Binary Type-II Heterojunction K7HNb6O19/g-C3N4: An Effective Photocatalyst for Hydrogen Evolution without a Co-Catalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Synthesis of K7HNb6O19·13H2O
2.3. Synthesis of g-C3N4
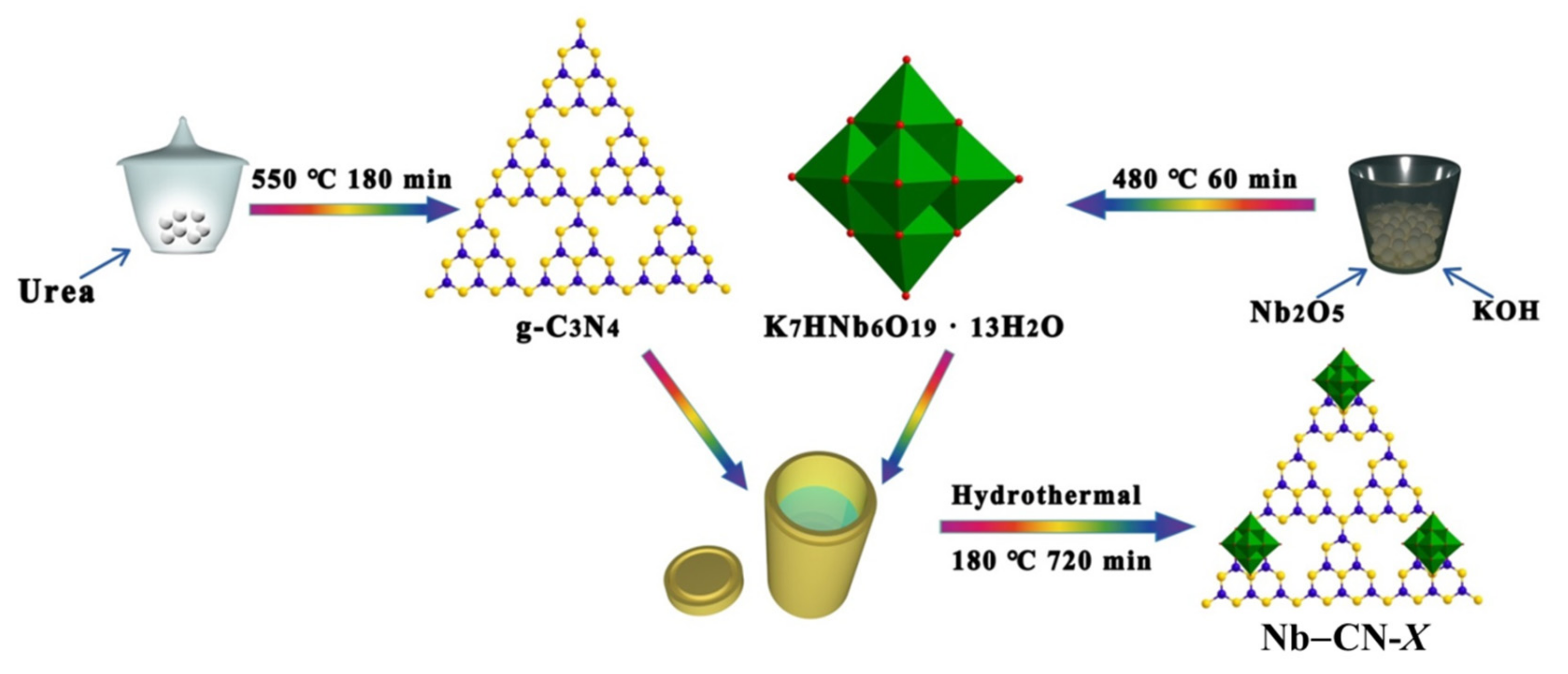
2.4. Synthesis of Nb–CN-X Composites
3. Results and Discussion
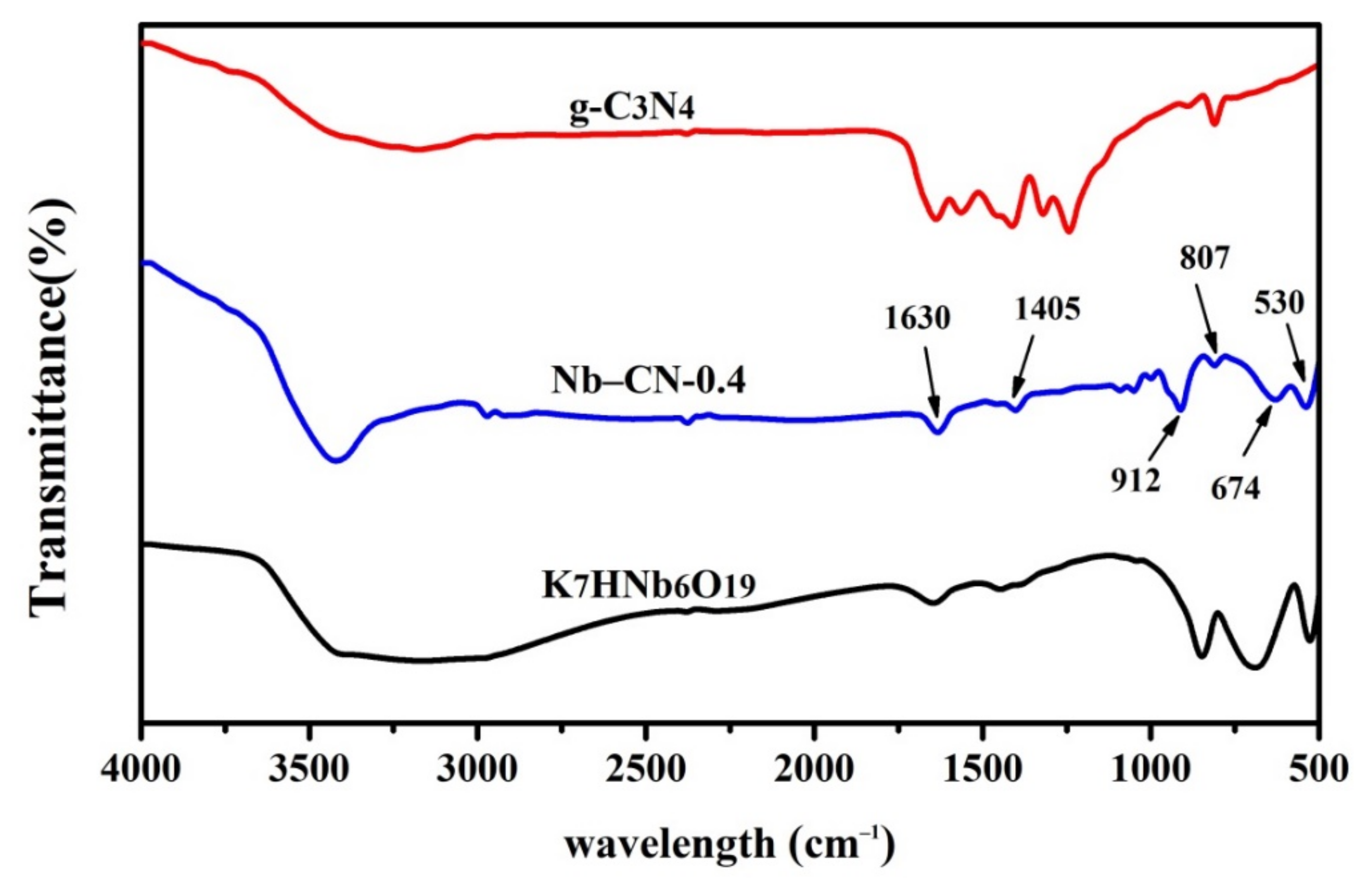
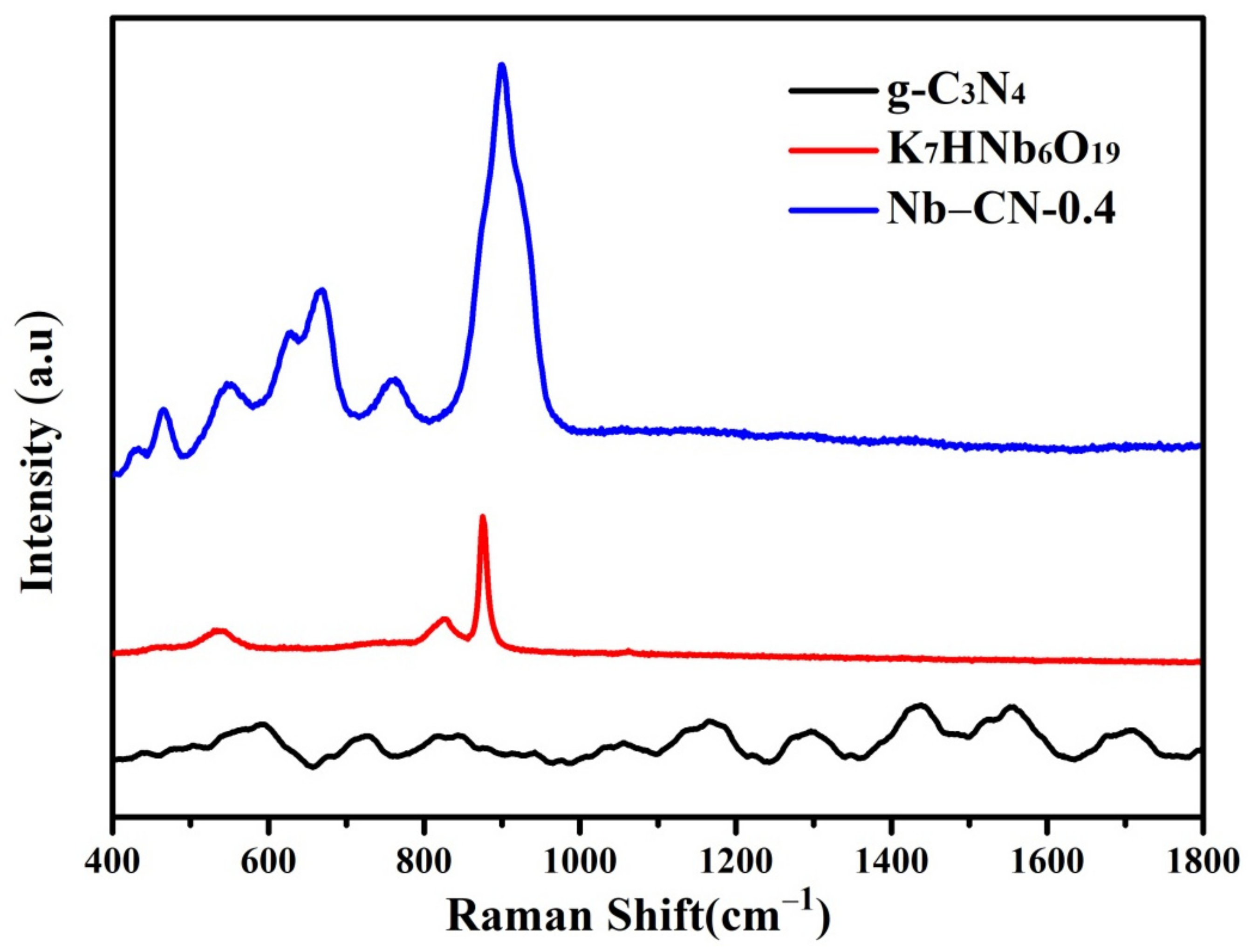
3.1. Morphology and Structure Analysis
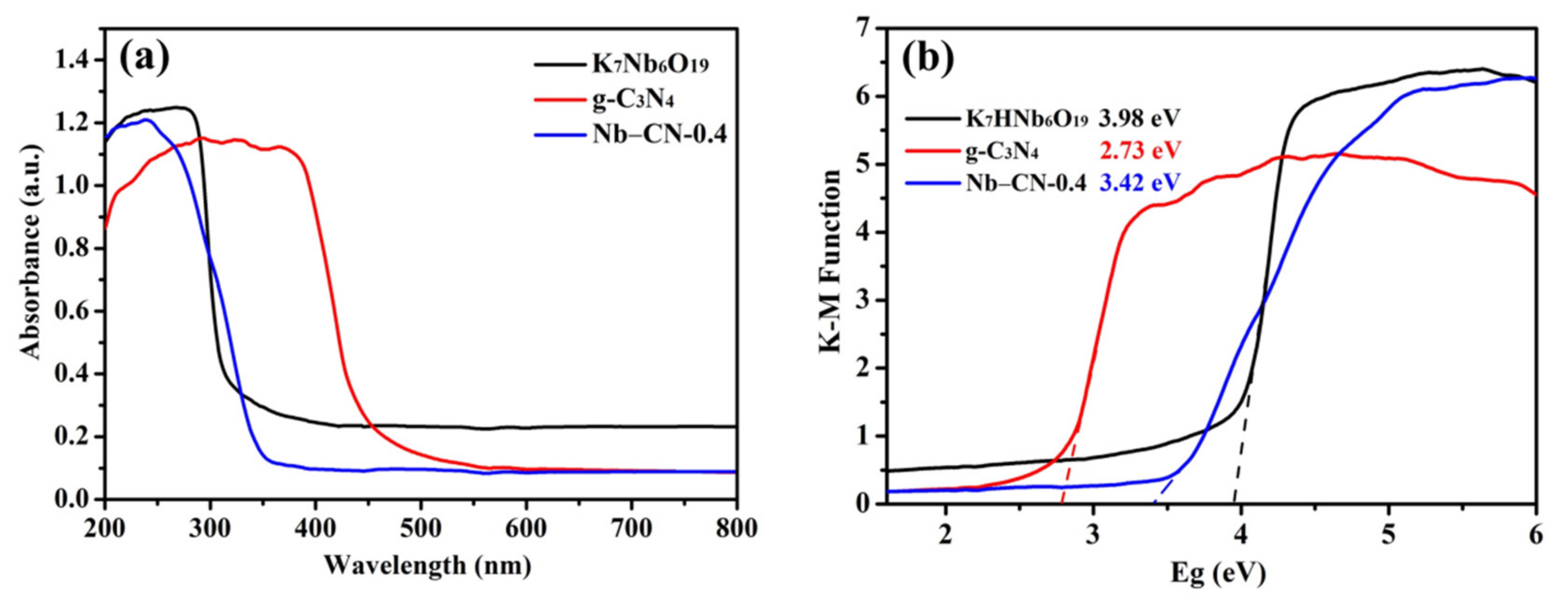
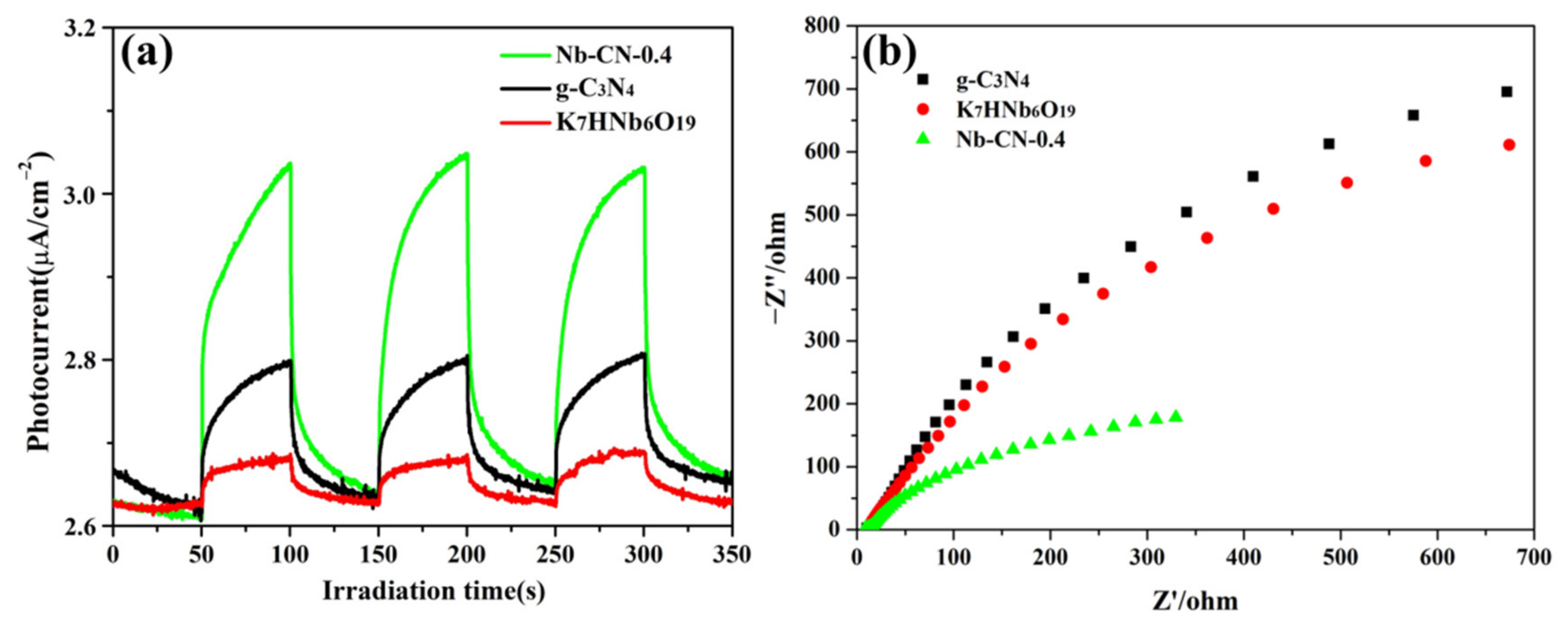
3.2. Optical and Electric Properties
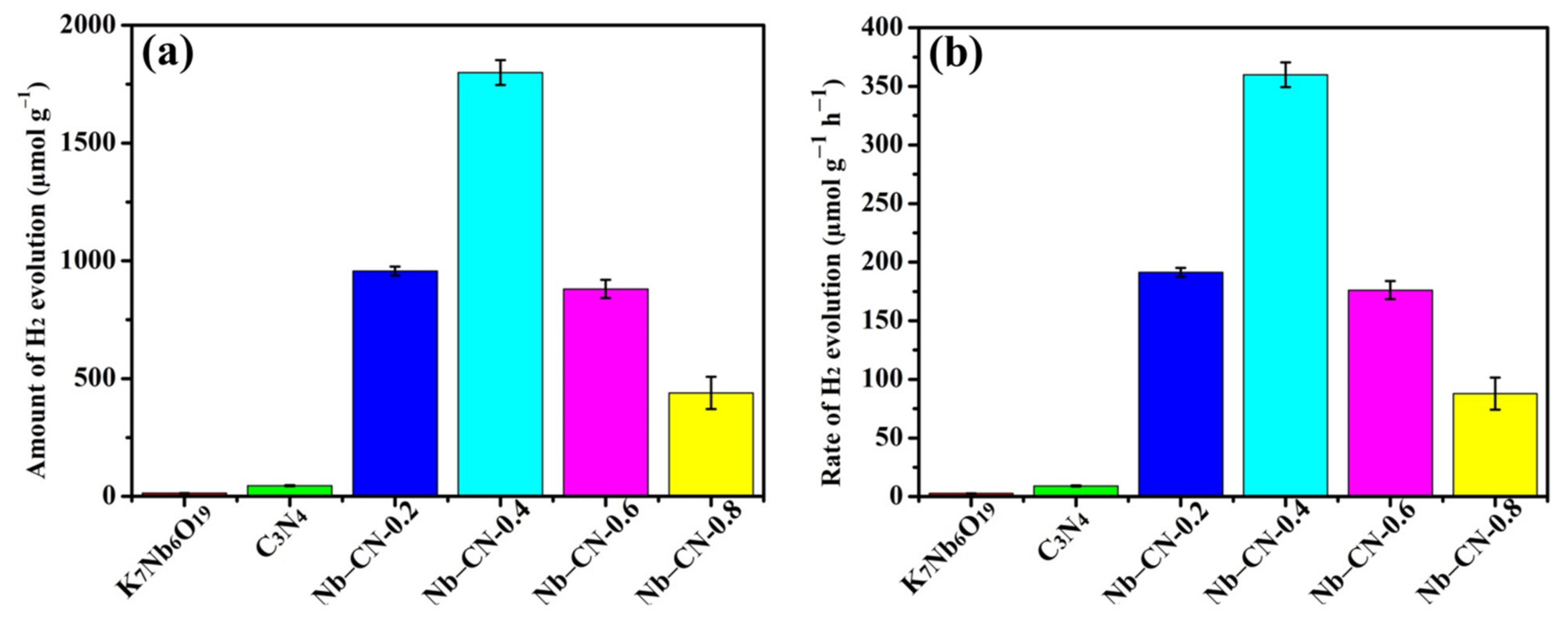
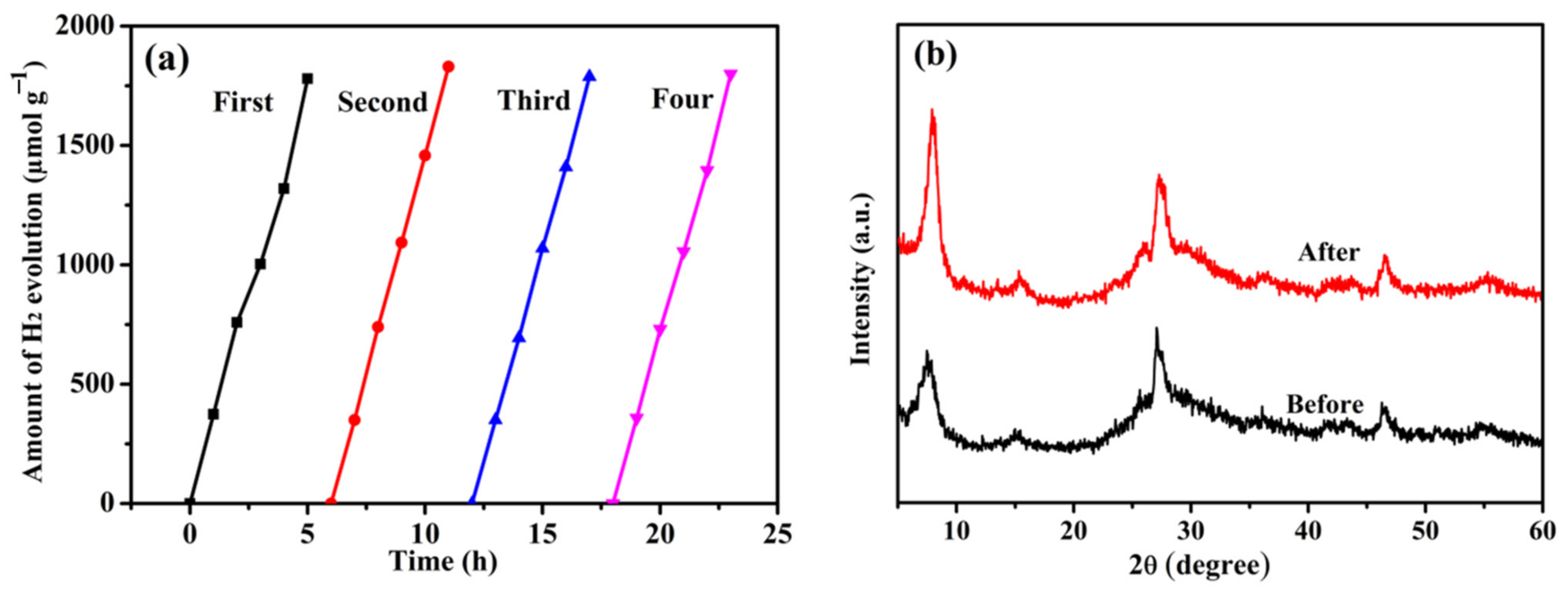
3.3. Photocatalytic H2 Production
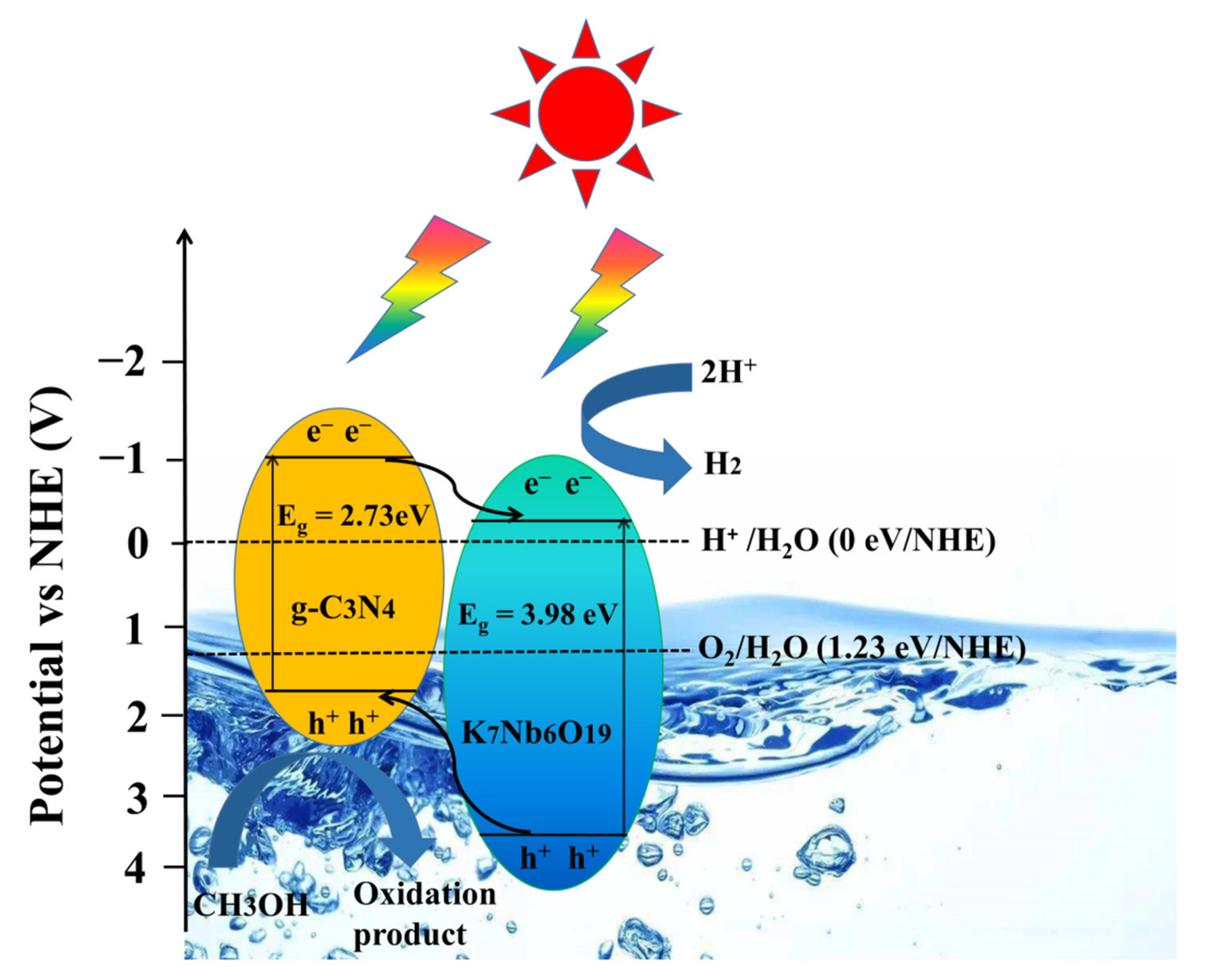
3.4. Investigation of Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, M.; Wang, X.; Jia, Y.; Chen, X.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X. Rational design of 1D/2D heterostructured photocatalyst for energy and environmental applications. Chem. Eng. J. 2020, 395, 125030. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Zeng, G.; Cui, L.; Lei, Q.; Min, C.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Sołowski, G.; Shalaby, M.S.; Abdallah, H.; Shaban, A.M.; Cenian, A. Production of hydrogen from biomass and its separation using membrane technology. Sust. Energ. Rev. 2019, 82, 3152–3167. [Google Scholar] [CrossRef]
- Yan, J.; Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Recent Progress on Black Phosphorus-Based Materials for Photocatalytic Water Splitting. Small Methods 2018, 2, 1800212. [Google Scholar] [CrossRef]
