Operando Photo-Electrochemical Catalysts Synchrotron Studies
Abstract
:1. Introduction
2. Photoelectrochemical Reactions and Processes
2.1. Hydrogen Evolution Reaction
2.2. Oxygen Evolution Reaction
3. Photoelectrodes
3.1. Photoanode
3.2. Photocathode
4. PEC in Situ and Operando Synchrotron Studies
5. Theoretical Interpretation and Methods of Calculations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopp, G.; Lean, J.L. A new, lower value of total solar irradiance: Evidence and climate significance. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef] [Green Version]
- Coddington, O.; Lean, J.L.; Pilewskie, P.; Snow, M.; Lindholm, D. A Solar Irradiance Climate Data Record. Bull. Am. Meteorol. Soc. 2016, 97, 1265–1282. [Google Scholar] [CrossRef]
- Available online: https://www.eia.gov/international/data/world/electricity/electricity-consumption (accessed on 26 February 2022).
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Rosser, T.E.; Gross, M.A.; Lai, Y.H.; Reisner, E. Precious-metal free photoelectrochemical water splitting with immobilised molecular Ni and Fe redox catalysts. Chem. Sci. 2016, 7, 4024–4035. [Google Scholar] [CrossRef] [Green Version]
- Landman, A.; Halabi, R.; Dias, P.; Dotan, H.; Mehlmann, A.; Shter, G.E.; Halabi, M.; Naseraldeen, O.; Mendes, A.; Grader, G.S.; et al. Decoupled Photoelectrochemical Water Splitting System for Centralized Hydrogen Production. Joule 2020, 4, 448–471. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Zhu, Q.; Sun, J.; Qin, F.; Yu, L.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Water splitting by electrolysis at high current densities under 1.6 volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, J.; Yan, D. Transition metal-based layered double hydroxides for photo(electro)chemical water splitting: A mini review. Nanoscale 2021, 13, 13593–13603. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Mushtaq, M.A.; Wu, S.; Xiang, X.; Yan, D. Research Progress in Organic Synthesis by Means of Photoelectrocatalysis. Chem. Rec. 2021, 21, 841–857. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Recent Development of Ni/Fe-Based Micro/Nanostructures toward Photo/Electrochemical Water Oxidation. Adv. Energy Mater. 2019, 10. [Google Scholar] [CrossRef]
- Wang, T.; Guo, L.; Jiang, Z.; Chen, S.; Xu, S.; Zhang, Y.; Zhang, J.; Li, R.; Peng, T. Ru-Pincer Complex-Bridged Cu-Porphyrin Polymer for Robust (Photo)Electrocatalytic H2 Evolution via Single-Atom Active Sites. Adv. Funct. Mater. 2021, 31, 2107290. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Sun, J.; He, R.; Chen, S.; Chu, C.W.; Ren, Z. Highly active catalyst derived from a 3D foam of Fe(PO3)2/Ni2P for extremely efficient water oxidation. Proc. Natl. Acad. Sci. USA 2017, 114, 5607–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Luo, Y.; Yu, Q.; Zhang, Z.; Zhang, S.; Liu, Z.; Ren, W.; Cheng, H.M.; Li, J.; Liu, B. A Durable and Efficient Electrocatalyst for Saline Water Splitting with Current Density Exceeding 2000 mA cm−2. Adv. Funct. Mater. 2021, 31, 2010367. [Google Scholar] [CrossRef]
- van Bokhoven, J.A.; Lamberti, C. X-ray Absorption and X-ray Emission Spectroscopy: Theory and Applications; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chen, Z.; Deutsch, T.G.; Dinh, H.N.; Domen, K.; Emery, K.; Forman, A.J.; Gaillard, N.; Garland, R.; Heske, C.; Jaramillo, T.F.; et al. Introduction. In Photoelectrochemical Water Splitting: Standards, Experimental Methods, and Protocols; Springer: New York, NY, USA, 2013; pp. 1–5. [Google Scholar]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [Green Version]
- Davydova, E.S.; Mukerjee, S.; Jaouen, F.; Dekel, D.R. Electrocatalysts for Hydrogen Oxidation Reaction in Alkaline Electrolytes. ACS Catal. 2018, 8, 6665–6690. [Google Scholar] [CrossRef]
- Kalanur, S.; Seo, H. Electrocatalysts for Photochemical Water-Splitting. In Methods for Electrocatalysis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 171–199. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Xu, S.; Carter, E.A. Theoretical Insights into Heterogeneous (Photo)electrochemical CO2 Reduction. Chem. Rev. 2019, 119, 6631–6669. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.U.; Kim, C.W.; Nguyen-Le, M.-T.; Kang, Y.S. General Review on the Components and Parameters of Photoelectrochemical System for CO2 Reduction with in Situ Analysis. ACS Sustain. Chem. Eng. 2019, 7, 7431–7455. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Li, Y.; Chen, J.; Yu, B.; Wang, J.; Zhang, L.; Zhang, J. Energy related CO2 conversion and utilization: Advanced materials/nanomaterials, reaction mechanisms and technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Polman, A.; Atwater, H.A. Photonic design principles for ultrahigh-efficiency photovoltaics. Nat. Mater. 2012, 11, 174–177. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M.A.; Townsend, T.K.; Osterloh, F.E. Quantum confinement controlled photocatalytic water splitting by suspended CdSe nanocrystals. Chem. Commun. 2012, 48, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Murgolo, S.; De Ceglie, C.; Di Iaconi, C.; Mascolo, G. Novel TiO2-based catalysts employed in photocatalysis and photoelectrocatalysis for effective degradation of pharmaceuticals (PhACs) in water: A short review. Curr. Opin. Green Sustain. Chem. 2021, 30, 100473. [Google Scholar] [CrossRef]
