Principle Study of the Attachment of Graphene onto Different Terminated Diamond (111) Surfaces. Adv. Condens. Matter Phys. 2019, 2019, 9098256. [Google Scholar] [CrossRef] [Green Version]
- Goyenola, C.; Stafstrom, S.; Hultman, L.; Gueorguiev, K.G. Structural patterns arising during synthetic growth of fuller-ene-like sulfocarbide. J. Phys. Chem. C 2012, 116, 21124–21131. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Czigány, Z.; Furlan, A.; Stafström, S.; Hultman, L. Intercalation of P atoms in Fullerene-like CPx. Chem. Phys. Lett. 2011, 501, 400–403. [Google Scholar] [CrossRef] [Green Version]
- Gueorguiev, G.; Goyenola, C.; Schmidt, S.; Hultman, L. CFx: A first-principles study of structural patterns arising during synthetic growth. Chem. Phys. Lett. 2011, 516, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-q.; Su, H.; Jing, Y.; Guo, J.; Tang, J. Methane adsorption on the surface of a model of shale: A density functional theory study. Appl. Surf. Sci. 2016, 387, 379–384. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Björkman, T.; Guļāns, A.; Krasheninnikov, A.; Nieminen, R.M. van der Waals Bonding in Layered Compounds from Advanced Density-Functional First-Principles Calculations. Phys. Rev. Lett. 2012, 108, 235502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Liu, G.-L.; Fan, D.-Z.; Zhang, G.-Y. Density functional theory study on the electronic structure and optical properties of S absorbed graphene. Phys. B Condens. Matter 2018, 545, 99–106. [Google Scholar] [CrossRef]