- Ng, B.; Putri, L.; Kong, X.; Teh, Y.; Pasbakhsh, P.; Chai, S. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2019, 7, 1903171. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, S.; Wang, X.; Yan, W.; Peng, S. Ramakarishna, Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B Environ. 2019, 257, 117855. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Dong, B.; Lan, Y. Polyoxometalate-Based Compounds for photo/electrocatalytic applications. Angew. Chem. Int. Ed. 2020, 59, 20779–20793. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Singh, A.; Alomari, S.; Goberna-Ferro’n, S.; Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; MacLeod, J.; Sonar, P.; et al. Polyoxometalates (POMs): From electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Zhao, J.; Wang, L.; Yang, G. State-of-the-art advances in the structural diversities and catalytic applications of polyoxoniobate-based materials. Coordin. Chem. Rev. 2021, 440, 213966. [Google Scholar] [CrossRef]
- Xu, Q.; Niu, Y.; Wang, G.; Li, Y.; Zhao, Y.; Singh, V.; Niu, J.; Wang, J. Polyoxoniobates as a superior Lewis base efficiently catalyzed Knoevenagel Condensation. Mol. Catal. 2018, 453, 93–99. [Google Scholar] [CrossRef]
- Dong, J.; Lv, H.; Sun, X.; Wang, Y.; Ni, Y.; Zou, B.; Zhang, N.; Yin, A.; Chi, Y.; Hu, C. A Versatile Self-Detoxifying Material Based on Immobilized Polyoxoniobate for Decontamination of Chemical Warfare Agent Simulants. Chem. Eur. J. 2018, 24, 19208–19215. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, X.; Qi, Y.; Niu, P.; Zheng, S. Giant Hollow Heterometallic Polyoxoniobates with Sodalite-Type Lanthanide–Tungsten–Oxide Cages: Discrete Nanoclusters and Extended Frameworks. Angew. Chem. Int. Ed. 2016, 55, 13793–13797. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The ‘‘holy grail’’ for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Yin, W.; Bai, L.; Zhu, Y.; Zhong, S.; Zhao, L.; Li, Z.; Bai, S. Embedding Metal in the Interface of a p-n Heterojunction with a Stack Design for Superior Z-Scheme Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces. 2016, 8, 23133–23142. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Liu, M.; Cheng, C.; Deng, J.; Shi, J. Localized NiS2 Quantum Dots on g-C3N4 Nanosheets for Efficient Photocatalytic Hydrogen Production from Water. ChemCatChem 2018, 10, 5441–5448. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, X.; Chen, S. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, S.; Zhang, X.; Jiang, Z.; Zheng, J.; Guo, C. Combination of experimental and theoretical investigation on Ti-doped gC3N4 with improved photo-catalytic activity. Appl. Surf. Sci. 2019, 489, 427–434. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Lu, R.; Yu, A. Time-Resolved Study on Xanthene Dye-Sensitized Carbon Nitride Photocatalytic Systems. ACS Appl. Mater. Interfaces 2015, 7, 21868–21874. [Google Scholar] [CrossRef]
- Li, X.; Bi, W.; Zhang, L.; Tao, S.; Chu, W.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Single-Atom Pt as Co-Catalyst for Enhanced Photocatalytic H2 Evolution. Adv. Mater. 2016, 28, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Raziq, F.; Sun, L.; Wang, Y.; Zhang, X.; Humayun, M.; Ali, S.; Bai, L.; Qu, Y.; Yu, H.; Jing, L. Synthesis of Large Surface-Area g-C3N4 Comodified with MnOx and Au-TiO2 as Efficient Visible-Light Photocatalysts for Fuel Production. Adv. Energy Mater. 2018, 8, 1701580. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Y.; Qin, J.; Li, Z.; Wang, J. Boron doped C3N4 nanodots/nonmetal element (S, P, F, Br) doped C3N4 nanosheets heterojunction with synergistic effect to boost the photocatalytic hydrogen production performance. Appl. Surf. Sci. 2020, 541, 148558. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, D.; Li, X.; Sun, Y.; Zheng, S. Synthesis of noble-metal-free ternary K7HNb6O19/Cd0.5Zn0.5S/g-C3N4 tandem heterojunctions for efficient photocatalytic performance under visible light. Appl. Organomet. Chem. 2019, 33, e5178. [Google Scholar] [CrossRef]
- Yan, G.; Feng, X.; Xiao, L.; Xi, W.; Tan, H.; Shi, H.; Wang, Y.; Li, Y. Tuning of the photocatalytic performance of g-C3N4 by polyoxometalates under visible light. Dalton Trans. 2017, 46, 16019–16024. [Google Scholar] [CrossRef]
- Wu, J.; Liao, L.; Yan, W.; Xue, Y.; Sun, Y.; Yan, X.; Chen, Y.; Xie, Y. Polyoxometalates Immobilized in Ordered Mesoporous Carbon Nitride as Highly Efficient Water Oxidation Catalysts. ChemSusChem 2012, 5, 1207–1212. [Google Scholar] [CrossRef]
- Liu, J.; Xie, S.; Geng, Z.; Huang, K.; Fan, L.; Zhou, W.; Qiu, L.; Gao, D.; Ji, L.; Duan, L.; et al. Carbon Nitride Supramolecular Hybrid Material Enabled HighEfficiency Photocatalytic Water Treatments. Nano Lett. 2016, 16, 6568–6575. [Google Scholar] [CrossRef]
- Long, Z.; Zhou, Y.; Chen, G.; Ge, W.; Wang, J. C3N4-H5PMo10V2O40: A dual-catalysis system for reductant-free aerobic oxidation of benzene to phenol. Sci. Rep. 2014, 4, 3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Chen, H.; He, Y.; Zhao, H.; Wang, R.; Zhang, L.; You, W. Biomolecule-mediated hydrothermal synthesis of polyoxoniobate–CdS nanohybrids with enhanced photocatalytic performance for hydrogen production and RhB degradation. Dalton Trans. 2017, 46, 9407–9414. [Google Scholar] [CrossRef]