- Chen, H.-J.; Yang, Y.-L.; Zou, X.-X.; Shi, X.-L.; Chen, Z.-G. Flexible hollow TiO2@CMS/carbon-fiber van der Waals heterostructures for simulated-solar light photocatalysis and photoelectrocatalysis. J. Mater. Sci. Technol. 2022, 98, 143–150. [Google Scholar] [CrossRef]
- He, Y.; Chen, K.; Leung, M.K.H.; Zhang, Y.; Li, L.; Li, G.; Xuan, J.; Li, J. Photocatalytic fuel cell—A review. Chem. Eng. J. 2022, 428, 131074. [Google Scholar] [CrossRef]
- Rey, A.; García-Muñoz, P.; Hernández-Alonso, M.D.; Mena, E.; García-Rodríguez, S.; Beltrán, F.J. WO3–TiO2 based catalysts for the simulated solar radiation assisted photocatalytic ozonation of emerging contaminants in a municipal wastewater treatment plant effluent. Appl. Catal. B Environ. 2014, 154–155, 274–284. [Google Scholar] [CrossRef]
- You, S.-M.; Wang, T.-H.; Doong, R.-A.; Millet, P. PEC water splitting using mats of calcined TiO2 rutile nanorods photosensitized by a thin layer of Ni-benzene dicarboxylic acid MOF. Electrochimia 2021, 393, 139014. [Google Scholar] [CrossRef]
- Braiek, Z.; Ben Naceur, J.; Jrad, F.; Ben Assaker, I.; Chtourou, R. Novel synthesis of graphene oxide/In2S3/TiO2 NRs heterojunction photoanode for enhanced photoelectrochemical (PEC) performance. Int. J. Hydrogen Energy 2022, 47, 3655–3666. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, J.; Li, J.; Huang, K.; Li, X.; Zhou, B.; Cai, W. Aerated visible-light responsive photocatalytic fuel cell for wastewater treatment with producing sustainable electricity in neutral solution. Chem. Eng. J. 2014, 252, 89–94. [Google Scholar] [CrossRef]
- Kee, M.-W.; Lam, S.-M.; Sin, J.-C.; Zeng, H.; Mohamed, A.R. Explicating charge transfer dynamics in anodic TiO2/ZnO/Zn photocatalytic fuel cell for ameliorated palm oil mill effluent treatment and synchronized energy generation. J. Photochem. Photobiol. A Chem. 2020, 391, 112353. [Google Scholar] [CrossRef]
- Fu, R.; Zeng, X.; Ma, L.; Gao, S.; Wang, Q.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. Enhanced photocatalytic and photoelectrochemical activities of reduced TiO2−x/BiOCl heterojunctions. J. Power Sources 2016, 312, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Jiang, J.; Ren, W.; Wang, H.; Zhang, R.; Xie, Y.; Chen, Y. Construction of ZnO/CdS three-dimensional hierarchical photoelectrode for improved photoelectrochemical performance. Renew. Energy 2020, 153, 241–248. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, W.; Cheng, Y.F. Preparation of Co3O4@ZnO core-shell nanocomposites with intrinsic p-n junction as high-performance photoelectrodes for photoelectrochemical cathodic protection under visible light. Appl. Surf. Sci. 2019, 476, 815–821. [Google Scholar] [CrossRef]
- Rabell, G.O.; Alfaro Cruz, M.R.; Juárez-Ramírez, I. Photoelectrochemical (PEC) analysis of ZnO/Al photoelectrodes and its photocatalytic activity for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 7770–7782. [Google Scholar] [CrossRef]
- Sahoo, P.; Sharma, A.; Padhan, S.; Thangavel, R. Construction of ZnO@NiO heterostructure photoelectrodes for improved photoelectrochemical performance. Int. J. Hydrogen Energy 2021, 46, 36176–36188. [Google Scholar] [CrossRef]
- Celebi, N.; Salimi, K. Yolk-shell ZnO@C-CeO2 ternary heterostructures with conductive N-doped carbon mediated electron transfer for highly efficient water splitting. J. Colloid Interface Sci. 2022, 605, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, A.A.; Zainal, Z.; Talib, Z.A.; Lim, H.N.; Holi, A.M.; Bahrudin, N.N. Enhanced photoelectrochemical performance of Bi2S3/Ag2S/ZnO novel ternary heterostructure nanorods. Arab. J. Chem. 2020, 13, 9166–9178. [Google Scholar] [CrossRef]
- Bai, S.; Han, J.; Zhang, K.; Zhao, Y.; Luo, R.; Li, D.; Chen, A. rGO decorated semiconductor heterojunction of BiVO4/NiO to enhance PEC water splitting efficiency. Int. J. Hydrogen Energy 2022, 47, 4375–4385. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Hu, X.; Meng, Y.; She, H.; Wang, L.; Huang, J.; Zhu, G. Constructing NiFe-metal-organic frameworks from NiFe-layered double hydroxide as a highly efficient cocatalyst for BiVO4 photoanode PEC water splitting. Chem. Eng. J. 2022, 433, 133592. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.-C.; Wei, Y.; Feng, D.; Zhang, H. Construction of a novel photoelectrochemical sensor for detecting trace amount of copper (II) ion. Electrochimia 2021, 370, 137736. [Google Scholar] [CrossRef]
- Nandal, N.; Prajapati, P.K.; Abraham, B.M.; Jain, S.L. CO2 to ethanol: A selective photoelectrochemical conversion using a ternary composite consisting of graphene oxide/copper oxide and a copper-based metal-organic framework. Electrochimica 2022, 404, 139612. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Jeffery Minggu, L.; Arifin, K.; Mohamad Yunus, R.; Mohamed, M.A.; Kassim, M.B. Recent advances on state-of-the-art copper (I/II) oxide as photoelectrode for solar green fuel generation: Challenges and mitigation strategies. Appl. Catal. A Gen. 2019, 582, 117104. [Google Scholar] [CrossRef]