- Akay, T.I.; Toffoli, D.; Ustunel, H. Combined effect of point defects and layer number on the adsorption of benzene and toluene on graphene. Appl. Surf. Sci. 2019, 480, 1063–1069. [Google Scholar] [CrossRef]
- Liu, M.; Liu, M.; She, L.; Zha, Z.; Pan, J.; Li, S.; Li, T.; He, Y.; Cai, Z.; Wang, J.; et al. Graphene-like nanoribbons periodically embedded with four- and eight-membered rings. Nat. Commun. 2017, 8, 14924. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, Y.; Xu, W.; Zhang, M.; Zhang, C. First-Principles Study on Methane Adsorption Performance of Ti-Modified Porous Graphene. Phys. Status Solidi B 2021, 258, 2100168. [Google Scholar] [CrossRef]
- Ding, Y. Research on the Mechanism of CH4, CO2, H2O and O2 Adsorption on Coal Molecule. Master’s Thesis, North China Electric Power University, Baoding, China, 2018. [Google Scholar]
- Li, K.; Li, H.; Yan, N.; Wang, T.; Zhao, Z. Adsorption and dissociation of CH4 on graphene: A density functional theory study. Appl. Surf. Sci. 2018, 459, 693–699. [Google Scholar] [CrossRef]
- Yuan, W.H. Study on the Adsorption Properties of Formaldehyde-Methane on Graphene; Beijing Institute of Technology: Beijing, China, 2016. [Google Scholar]
- Zhou, J.; Xuan, H.Y.; Xiao, R.J.; Zhang, X.Y.; Man, M.O.; Fang, Z.J. First-principles study on adsorption property of carbon surface to methane. J. Guangxi Univ. Nat. Sci. Ed. 2018. [Google Scholar]
- Liu, J.; Chen, Y.Z.; Xie, Y. The calculation of adsorption properties of H2S, CO2 and CH4 on Fe doped MoS2 based on first-principles. J. At. Mol. Phys. 2020, 37, 501–507. [Google Scholar]