- Gan, Q.; Shi, W.; Xing, Y.; Hou, Y. A Polyoxoniobate/g-C3N4 Nanoporous Material with High Adsorption Capacity of Methylene Blue from Aqueous Solution. Front. Chem. 2018, 6, 7–16. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Y.; Han, Z.; Sun, Y.; Wang, X.; Zhang, Q.; Zou, Z. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production. Chin. J. Catal. 2021, 42, 164–174. [Google Scholar] [CrossRef]
- Filowitz, M.; Ho, R.K.; Klemperer, W.G.; Shum, W. 17O Nuclear Magnetic Resonance Spectroscopy of Polyoxometalates. 1. Sensitivity and Resolution. Inorg. Chem. 1979, 18, 93–103. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2 Evolution from Water using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.; Yang, G.; Yang, B.; Zhao, Y. Construction of Novel Three Dimensionally Ordered Macroporous Carbon Nitride for Highly Efficient Photocatalytic Activity. Appl. Catal. B Environ. 2016, 198, 276–285. [Google Scholar] [CrossRef]
- Heng, S.; Li, L.; Li, W.; Li, H.; Pang, J.; Zhang, M.; Bai, Y.; Dang, D. Enhanced Photocatalytic Hydrogen Production of the Polyoxoniobate Modified with RGO and PPy. Nanomaterials 2020, 10, 2449. [Google Scholar] [CrossRef]
- Heng, S.; Song, Q.; Liu, S.; Guo, H.; Pang, J.; Qu, X.; Bai, Y.; Li, L.; Dang, D. Construction of 2D polyoxoniobate/RGO heterojunction photocatalysts for the enhanced photodegradation of tetracycline. Appl. Surf. Sci. 2021, 553, 149505. [Google Scholar] [CrossRef]
- Bi, J.; Zhu, L.; Wu, J.; Xu, Y.; Wang, Z.; Zhang, X.; Han, Y. Optimizing electronic structure and charge transport of sulfur/potassium co-doped graphitic carbon nitride with efficient photocatalytic hydrogen evolution performance. Appl. Organomet. Chem. 2019, 33, e5163. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Black, J.R.; Nyman, M.; Casey, W.H. Rates of Oxygen Exchange between the [HxNb6O19]8−x(aq) Lindqvist Ion and Aqueous Solutions. J. Am. Chem. Soc. 2006, 128, 14712–14720. [Google Scholar] [CrossRef]