- Marathey, P.; Khanna, S.; Patel, B.; Ray, A. Investigation of spray pyrolyzed copper oxide as a photocathode in photoelectrochemical energy conversion. Mater. Today Proc. 2020, 28, 883–887. [Google Scholar] [CrossRef]
- Li, C.; Hisatomi, T.; Watanabe, O.; Nakabayashi, M.; Shibata, N.; Domen, K.; Delaunay, J.-J. Simultaneous enhancement of photovoltage and charge transfer in Cu2O-based photocathode using buffer and protective layers. Appl. Phys. Lett. 2016, 109, 033902. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Li, X.; Zhang, G. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Li, S.; Zhang, B.; Wang, M.; Shen, Y. Carbon coated Cu2O nanowires for photo-electrochemical water splitting with enhanced activity. Appl. Surf. Sci. 2015, 358, 404–411. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, T.; Gong, J. Single-crystal silicon-based electrodes for unbiased solar water splitting: Current status and prospects. Chem. Soc. Rev. 2019, 48, 2158–2181. [Google Scholar] [CrossRef] [PubMed]
- Behara, D.K.; Sharma, G.P.; Upadhyay, A.P.; Gyanprakash, M.; Pala, R.G.S.; Sivakumar, S. Synchronization of charge carrier separation by tailoring the interface of Si–Au–TiO2 heterostructures via click chemistry for PEC water splitting. Chem. Eng. Sci. 2016, 154, 150–169. [Google Scholar] [CrossRef]
- Dhara, A.; Show, B.; Baral, A.; Chabri, S.; Sinha, A.; Bandyopadhyay, N.R.; Mukherjee, N. Core-shell CuO-ZnO p-n heterojunction with high specific surface area for enhanced photoelectrochemical (PEC) energy conversion. Sol. Energy 2016, 136, 327–332. [Google Scholar] [CrossRef]
- Takahashi, Y.; Jeem, M.; Zhang, L.; Watanabe, S. The origin of opto-functional enhancement in ZnO/CuO nanoforest structure fabricated by submerged photosynthesis. Appl. Mater. Today 2022, 26, 101359. [Google Scholar] [CrossRef]
- Zou, K.; Fu, Y.; Yang, R.; Zhang, X.; Du, C.; Chen, J. CuO-ZnO heterojunction derived from Cu(2+)-doped ZIF-8: A new photoelectric material for ultrasensitive PEC immunoassay of CA125 with near-zero background noise. Anal. Chim. Acta 2020, 1099, 75–84. [Google Scholar] [CrossRef]
- Kaur, G.; Divya; Satsangi, V.R.; Dass, S.; Shrivastav, R. 3D-nano-hetero-structured n/n junction, CuO/Ru–ZnO thin films, for hydrogen generation with enhanced photoelectrochemical performances. Int. J. Hydrogen Energy 2020, 45, 21051–21067. [Google Scholar] [CrossRef]
- Minguzzi, A.; Naldoni, A.; Lugaresi, O.; Achilli, E.; D’Acapito, F.; Malara, F.; Locatelli, C.; Vertova, A.; Rondinini, S.; Ghigna, P. Observation of charge transfer cascades in alpha-Fe2O3/IrOx photoanodes by operando X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 5715–5720. [Google Scholar] [CrossRef]
- Malara, F.; Minguzzi, A.; Marelli, M.; Morandi, S.; Psaro, R.; Dal Santo, V.; Naldoni, A. α-Fe2O3/NiOOH: An Effective Heterostructure for Photoelectrochemical Water Oxidation. ACS Catal. 2015, 5, 5292–5300. [Google Scholar] [CrossRef]
- Barroso, M.; Mesa, C.A.; Pendlebury, S.R.; Cowan, A.J.; Hisatomi, T.; Sivula, K.; Gratzel, M.; Klug, D.R.; Durrant, J.R. Dynamics of photogenerated holes in surface modified alpha-Fe2O3 photoanodes for solar water splitting. Proc. Natl. Acad. Sci. USA 2012, 109, 15640–15645. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Esparza, A.T.; Shinagawa, T.; Ould-Chikh, S.; Qureshi, M.; Peng, X.Y.; Wei, N.N.; Anjum, D.H.; Clo, A.; Weng, T.C.; Nordlund, D.; et al. An Oxygen-Insensitive Hydrogen Evolution Catalyst Coated by a Molybdenum-Based Layer for Overall Water Splitting. Angew. Chem.-Int. Ed. 2017, 56, 5780–5784. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.D.; Cornuz, M.; Sivula, K.; Gratzel, M. Light-induced water splitting with hematite: Improved nanostructure and iridium oxide catalysis. Angew. Chem. Int. Ed. Engl. 2010, 49, 6405–6408. [Google Scholar] [CrossRef] [PubMed]
- Bledowski, M.; Wang, L.; Neubert, S.; Mitoraj, D.; Beranek, R. Improving the Performance of Hybrid Photoanodes for Water Splitting by Photodeposition of Iridium Oxide Nanoparticles. J. Phys. Chem. C 2014, 118, 18951–18961. [Google Scholar] [CrossRef]
- Tsyganok, A.; Ghigna, P.; Minguzzi, A.; Naldoni, A.; Murzin, V.; Caliebe, W.; Rothschild, A.; Ellis, D.S. Operando X-ray Absorption Spectroscopy (XAS) Observation of Photoinduced Oxidation in FeNi (Oxy)hydroxide Overlayers on Hematite (alpha-Fe2O3) Photoanodes for Solar Water Splitting. Langmuir ACS J. Surf. Colloids 2020, 36, 11564–11572. [Google Scholar] [CrossRef] [PubMed]
- Tsyganok, A.; Klotz, D.; Malviya, K.D.; Rothschild, A.; Grave, D.A. Different Roles of Fe1–xNixOOH Cocatalyst on Hematite (α-Fe2O3) Photoanodes with Different Dopants. ACS Catal. 2018, 8, 2754–2759. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Drevon, D.; Gorlin, M.; Chernev, P.; Xi, L.; Dau, H.; Lange, K.M. Uncovering The Role of Oxygen in Ni-Fe(OxHy) Electrocatalysts using In situ Soft X-ray Absorption Spectroscopy during the Oxygen Evolution Reaction. Sci. Rep. 2019, 9, 1532. [Google Scholar] [CrossRef]