- Kumar, K.V.; Preuss, K.; Titirici, M.; Rodriguez-Reinoso, F. Nanoporous Materials for the Onboard Storage of Natural Gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.-H.; Song, M.; Liu, X.; Zhao, Y.; Zhang, M.; Zhang, C.-R. First-Principles Study on Methane (CH4) Storage Properties of Graphdiyne. J. Phys. Chem. C 2020, 124, 8110–8118. [Google Scholar] [CrossRef]
- Zhou, X.; Gall, D.; Khare, S.V. Mechanical properties and electronic structure of anti-ReO3 structured cubic nitrides, M3N, of d block transition metals M: An ab initio study. J. Alloy. Compd. 2014, 595, 80–86. [Google Scholar] [CrossRef]
- Tawfik, S.A.; Cui, X.Y.; Ringer, S.P.; Stampfl, C. Multiple CO2 capture in stable metal-doped graphene: A theoretical trend study. RSC Adv. 2015, 5, 50975–50982. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Meng, F. Structure and gap opening of graphene with Fe doped bridged trivacancy. Comput. Mater. Sci. 2016, 117, 65–70. [Google Scholar] [CrossRef]
- Liu, X. Theoretical Studies of Interaction between Metal and Graphene and Molecular Clusters. Master’s Thesis, Jilin University, Changchun, China, 2011. [Google Scholar]
- Wu, M.; Cao, C.; Jiang, J.Z. Electronic structure of substitutionally Mn-doped graphene. New J. Phys. 2010, 12, 63020. [Google Scholar] [CrossRef]
- Chen, J.-J.; Li, W.-W.; Li, X.-L.; Yu, H.-Q. Improving Biogas Separation and Methane Storage with Multilayer Graphene Nanostructure via Layer Spacing Optimization and Lithium Doping: A Molecular Simulation Investigation. Environ. Sci. Technol. 2012, 46, 10341–10348. [Google Scholar] [CrossRef]