- Xing, W.; Tu, W.; Han, Z.; Hu, Y.; Meng, Q.; Chen, G. Template-induced high-crystalline g-C3N4 nanosheets for enhanced photocatalytic H2 evolution. ACS Energy Lett. 2018, 3, 514–519. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, M.; Wei, Z.; Pan, M.; Su, C. Ultrathin Graphitic Carbon Nitride Nanosheets for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 1010–1018. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Kong, Y.; Lv, C.; Zhang, C.; Chen, G. Cyano group modified g-C3N4: Molten salt method achievement and promoted photocatalytic nitrogen fixation activity. Appl. Surf. Sci. 2020, 515, 146009. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Xu, J.; Liu, Q.; Xu, H.; Tang, H.; Li, G.; Jiang, Y.; Qu, F.; Lin, Z.; et al. Probing supramolecular assembly and charge carrier dynamics toward enhanced photocatalytic hydrogen evolution in 2D graphitic carbon nitride nanosheets. Appl. Catal. B Environ. 2019, 256, 117867. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Xing, Y.; Liu, X.; Yang, Y.; Wang, X.; Song, S. Preparation of Carbon-Rich g-C3N4 Nanosheets with Enhanced Visible Light Utilization for Efficient Photocatalytic Hydrogen Production. Small 2017, 13, 1701552. [Google Scholar] [CrossRef]
- Aureliano, M.; Ohlin, C.A.; Vieira, M.O.; Marques, M.P.; Caseye, W.H.; Carvalho, L.B. Characterization of decavanadate and decaniobate solutions by Raman spectroscopy. Dalton Trans. 2016, 45, 7391–7399. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Ou-yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon. 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Dong, H.; Guo, X.; Yang, C.; Ouyang, Z. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl. Catal. B Environ. 2018, 230, 65–76. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO3. J. Appl. Phys. 1977, 48, 1914. [Google Scholar] [CrossRef]