- Lin, F.; Boettcher, S.W. Adaptive semiconductor/electrocatalyst junctions in water-splitting photoanodes. Nat. Mater. 2014, 13, 81–86. [Google Scholar] [CrossRef]
- Liu, L.; Ji, Z.; Zou, W.; Gu, X.; Deng, Y.; Gao, F.; Tang, C.; Dong, L. In Situ Loading Transition Metal Oxide Clusters on TiO2 Nanosheets As Co-catalysts for Exceptional High Photoactivity. ACS Catal. 2013, 3, 2052–2061. [Google Scholar] [CrossRef]
- Malara, F.; Fabbri, F.; Marelli, M.; Naldoni, A. Controlling the Surface Energetics and Kinetics of Hematite Photoanodes Through Few Atomic Layers of NiOx. ACS Catal. 2016, 6, 3619–3628. [Google Scholar] [CrossRef]
- Steier, L.; Herraiz-Cardona, I.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Tilley, S.D.; Grätzel, M. Understanding the Role of Underlayers and Overlayers in Thin Film Hematite Photoanodes. Adv. Funct. Mater. 2014, 24, 7681–7688. [Google Scholar] [CrossRef]
- Achilli, E.; Minguzzi, A.; Visibile, A.; Locatelli, C.; Vertova, A.; Naldoni, A.; Rondinini, S.; Auricchio, F.; Marconi, S.; Fracchia, M.; et al. 3D-printed photo-spectroelectrochemical devices for in situ and in operando X-ray absorption spectroscopy investigation. J. Synchrotron Radiat. 2016, 23, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Minguzzi, A.; Lugaresi, O.; Locatelli, C.; Rondinini, S.; D’Acapito, F.; Achilli, E.; Ghigna, P. Fixed energy X-ray absorption voltammetry. Anal. Chem. 2013, 85, 7009–7013. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Takanabe, K. Insights on Measuring and Reporting Heterogeneous Photocatalysis: Efficiency Definitions and Setup Examples. Chem. Mater. 2016, 29, 158–167. [Google Scholar] [CrossRef]
- Trześniewski, B.J.; Smith, W.A. Photocharged BiVO4 photoanodes for improved solar water splitting. J. Mater. Chem. A 2016, 4, 2919–2926. [Google Scholar] [CrossRef] [Green Version]
- Zachaus, C.; Abdi, F.F.; Peter, L.M.; van de Krol, R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 2017, 8, 3712–3719. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Iwashina, K.; Kudo, A. Facile fabrication of an efficient BiVO4 thin film electrode for water splitting under visible light irradiation. Proc. Natl. Acad. Sci. USA 2012, 109, 11564–11569. [Google Scholar] [CrossRef] [Green Version]
- Seabold, J.A.; Choi, K.S. Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst. J. Am. Chem. Soc. 2012, 134, 2186–2192. [Google Scholar] [CrossRef]
- Xi, L.; Schellenberger, M.; Praeg, R.F.; Gao, D.; Drevon, D.; Plate, P.; Bogdanoff, P.; van de Krol, R.; Lange, K.M. Structural Monitoring of NiBi Modified BiVO4 Photoanodes Using in Situ Soft and Hard X-ray Absorption Spectroscopies. ACS Appl. Energy Mater. 2019, 2, 4126–4134. [Google Scholar] [CrossRef]
- Schwanke, C.; Xi, L.; Lange, K.M. A soft XAS transmission cell for operando studies. J. Synchrotron Radiat. 2016, 23, 1390–1394. [Google Scholar] [CrossRef]
- Xi, L.; Schwanke, C.; Xiao, J.; Abdi, F.F.; Zaharieva, I.; Lange, K.M. In Situ L-Edge XAS Study of a Manganese Oxide Water Oxidation Catalyst. J. Phys. Chem. C 2017, 121, 12003–12009. [Google Scholar] [CrossRef]
- Xi, L.; Schwanke, C.; Zhou, D.; Drevon, D.; van de Krol, R.; Lange, K.M. In situ XAS study of CoBi modified hematite photoanodes. Dalton Trans. 2017, 46, 15719–15726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Mitsutomi, Y.; Mineo, T.; Nagasaka, M.; Yuzawa, H.; Kosugi, N.; Kondoh, H. Direct Observation of Active Nickel Oxide Cluster in Nickel–Borate Electrocatalyst for Water Oxidation by In Situ O K-Edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2015, 119, 19279–19286. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Schelhas, L.T.; Li, M.; Ager, J.W. Undoped and Ni-Doped CoOx Surface Modification of Porous BiVO4 Photoelectrodes for Water Oxidation. J. Phys. Chem. C 2016, 120, 23449–23457. [Google Scholar] [CrossRef] [Green Version]
- McDonald, K.J.; Choi, K.-S. A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ. Sci. 2012, 5, 8553–8557. [Google Scholar] [CrossRef]
- Luo, W.; Yang, Z.; Li, Z.; Zhang, J.; Liu, J.; Zhao, Z.; Wang, Z.; Yan, S.; Yu, T.; Zou, Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, H.; Yang, Z.; Long, X.; Wang, Z.; Zhu, Z.; Qu, Y.; Yang, S. Origin of the Different Photoelectrochemical Performance of Mesoporous BiVO4 Photoanodes between the BiVO4 and the FTO Side Illumination. J. Phys. Chem. C 2015, 119, 23350–23357. [Google Scholar] [CrossRef]
- Ha, D.-H.; Moreau, L.M.; Honrao, S.; Hennig, R.G.; Robinson, R.D. The Oxidation of Cobalt Nanoparticles into Kirkendall-Hollowed CoO and Co3O4: The Diffusion Mechanisms and Atomic Structural Transformations. J. Phys. Chem. C 2013, 117, 14303–14312. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hung, S.F.; Chen, H.Y.; Chan, T.S.; Chen, H.M.; Liu, B. In Operando Identification of Geometrical-Site-Dependent Water Oxidation Activity of Spinel Co3O4. J. Am. Chem. Soc. 2016, 138, 36–39. [Google Scholar] [CrossRef]