- Rad, A.S.; Pazoki, H.; Mohseni, S.; Zareyee, D.; Peyravi, M. Surface study of platinum decorated graphene towards ad-sorption of NH3 and CH4. Mater. Chem. Phys. 2016, 182, 32–38. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, X.-L. Simulations on methane uptake in tunable pillared porous graphene hybrid architectures. J. Mol. Graph. Model. 2018, 85, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; El-Merraoui, M.; Steele, W.; Kaneko, K. Methane adsorption on single-walled carbon nanotube: A density functional theory model. Chem. Phys. Lett. 2002, 352, 334–341. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.; Zhao, Y.; Zhang, M.; Tian, R.; Zhang, C. Methane adsorption properties of N-doped graphdiyne: A first-principles study. Struct. Chem. 2021, 32, 1517–1527. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Xu, W.; Ju, J.; Zhao, Y.; Zhang, M.; Sang, C.; Zhang, C. First-principles study of the impact of hydrogen on the adsorption properties of Ti-decorated graphdiyne storage methane. Chem. Phys. Lett. 2022, 790, 139329. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Harun, N.Y.; Bilad, M.R.; Jaafar, J.; Aziz, F.; Salleh, W.N.W.; Ismail, A.F. Novel Activated Carbon Nanofibers Composited with Cost-Effective Graphene-Based Materials for Enhanced Adsorption Performance toward Methane. Polymers 2020, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
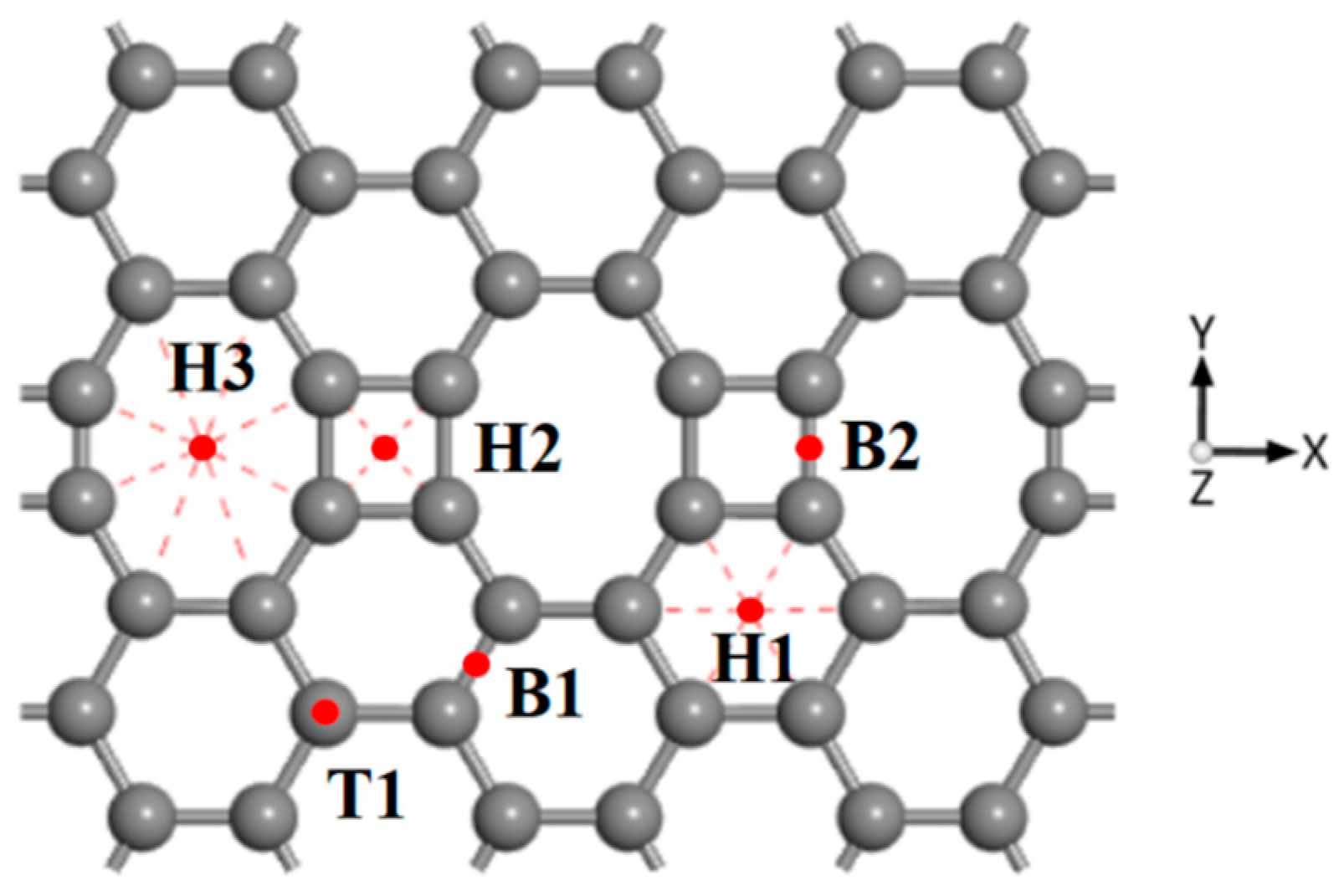
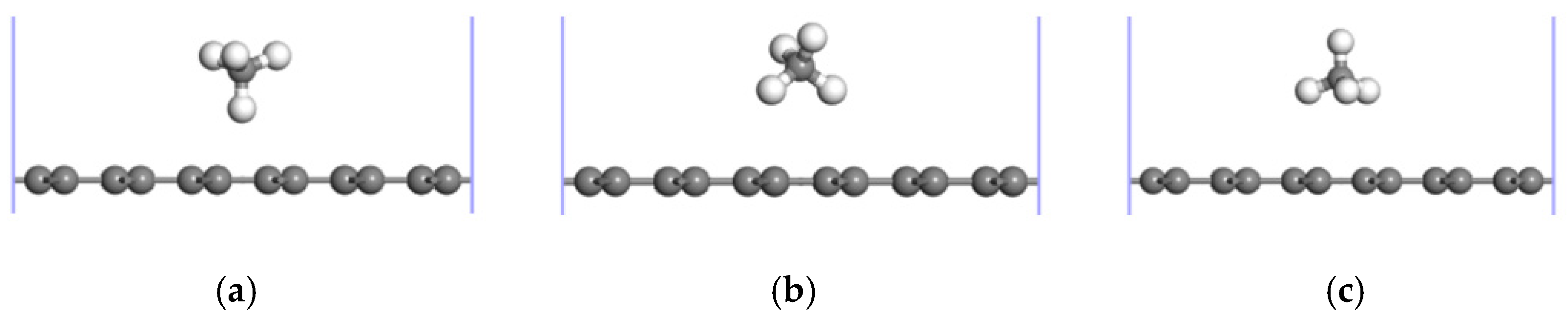
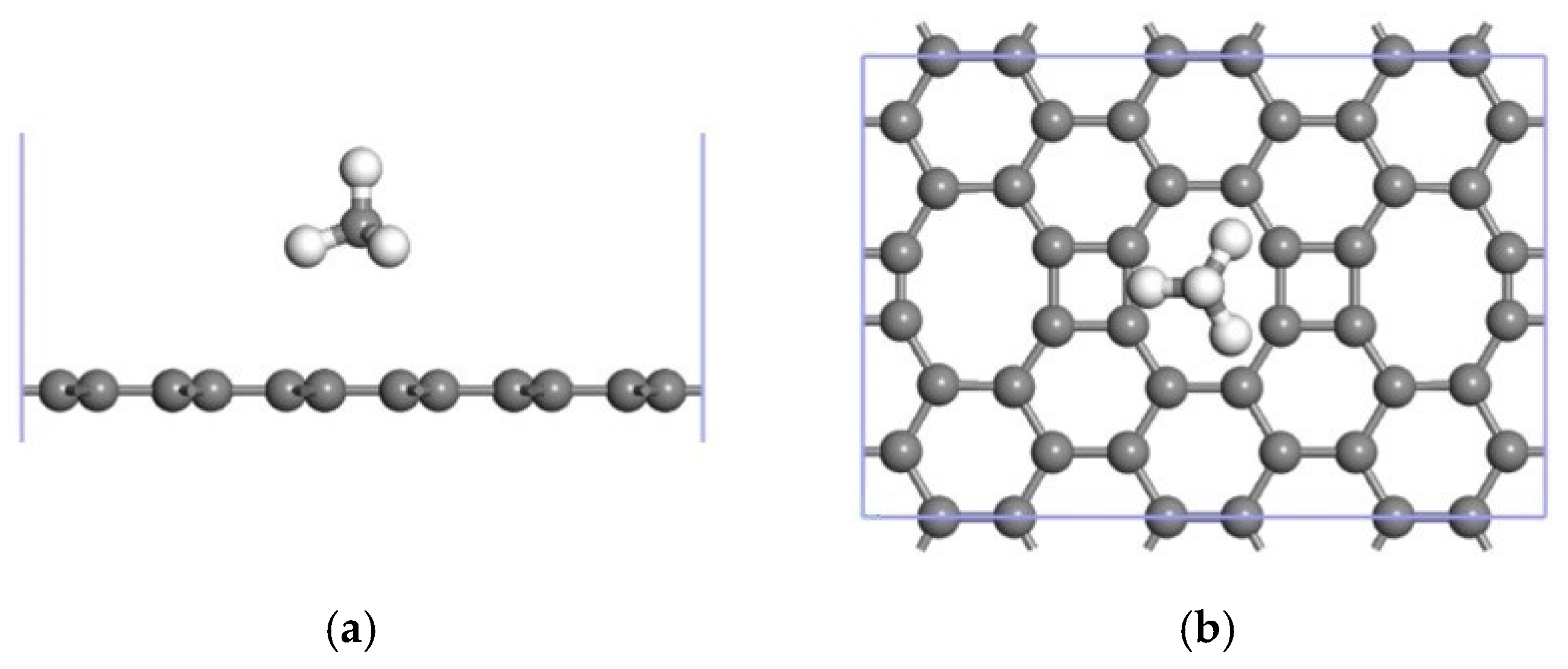
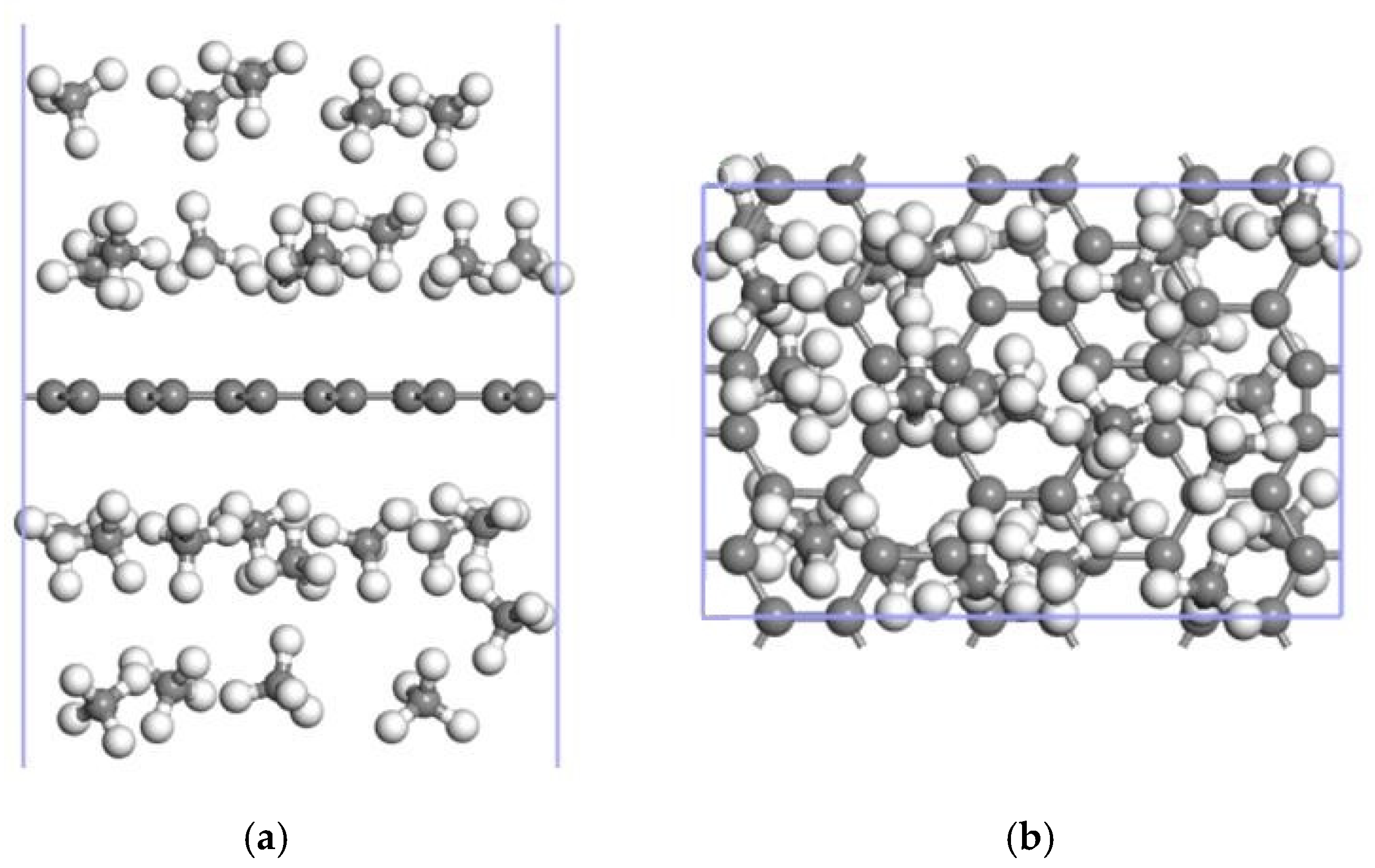

| The Absorption Point of CH4 | (Å) | ∠H-C-H (°) | |
|---|---|---|---|
| H1 | −0.824 | 3.291 | 109.507 |