- Nekoueia, F.; Nekoueib, S.; Pouzeshc, M.; Liu, Y. Porous-CdS/Cu2O/graphitic-C3N4 dual p-n junctions as highly efficient photo/catalysts for degrading ciprofloxacin and generating hydrogen using solar energy. Chem. Eng. J. 2020, 385, 123710. [Google Scholar] [CrossRef]
- Wang, Y.; Bayazit, M.K.; Moniz, S.J.; Ruan, Q.; Lau, C.; Martsinovich, N.; Tang, J. Linker-controlled polymeric photocatalyst for highly efficient hydrogen evolution from water. Energy Environ. Sci. 2017, 10, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Huang, Q.; Zhu, B.; Yu, J. Trace-level phosphorus and sodium co-doping of g-C3N4 for enhanced photocatalytic H2 production. J. Power Sources 2017, 351, 151–159. [Google Scholar] [CrossRef]
- Lia, H.; Wang, Z.; Lua, Y.; Liu, S.; Chen, X.; Wei, G.; Ye, G.; Chen, J. Microplasma electrochemistry (MIPEC) methods for improving the photocatalytic performance of g-C3N4 in degradation of RhB. Appl. Surf. Sci. 2020, 531, 147307. [Google Scholar] [CrossRef]
- Cai, S.; Wang, L.; Heng, S.; Li, H.; Bai, Y.; Dang, D. Interaction of Single-Atom Platinum–Oxygen Vacancy Defects for the Boosted Photosplitting Water H2 Evolution and CO2 Photoreduction: Experimental and Theoretical Study. J. Phys. Chem. C 2020, 124, 24566–24579. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, H.; Chen, W.; Li, Y.; Wang, E. A copper (II)–ethylenediamine modified polyoxoniobate with photocatalytic H2 evolution activity under visible light irradiation. Dalton Trans. 2012, 41, 9882. [Google Scholar] [CrossRef] [Green Version]