- Lu, Y.R.; Wang, Y.F.; Chang, H.W.; Huang, Y.C.; Chen, J.L.; Chen, C.L.; Lin, Y.C.; Lin, Y.G.; Pong, W.F.; Ohigashi, T.; et al. Effect of Fe2O3 coating on ZnO nanowires in photoelectrochemical water splitting: A synchrotron X-ray spectroscopic and spectromicroscopic investigation. Sol. Energy Mater. Sol. Cells 2020, 209, 110469. [Google Scholar] [CrossRef]
- Hamalainen, K.; Siddons, D.P.; Hastings, J.B.; Berman, L.E. Elimination of the inner-shell lifetime broadening in X-ray-absorption spectroscopy. Phys. Rev. Lett. 1991, 67, 2850–2853. [Google Scholar] [CrossRef]
- Krause, M.O.; Oliver, J.H. Natural widths of atomic K and L levels, Kα X-ray lines and several KLL Auger lines. J. Phys. Chem. Ref. Data 1979, 8, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Stöhr, J. NEXAFS Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Huse, N.; Wen, H.; Nordlund, D.; Szilagyi, E.; Daranciang, D.; Miller, T.A.; Nilsson, A.; Schoenlein, R.W.; Lindenberg, A.M. Probing the hydrogen-bond network of water via time-resolved soft X-ray spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 3951–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasaka, M.; Yuzawa, H.; Horigome, T.; Kosugi, N. In operando observation system for electrochemical reaction by soft X-ray absorption spectroscopy with potential modulation method. Rev. Sci. Instrum. 2014, 85, 104105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreck, S.; Gavrila, G.; Weniger, C.; Wernet, P. A sample holder for soft X-ray absorption spectroscopy of liquids in transmission mode. Rev. Sci. Instrum. 2011, 82, 103101. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Wang, F.; Schwanke, C.; Abdi, F.F.; Golnak, R.; Fiechter, S.; Ellmer, K.; van de Krol, R.; Lange, K.M. In Situ Structural Study of MnPi-Modified BiVO4 Photoanodes by Soft X-ray Absorption Spectroscopy. J. Phys. Chem. C 2017, 121, 19668–19676. [Google Scholar] [CrossRef] [Green Version]
- Baran, T.; Fracchia, M.; Vertova, A.; Achilli, E.; Naldoni, A.; Malara, F.; Rossi, G.; Rondinini, S.; Ghigna, P.; Minguzzi, A.; et al. Operando and Time-Resolved X-ray Absorption Spectroscopy for the Study of Photoelectrode Architectures. Electrochimia 2016, 207, 16–21. [Google Scholar] [CrossRef]
- Fracchia, M.; Cristino, V.; Vertova, A.; Rondinini, S.; Caramori, S.; Ghigna, P.; Minguzzi, A. Operando X-ray absorption spectroscopy of WO3 photoanodes. Electrochimia 2019, 320, 134561. [Google Scholar] [CrossRef]
- Ma, J.; Mao, K.; Low, J.; Wang, Z.; Xi, D.; Zhang, W.; Ju, H.; Qi, Z.; Long, R.; Wu, X.; et al. Efficient Photoelectrochemical Conversion of Methane into Ethylene Glycol by WO3 Nanobar Arrays. Angew. Chem. Int. Ed. Engl. 2021, 60, 9357–9361. [Google Scholar] [CrossRef]
- Wojtyła, S.; Baran, T. Electrochemically prepared copper/indium oxides photocathode for efficient photoelectrochemical hydrogen production. Sol. Energy Mater. Sol. Cells 2020, 206, 110262. [Google Scholar] [CrossRef]
- Lin, Y.-G.; Hsu, Y.-K.; Lin, Y.-C.; Chen, Y.-C. Electrodeposited Fe2TiO5 nanostructures for photoelectrochemical oxidation of water. Electrochimia 2016, 213, 898–903. [Google Scholar] [CrossRef]
- Cao, C.; Xie, X.; Zeng, Y.; Shi, S.; Wang, G.; Yang, L.; Wang, C.-Z.; Lin, S. Highly efficient and stable p-type ZnO nanowires with piezotronic effect for photoelectrochemical water splitting. Nano Energy 2019, 61, 550–558. [Google Scholar] [CrossRef]
- Chang, H.W.; Fu, Y.; Lee, W.Y.; Lu, Y.R.; Huang, Y.C.; Chen, J.L.; Chen, C.L.; Chou, W.C.; Chen, J.M.; Lee, J.F.; et al. Visible light-induced electronic structure modulation of Nb- and Ta-doped alpha-Fe2O3 nanorods for effective photoelectrochemical water splitting. Nanotechnology 2018, 29, 064002. [Google Scholar] [CrossRef] [PubMed]
- Daniel, Q.; Ambre, R.B.; Zhang, B.; Philippe, B.; Chen, H.; Li, F.; Fan, K.; Ahmadi, S.; Rensmo, H.; Sun, L. Re-Investigation of Cobalt Porphyrin for Electrochemical Water Oxidation on FTO Surface: Formation of CoOx as Active Species. ACS Catal. 2017, 7, 1143–1149. [Google Scholar] [CrossRef]
- Gajda-Schrantz, K.; Tymen, S.; Boudoire, F.; Toth, R.; Bora, D.K.; Calvet, W.; Gratzel, M.; Constable, E.C.; Braun, A. Formation of an electron hole doped film in the alpha-Fe2O3 photoanode upon electrochemical oxidation. Phys. Chem. Chem. Phys. 2013, 15, 1443–1451. [Google Scholar] [CrossRef]
- Buriak, J.M.; Schanze, K.S. ACS Applied Materials & Interfaces and Chemistry of Materials to Exclusively Publish Full Articles in 2019Please submit letters to ACS Materials Letters, which launches in early 2019. ACS Appl. Mater. Interfaces 2019, 11, 4703–4704. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef] [Green Version]
- Biroju, R.K.; Das, D.; Sharma, R.; Pal, S.; Mawlong, L.P.L.; Bhorkar, K.; Giri, P.K.; Singh, A.K.; Narayanan, T.N. Hydrogen Evolution Reaction Activity of Graphene-MoS2 van der Waals Heterostructures. Acs Energy Lett. 2017, 2, 1355–1361. [Google Scholar] [CrossRef]