| H2 | −0.833 | 3.222 | 109.878 |
| H3 | −0.847 | 3.081 | 109.551 |
| T1 | −0.835 | 3.278 | 109.554 |
| B1 | −0.832 | 3.251 | 109.811 |
| B2 | −0.830 | 3.251 | 109.560 |
| The Absorption Point of a Single Mn Atom | Distance (Å) | |||||
|---|---|---|---|---|---|---|
| H1 | −2.922 | 2.037 | 2.037 | 2.104 | 2.105 | 0.29 |
| H2 | −3.453 | 2.967 | 2.974 | 2.974 | 2.981 | 0.39 |
| H3 | −3.218 | 2.113 | 2.128 | 2.430 | 2.431 | 0.33 |
| Number of CH4 Molecules | (Å) | (Å) | PBW (wt%) | ||
|---|---|---|---|---|---|
| 1 | −1.717 | −1.717 | 3.824 | 1.967 | 2.79 |
| 2 | −1.289 | −0.862 | 3.789 | 3.614 | 5.43 |
| 3 | −1.177 | −0.953 | 3.590 | 4.483 | 7.92 |
| 4 | −1.103 | −0.879 | 4.100 | 4.956 | 10.29 |
| 5 | −1.047 | −0.824 | 3.551 | 7.299 | 12.54 |
| 6 | −1.022 | −0.897 | 3.448 | 7.293 | 14.68 |
| 7 | −1.018 | −0.978 | 3.397 | 3.928 | 16.72 |
| 8 | −0.995 | −0.832 | 4.840 | 5.319 | 18.66 |
| 9 | −0.971 | −0.775 | 7.176 | 5.724 | 20.52 |
| 10 | −0.956 | −0.825 | 7.156 | 5.777 | 22.29 |
| 11 | −0.943 | −0.810 | 7.329 | 5.612 | 23.98 |
| 12 | −0.927 | −0.755 | 7.460 | 8.228 | 25.60 |
| 13 | −0.918 | −0.806 | 7.831 | 9.377 | 27.16 |
| 14 | −0.913 | −0.856 | 8.035 | 7.837 | 28.65 |
| 15 | −0.907 | −0.816 | 9.132 | 7.743 | 30.08 |
| 16 | −0.897 | −0.755 | 11.550 | 10.780 | 31.46 |
| 30 | −0.867 | 46.25 |
| Before Adsorption (e) | After Adsorption (e) | |||||||
|---|---|---|---|---|---|---|---|---|
| Atom | s | p | d | Charge | s | p | d | Charge |
| H1 | 0.73 | 0.27 | 0.87 | 0.13 | ||||
| H2 | 0.73 | 0.27 | 0.87 | 0.13 | ||||
| H3 | 0.73 | 0.27 | 0.72 | 0.28 | ||||