- Geng, Q.; Liu, Q.; Ma, P.; Wang, J.; Niu, J. Synthesis, crystal structure and photocatalytic properties of an unprecedented arsenic-disubstituted Lindqvist-type peroxopolyoxoniobate ion: {As2Nb4(O2)4O14H1.5}4.5−. Dalton Trans. 2014, 43, 9843. [Google Scholar] [CrossRef]
- Niu, J.; Li, F.; Zhao, J.; Ma, P.; Zhang, D.; Ulrich Kortz, B.B.; Wang, J. Tetradecacobalt(II)-Containing 36-Niobate [Co14(OH)16(H2O)8Nb36O106]20− and Its Photocatalytic H2 Evolution Activity. Chem. Eur. J. 2014, 20, 9852–9857. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Zhang, Z.; Li, Y.; Gao, Y.; Wang, E. Polyoxoniobate-based 3D framework materials with photocatalytic hydrogen evolution activity. Chem. Commun. 2014, 50, 6017. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Zhang, X.; Chi, Y.; Yang, S.; Li, J.; Hu, C. Controllable Assembly of Vanadium-Containing Polyoxoniobate-Based Three-Dimensional Organic–Inorganic Hybrid Compounds and Their Photocatalytic Properties. Inorg. Chem. 2016, 55, 7501–7507. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Q.; Heng, S.; Wang, W.; Guo, H.; Li, H.; Dang, D. Binary Type-II Heterojunction K7HNb6O19/g-C3N4: An Effective Photocatalyst for Hydrogen Evolution without a Co-Catalyst. Nanomaterials 2022, 12, 849. https://doi.org/10.3390/nano12050849
Song Q, Heng S, Wang W, Guo H, Li H, Dang D. Binary Type-II Heterojunction K7HNb6O19/g-C3N4: An Effective Photocatalyst for Hydrogen Evolution without a Co-Catalyst. Nanomaterials. 2022; 12(5):849. https://doi.org/10.3390/nano12050849
Chicago/Turabian StyleSong, Qi, Shiliang Heng, Wenbin Wang, Huili Guo, Haiyan Li, and Dongbin Dang. 2022. "Binary Type-II Heterojunction K7HNb6O19/g-C3N4: An Effective Photocatalyst for Hydrogen Evolution without a Co-Catalyst" Nanomaterials 12, no. 5: 849. https://doi.org/10.3390/nano12050849