- Kleiman-Shwarsctein, A.; Huda, M.N.; Walsh, A.; Yan, Y.F.; Stucky, G.D.; Hu, Y.S.; Al-Jassim, M.M.; McFarland, E.W. Electrodeposited Aluminum-Doped alpha-Fe2O3 Photoelectrodes: Experiment and Theory. Chem. Mater. 2010, 22, 510–517. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Li, F.W.; Gao, L.L.; Long, X.F.; Hu, Y.P.; Wang, C.L.; Wei, S.Q.; Jin, J.; Ma, J.T. Facile regrowth of Mg-Fe2O3/P-Fe2O3 homojunction photoelectrode for efficient solar water oxidation. J. Mater. Chem. A 2018, 6, 13412–13418. [Google Scholar] [CrossRef]
- Meng, X.Y.; Qin, G.W.; Li, S.; Wen, X.H.; Ren, Y.P.; Pei, W.L.; Zuo, L. Enhanced photoelectrochemical activity for Cu and Ti doped hematite: The first principles calculations. Appl. Phys. Lett. 2011, 98, 112104. [Google Scholar] [CrossRef]
- Oral, B.; Can, E.; Yildirim, R. Analysis of photoelectrochemical water splitting using machine learning. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Reier, T.; Pawolek, Z.; Cherevko, S.; Bruns, M.; Jones, T.; Teschner, D.; Selve, S.; Bergmann, A.; Nong, H.N.; Schlogl, R.; et al. Molecular Insight in Structure and Activity of Highly Efficient, Low-Ir Ir-Ni Oxide Catalysts for Electrochemical Water Splitting (OER). J. Am. Chem. Soc. 2015, 137, 13031–13040. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Upadhyay, S.; Satsangi, V.R.; Shrivastav, R.; Waghmare, U.V.; Dass, S. Improved Photoelectrochemical Water Splitting Performance of Cu2O/SrTiO3 Heterojunction Photoelectrode. J. Phys. Chem. C 2014, 118, 25320–25329. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, H.J.; Gao, G.P.; Butburee, T.; Lyu, M.Q.; Thaweesak, S.; Yun, J.H.; Du, A.J.; Liu, G.; Wang, L.Z. Synergistic crystal facet engineering and structural control of WO3 films exhibiting unprecedented photoelectrochemical performance. Nano Energy 2016, 24, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; Shao, M.F.; Ning, F.Y.; Xu, S.M.; Li, Z.H.; Wei, M.; Evans, D.G.; Duan, X. Au nanoparticles sensitized ZnO nanorod@nanoplatelet core-shell arrays for enhanced photoelectrochemical water splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tian, W.J.; Li, Y.G.; Sun, H.Q.; Tade, M.O.; Wang, S.B. Heterostructured WO3@CoWO4 bilayer nanosheets for enhanced visible-light photo, electro and photoelectro-chemical oxidation of water. J. Mater. Chem. A 2018, 6, 6265–6272. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhu, Z.L.; Chen, H.N.; Bai, Y.; Xiao, S.; Zheng, X.L.; Xue, Q.Z.; Yang, S.H. Iron-doping-enhanced photoelectrochemical water splitting performance of nanostructured WO3: A combined experimental and theoretical study. Nanoscale 2015, 7, 2933–2940. [Google Scholar] [CrossRef]
- Simfukwe, J.; Mapasha, R.E.; Braun, A.; Diale, M. Ab Initio Studies of Bimetallic-Doped {0001} Hematite Surface for Enhanced Photoelectrochemical Water Splitting. Catalysts 2021, 11, 940. [Google Scholar] [CrossRef]
- Bunker, G. Introduction to XAFS: A Practical Guide to X-ray Absorption Fine Structure Spectroscopy; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Guda, A.A.; Guda, S.A.; Lomachenko, K.A.; Soldatov, M.A.; Pankin, I.A.; Soldatov, A.V.; Braglia, L.; Bugaev, A.L.; Martini, A.; Signorile, M.; et al. Quantitative structural determination of active sites from in situ and operando XANES spectra: From standard ab initio simulations to chemometric and machine learning approaches. Catal. Today 2019, 336, 3–21. [Google Scholar] [CrossRef]
- Rankine, C.D.; Penfold, T.J. Progress in the Theory of X-ray Spectroscopy: From Quantum Chemistry to Machine Learning and Ultrafast Dynamics. J. Phys. Chem. A 2021, 125, 4276–4293. [Google Scholar] [CrossRef] [PubMed]
- Guda, A.A.; Guda, S.A.; Martini, A.; Bugaev, A.L.; Soldatov, M.A.; Soldatov, A.V.; Lamberti, C. Machine learning approaches to XANES spectra for quantitative 3D structural determination: The case of CO2 adsorption on CPO-27-Ni MOF. Radiat. Phys. Chem. 2020, 175, 108430. [Google Scholar] [CrossRef]
- Carbone, M.R.; Yoo, S.; Topsakal, M.; Lu, D.Y. Classification of local chemical environments from X-ray absorption spectra using supervised machine learning. Phys. Rev. Mater. 2019, 3, 033604. [Google Scholar] [CrossRef]
- Timoshenko, J.; Halder, A.; Yang, B.; Seifert, S.; Pellin, M.J.; Vajda, S.; Frenkel, A.I. Subnanometer Substructures in Nanoassemblies Formed from Clusters under a Reactive Atmosphere Revealed Using Machine Learning. J. Phys. Chem. C 2018, 122, 21686–21693. [Google Scholar] [CrossRef]
- Timoshenko, J.; Lu, D.Y.; Lin, Y.W.; Frenkel, A.I. Supervised Machine-Learning-Based Determination of Three-Dimensional Structure of Metallic Nanoparticles. J. Phys. Chem. Lett. 2017, 8, 5091–5098. [Google Scholar] [CrossRef]
- Martini, A.; Bugaev, A.L.; Guda, S.A.; Guda, A.A.; Priola, E.; Borfecchia, E.; Smolders, S.; Janssens, K.; De Vos, D.; Soldatov, A.V. Revisiting the Extended X-ray Absorption Fine Structure Fitting Procedure through a Machine Learning-Based Approach. J. Phys. Chem. A 2021, 125, 7080–7091. [Google Scholar] [CrossRef]

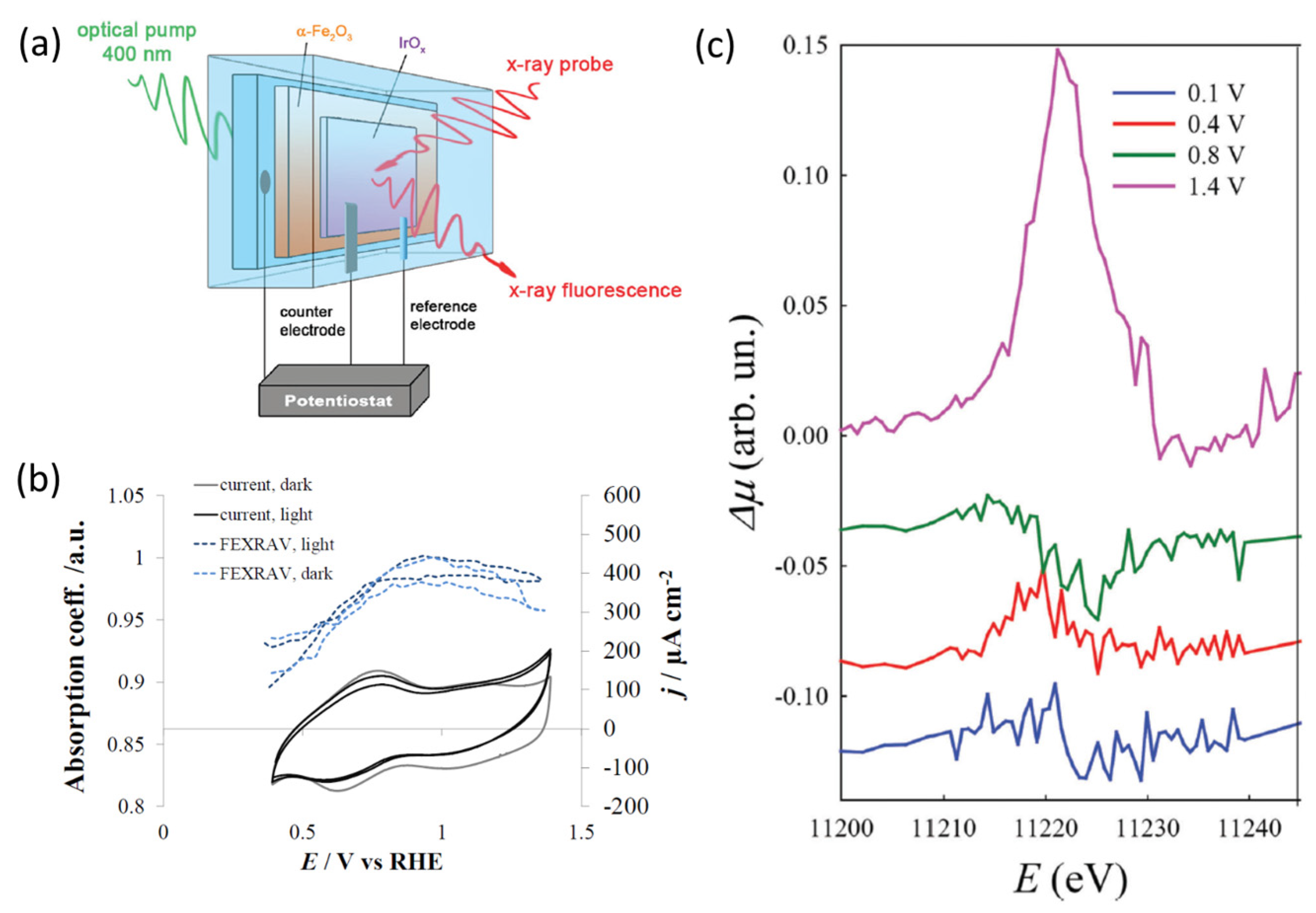
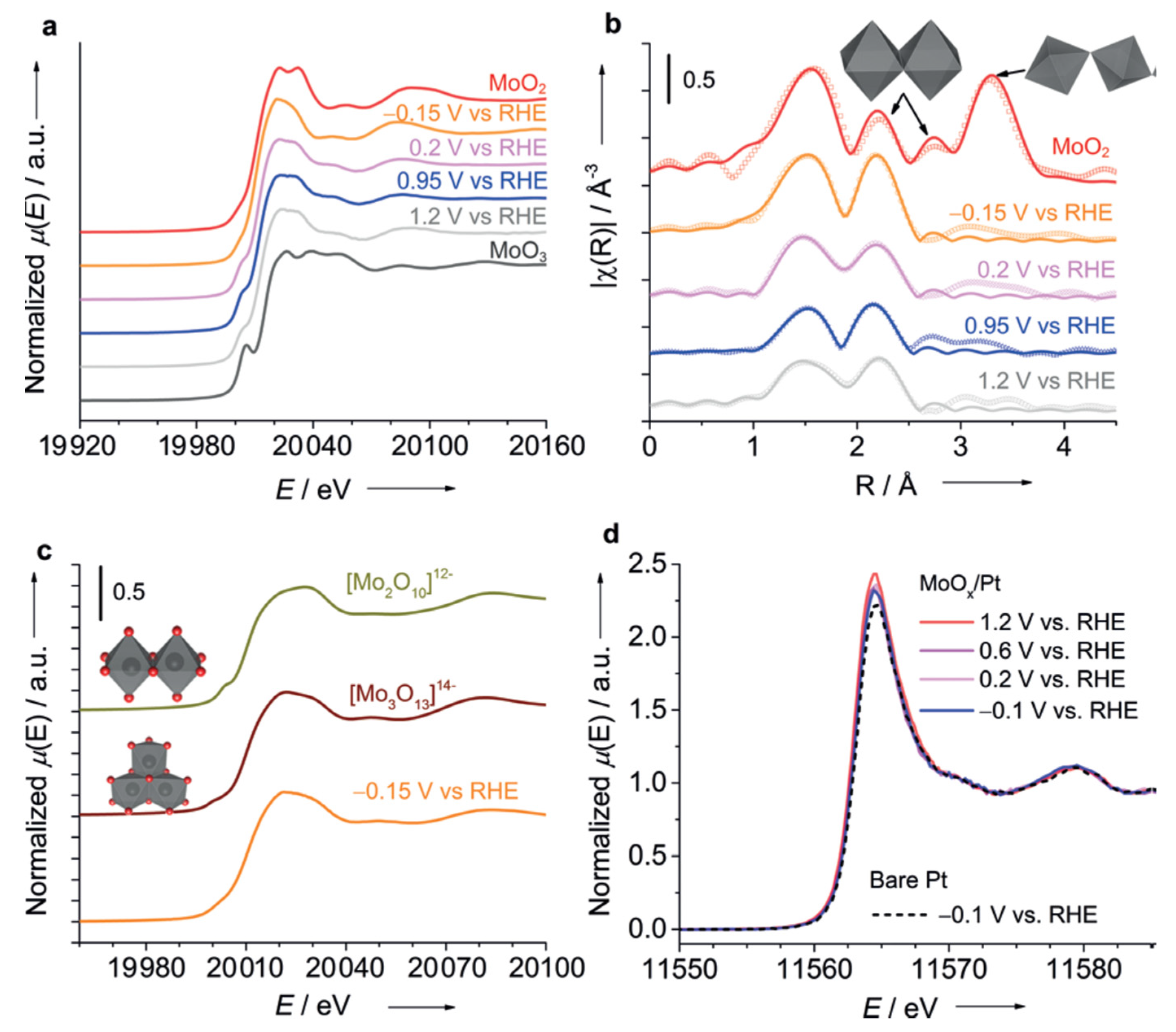

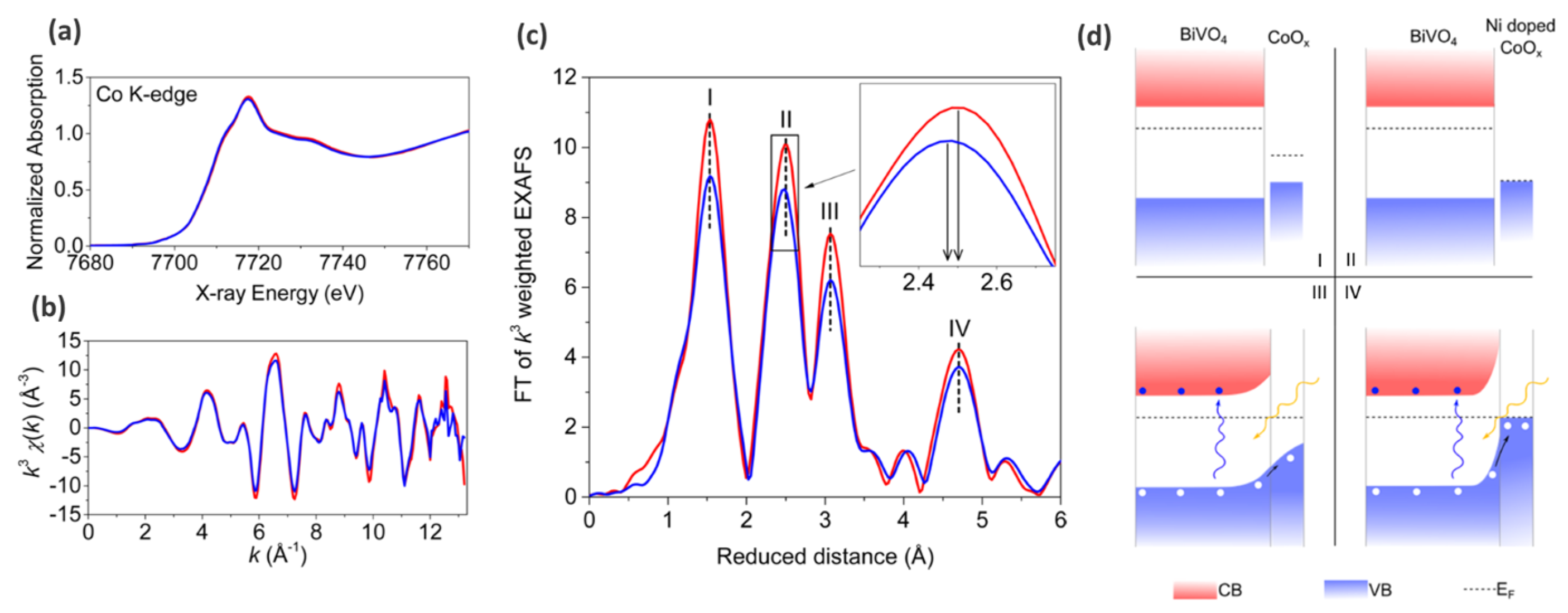
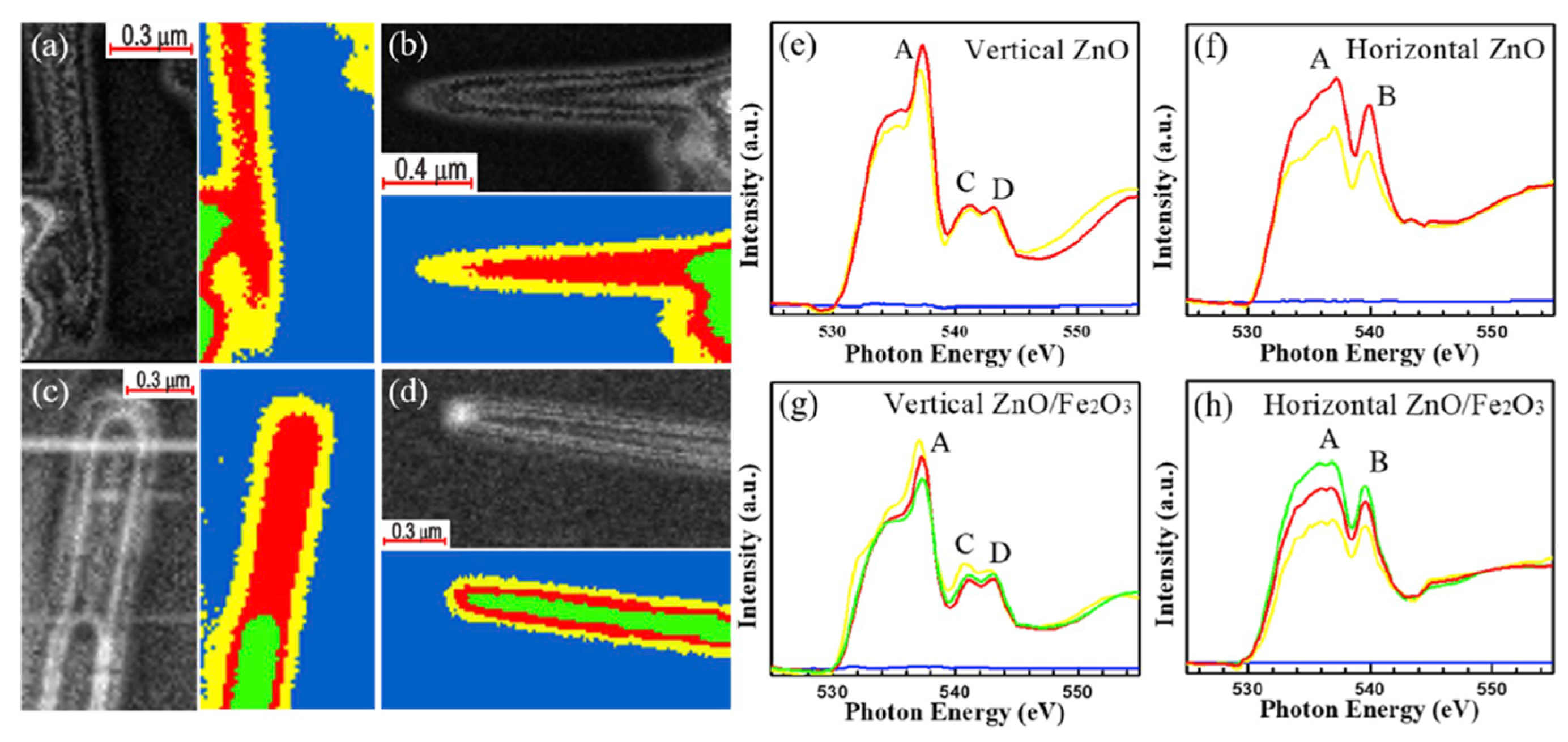

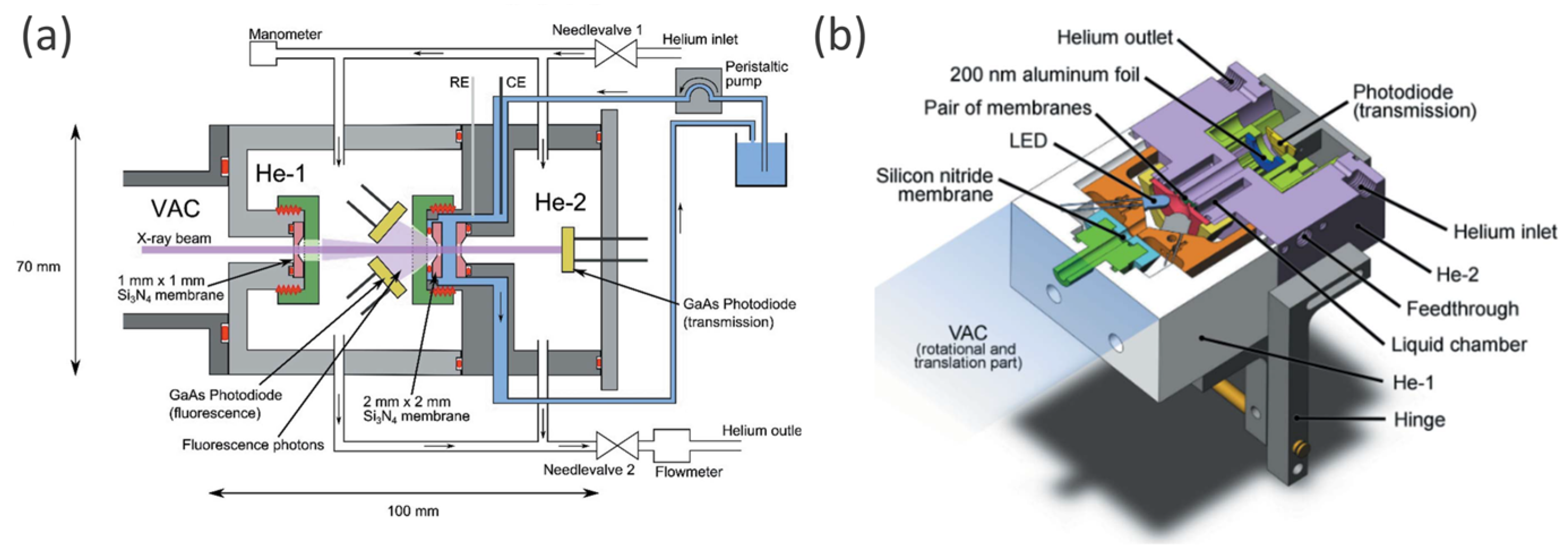
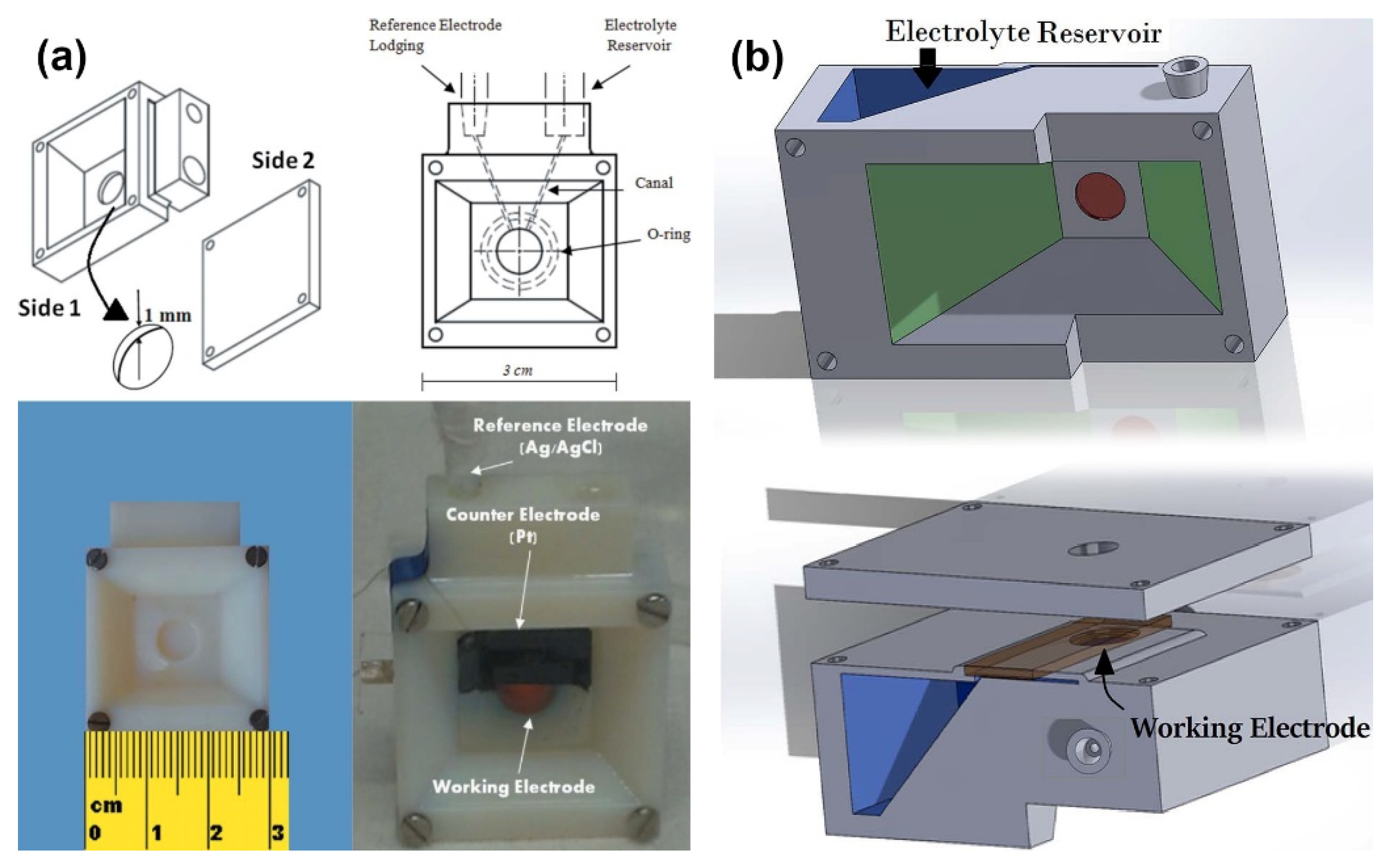