| H4 | 0.73 | 0.27 | 0.64 | 0.36 | ||||
| C | 1.51 | 3.59 | −1.10 | 1.49 | 3.61 | −1.09 | ||
| C1 | 1.03 | 3.00 | −0.03 | 1.05 | 3.03 | −0.08 | ||
| C2 | 1.03 | 3.00 | −0.04 | 1.05 | 3.03 | −0.09 | ||
| C3 | 1.05 | 3.03 | −0.09 | 1.05 | 3.04 | −0.10 | ||
| C4 | 1.05 | 3.04 | −0.09 | 1.05 | 3.04 | −0.10 | ||
| Mn | 2.00 | 6.00 | 6.21 | 0.69 | 2.01 | 6.00 | 6.13 | 0.98 |
| Number of CH4 Molecules | (Å) | (Å) | PBW (wt%) | ||
|---|---|---|---|---|---|
| 1 | −1.726 | −1.726 | 3.789 | 1.943 | 2.55 |
| 2 | −1.296 | −0.866 | 3.828 | 4.045 | 4.96 |
| 3 | −1.149 | −0.855 | 3.590 | 4.406 | 7.27 |
| 4 | −1.105 | −0.974 | 3.483 | 3.996 | 9.46 |
| 5 | −1.058 | −0.869 | 4.196 | 7.647 | 11.55 |
| 6 | −1.030 | −0.891 | 3.937 | 4.558 | 13.55 |
| 7 | −1.015 | −0.926 | 3.379 | 7.596 | 15.46 |
| 8 | −0.993 | −0.835 | 4.498 | 5.836 | 17.28 |
| 9 | −0.967 | −0.760 | 7.187 | 5.871 | 19.03 |
| 10 | −0.950 | −0.796 | 7.167 | 5.827 | 20.71 |
| 11 | −0.936 | −0.797 | 7.385 | 6.282 | 22.32 |
| 12 | −0.926 | −0.814 | 7.574 | 6.176 | 23.86 |
| 13 | −0.917 | −0.806 | 7.885 | 9.321 | 25.35 |
| 14 | −0.909 | −0.813 | 7.931 | 8.754 | 26.77 |
| 15 | −0.900 | −0.777 | 9.242 | 11.033 | 28.15 |
| 16 | −0.889 | −0.727 | 11.369 | 9.523 | 29.47 |
| 17 | −0.882 | −0.756 | 11.336 | 9.868 | 30.75 |
| 18 | −0.875 | −0.769 | 11.578 | 10.493 | 31.98 |
| 19 | −0.871 | −0.787 | 11.450 | 10.427 | 33.16 |
| 20 | −0.868 | −0.726 | 11.600 | 11.992 | 34.31 |
| 40 | −0.862 | 51.09 |
| Adsorption Structure | Modified Elements | Temp; Pressure | PBW (wt%) | Interpretation | Ref. |
|---|---|---|---|---|---|
| 2Mn-ELD-42C | Mn | - | 51.09 | ELD: Extended line defect | This work |
| 2Ti-GDY | Ti | - | 55.24 | GDY: graphdiyne | [44] |
| CNT-PG | - | 298 k; 40 bar | 44.70 | CNT-PG: carbon nanotube-porous graphene | [52] |
| SWNT | - | 303 k; 3.55 MPa | 19.80 | SWNT: single-walled carbon nanotubes | [53] |
| 2Ti-GY | Ti | - | 48.40 | GY: graphyne | [8] |
| 2Mn-GR | Mn | - | 32.93 | GR: graphene | [6] |
| 2Mn-N-GDY | Mn, N | - | 58.50 | GDY: graphdiyne | [54] |
| Ti-GDY | Ti | - | 63.54 | Co-mixing H2 and CH4 | [55] |
| GRHA/ACNF | - | 298 k; 12 bar | 66.40 | GRHA: graphene-derived rice husk ashes; ACNF: activated carbonNanofibers | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yang, S.; Zhang, X.; Xia, Y.; Li, J. Extended Line Defect Graphene Modified by the Adsorption of Mn Atoms and Its Properties of Adsorbing CH4. Nanomaterials 2022, 12, 697. https://doi.org/10.3390/nano12040697
Zhang C, Yang S, Zhang X, Xia Y, Li J. Extended Line Defect Graphene Modified by the Adsorption of Mn Atoms and Its Properties of Adsorbing CH4. Nanomaterials. 2022; 12(4):697. https://doi.org/10.3390/nano12040697
Chicago/Turabian StyleZhang, Chenxiaoyu, Shaobin Yang, Xu Zhang, Yingkai Xia, and Jiarui Li. 2022. "Extended Line Defect Graphene Modified by the Adsorption of Mn Atoms and Its Properties of Adsorbing CH4" Nanomaterials 12, no. 4: 697. https://doi.org/10.3390/nano12040697
APA StyleZhang, C., Yang, S., Zhang, X., Xia, Y., & Li, J. (2022). Extended Line Defect Graphene Modified by the Adsorption of Mn Atoms and Its Properties of Adsorbing CH4. Nanomaterials, 12(4), 697. https://doi.org/10.3390/nano12040697