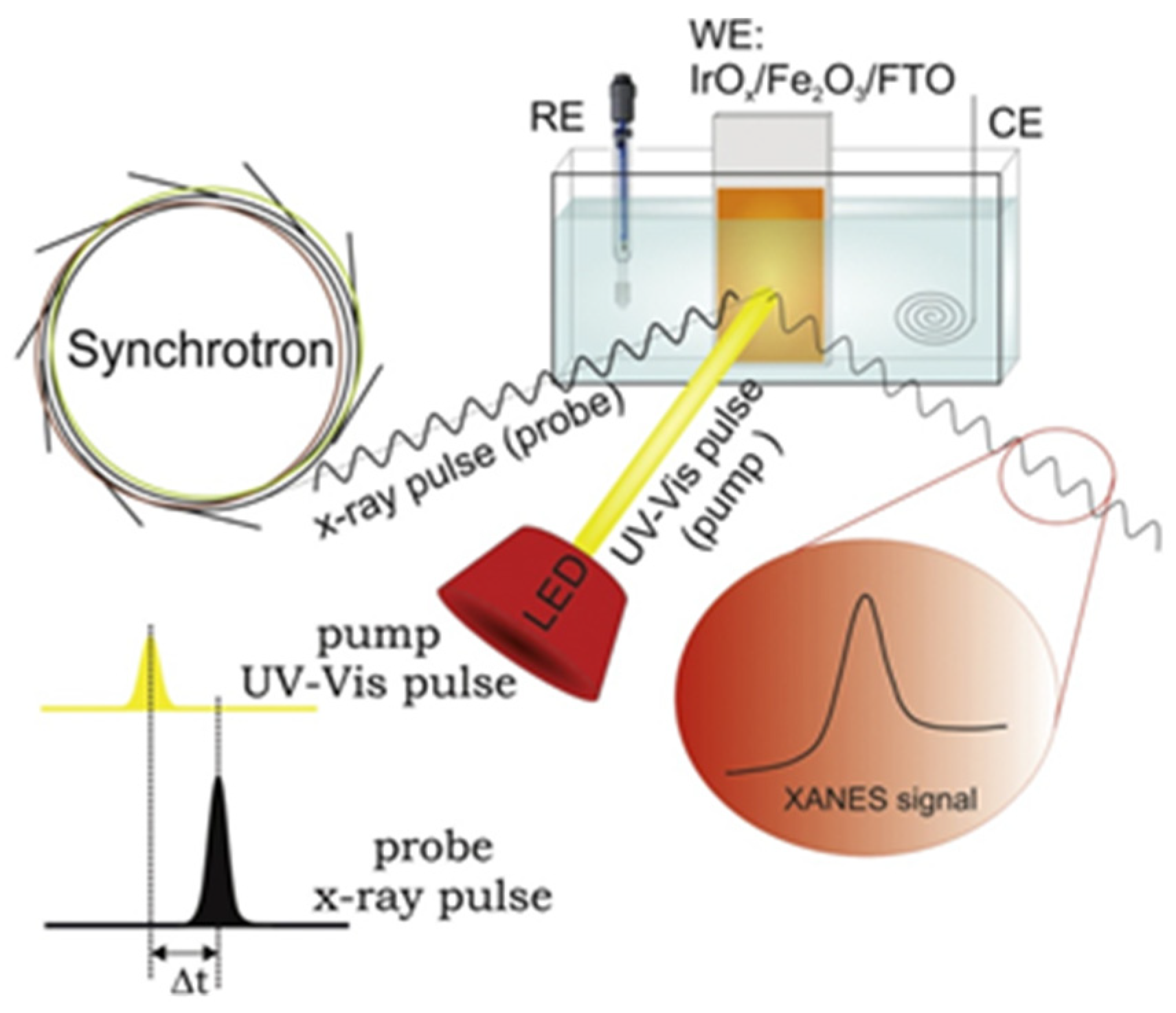
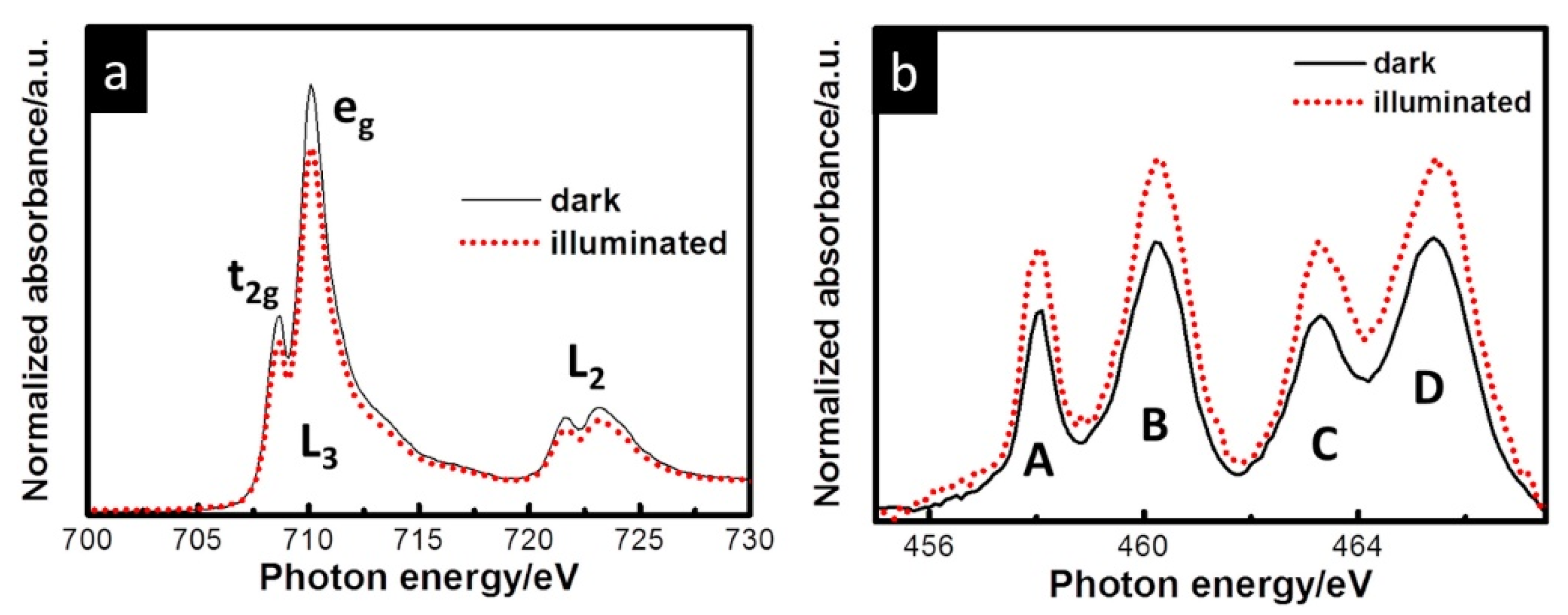

| Reaction | Mechanism | Photoelectrode Material | Electrolyte, Illumination, Applied Potential (vs. RHE) | Method of Characterization | PEC-Cell | Ref. |
|---|---|---|---|---|---|---|
| OER in alkaline media | The h+ transfer from α-Fe2O3 to IrOx overlayer was observed upon anodic photocurrent. An increase of h+ transfer was observed for higher pump-probe delay. At lower V, partial reduction of Ir occurs. | IrOx/α-Fe2O3@FTO | Aqueous 0.1 M K2HPO4 solution (pH 9.1); simultaneous (Δt = 0) and pump-and-probe (Δt = 600 ns) UV-vis illumination by LED at 400 nm; 1.56 V and slightly lower potential of 1.46 V at which there are no net anodic photocurrents. | operando time-resolved XAS in the fluorescence mode | Highly transparent to both UV–vis and X-ray radiation three-electrode cell, equipped with platinum and Ag/AgCl as counter and reference electrodes, respectively. | [102] |
| OER | Photoelectrons partially fill empty W 5d (t2g) orbitals. Progressive solid state redox transition accompanied by structural rearrangement of the photoanode material under OCP conditions. In contrast, at lower potential the formation of a WO3-x phase and/or Na+ intercalation was suggested. | WO3 mesoporous films onto FTO glass | Aqueous 0.1 M Na2SO4 (in Milli-Q grade water); backside illumination by means of a 400 nm LED; 0.35 V at which there are no photocurrents and quite higher value of 1.1 V where bubble formation gives no negative effect to the XAS signal. | operando XAS in the fluorescence mode: Δμ differential spectra and FEXRAV | Three-electrode cell made of two polyethylene terephthalate walls divided by a thick silicon rubber spacer with the W-shape internal structure. Thin Mylar® foil was used as the windows. The cell was equipped with Ag/AgCl and a Pt wire as a reference and counter electrodes, respectively. | [103] |
| CH4 conversion into ethylene glycol | Hydrogen atom abstracts from the CH4 producing methyl radicals CH3. Subsequent reactions lead to the formation of CH3OH attacked by highly reactive OH. Then, these formed hydroxymethyl radicals couple. | WO3 nanobar arrays onto FTO substrate | acidic medium of 0.1 M Na2SO4 (pH 2); LED light irradiation at 365 nm; 1.3 V applied potential. | in situ DRIFT spectroscopy | H-type cell with Nafion proton-exchange membrane separator. Ag/AgCl electrode and Pt sheet were used as the reference and counter electrode, respectively. | [104] |
| HER | Photoelectrons from CuO are injected into CB of In2O3, while holes from VB of In2O3 to CuO and further drain to support. However, unwanted electron trapping in copper oxide, causing its reduction to Cu2O was observed. | CuO/In2O3@FTO thin films | 0.1 M NaOH (pH = 13); illumination by means of a 400 nm LED; 0.4 V bias potential. | ex situ XAS in the total fluorescence yield mode: before and after 12 h stability test | Home-made gas-tight two compartment cell with three-electrode setup. One side held Ag/AgCl as a reference electrode along with a gold rod as a counter electrode, while the other side held a working electrode. | [105] |
| Water oxidation | Existence of local Fe2TiO5 structure in hematite formed a heterojunction, which facilitates the hole transport from hematite to Fe2TiO5 and improved the performance. | Ti-doped hematite then films | 1 M NaOH solution; Illumination by Xe lamp (150 W) with an AM 1.5 filter; 0.207 V potential. | in situ soft XAS | Conventional three-electrode system consisted of square platinum sheet as a counter electrode and an Ag/AgCl reference electrode | [106] |
| HER | Prepared Sb-doped ZnO NWs showed p-type behavior, leading to higher efficiency of photogenerated electron–holes separation. The piezotronic effect was used and tuned by applying different strains on the p-type ZnO NWs through a self-designed device in the PEC measurements, that improve PEC performance. | Sb-doped ZnO nanowires on a thin stainless steel | 0.2 M Na2SO4 solution, 500W Xe-lamp (100 mW/cm2), −0.2 VRHE | Synchrotron-based XANES in O K-edge and Zn L-edge of the samples | Three-electrode cell: WE, CE (Pt-foil), RE (Ag/AgCl) | [107] |
| HER | Nb- and Ta-doped α-Fe2O3 nanorods showed higher conductivity and therefore better PEC performance by facilitating charge transfer reducing electron–hole recombination. It was also estimated that Nb-doped hematite exhibits better since changes absorption intensity of materials more than Ta-doped does. | Nb- and Ta doped α-Fe2O3 nanorods on FTO glass plates | 0.5 M Na2SO4 solution, 500 W Xe-lamp (100 mW/cm2), −0.2 VRHE | Synchrotron-based XAS | Three-electrode cell: WE, CE (Pt-foil), RE (Ag/AgCl) | [108] |
| HER | (1) N 1s, Co 2p, C 1s revealed decomposition of porphyrin complexes under PEC conditions; (2) SOXPES allowed to probe Co states at different depths ranging from 2.5 nm to 9.5 nm | Cobalt porphyrin complexes on FTO glass plates, CoOx thin films | 0.1 M borate buffer | Hard X-ray Photoelectron spectroscopy (HAXPES), Soft X-ray Photoelectron spectroscopy (SOXPES) | Three-electrode cell: WE, CE (Pt-foil), RE (Ag/AgCl) | [109] |
| HER | XPS revealed the presence of Fe2+ features, which disappears during anodization of hematite film, wherein Fe3+ features concomitantly become enhanced. Bulk-sensitive analytical methods confirmed hematite structure of the photoanode. Therefore, only minute amounts of Fe2+ can be in or on the hematite photoanode and thus become converted, most likely at the hematite surface. | α-Fe2O3 on FTO glass plates | 1 M KOH, solar simulator light source, 200 mV to 500 mV | Synchrotron-based XPS and NEXAFS spectra | Gas-tight Teflon cell with three-electrode cell: WE, CE (Pt-plate), RE (Ag/AgCl) | [110] |
| OER |
| IrOx/α-Fe2O3-FTO photoanodes | aqueous 1 M K2HPO4 (pH = 9.8) Light Emitting Diode (LED) with a peak wavelength of 400 nm (LED engine, 5 mW, width of the emission ≈ 15 nm) focalized using BK7 glass spherical lenses; The radiant flux from the diode was about 0.25 W) Potential: 0.1 VRHE, 0.4 VRHE, 0.8 VRHE, 1.4 VRHE | operando Ir L3-edge XAS (at LISA-BM08 beamline at ESRF.) fluorescence mode + FEXRAV (at E = 11,221 eV) | Three electrode custom cell built using a 3D printer, reported in ref. [76] | [62] |
| HER + OER |
| Mo-coated Pt disk electrode | 0.1 M KClO4, pH 1.8 Applied potential: −0.15 VRHE, 0.2 VRHE, 0.95 VRHE, 1.2 VRHE. A Xe lamp (CERMAX PE300-BF, 300 W) was used as the light source, and the irradiation wavelength was controlled with the combination of a cold mirror and a water filter (300 < λ < 800 nm). | operando Mo K-edge XAS measurements (both XANES + EXAFS range) and Pt L3-edge HERFD-XANES under potential control for electrolysis under O2 saturation. | Three electrodes custom made used for operando XAS experiment. . The cell equipped with SiOx-Glassy Carbon window transparent for X-rays, which also playing a role of support for Mo-coated Pt WE. Mo was freshly electrodeposited on Pt in the XAS cells before each operando XAS run. | [65] |
| OER |
| NiBi decorated BiVO4 photoanode | The electrolyte is 0.2 M Bi buffer solution (pH 9.2). For DEMS experiments the light intensity was adjusted to 1.0 suns in the range of 400−900 nm. For XAS experiments, one while LED is used to illuminate the BiVO4 photoanode. Applied potential: OCP, 1.15 V, 1.45 V, 1.75 V and 2.05 V | in situ soft (Ni L-edges, O K-edge) and hard (Ni K-edge) XAS spectroscopy | The electrolyte solution is confined between two Si3N4 membranes (100 nm thickness). One of these Si3N4 membrane is coated with Ti and Au and as WE, platinum wire served as CE, and 1 mm diameter wire Ag/AgCl served as RE. The cell allows to measure in situ soft XAS in transmission mode [84]. | [111] |
| OER |
| Ni-doped CoOx (nitrogen flow assisted electrostatic spray pyrolysis) modified BiVO4 photoanode | Illumination: 500 W xenon lamp coupled to AM 1.5 filter (light intensity 100 mW cm−2); Electrolyte: aqueous 0.5 M Na2SO4; (no potential, ex situ XAS measurements) | Stady state hard XAS spectroscopy (Co K-edge) | Three-electrode cell: Ag/AgCl—reference electrode (RE); Platinum foil—counter electrode CE; | [88] |
| OER |
| Fe2O3 coating on ZnO nanowires (core–shell) | 1M NaOH solution AM 1.5 G filtered solar light 100 mW cm−2. Monochromator light for the excitation to measure the photoconversion of incident photons to electrons. | in situ soft (O K-edge, Zn L2,3-edge) and hard (Zn K-edge) XANES + STXM-XANES microscopy. | Two electrode modes: ZnO/Fe2O3 core–shell nanowires—WE; square platinum sheet—CE; | [94] |
| CO2 reduction | Working conditions strongly affect the structure of Bi2O3 nanotubes leading to formation of structural defects. | Tetragonal β-Bi2O3 nanotubes (NTs) on p-type Si nanowire arrays | Electrolite—CO2 bubbled 0.5 M KHCO3 with a volume of 35 mL. Irradiation—AM 1.5 G solar simulator with a light density of 50 mW/cm2. Applied potential—from −0.3 V to 0.2 V. | Operando XAS (XANES, EXAFS) | In situ PEC-cell with three electrodes: working electrode (1 × 1 cm2 carbon fiber paper with B2O3 NTs), counter electrode (graphite), reference electrode (Ag/AgCl). | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatov, M.A.; Medvedev, P.V.; Roldugin, V.; Novomlinskiy, I.N.; Pankin, I.; Su, H.; Liu, Q.; Soldatov, A.V. Operando Photo-Electrochemical Catalysts Synchrotron Studies. Nanomaterials 2022, 12, 839. https://doi.org/10.3390/nano12050839
Soldatov MA, Medvedev PV, Roldugin V, Novomlinskiy IN, Pankin I, Su H, Liu Q, Soldatov AV. Operando Photo-Electrochemical Catalysts Synchrotron Studies. Nanomaterials. 2022; 12(5):839. https://doi.org/10.3390/nano12050839
Chicago/Turabian StyleSoldatov, Mikhail A., Pavel V. Medvedev, Victor Roldugin, Ivan N. Novomlinskiy, Ilia Pankin, Hui Su, Qinghua Liu, and Alexander V. Soldatov. 2022. "Operando Photo-Electrochemical Catalysts Synchrotron Studies" Nanomaterials 12, no. 5: 839. https://doi.org/10.3390/nano12050839
APA StyleSoldatov, M. A., Medvedev, P. V., Roldugin, V., Novomlinskiy, I. N., Pankin, I., Su, H., Liu, Q., & Soldatov, A. V. (2022). Operando Photo-Electrochemical Catalysts Synchrotron Studies. Nanomaterials, 12(5), 839. https://doi.org/10.3390/nano12050839







