Synthesis of Magnetic Fe3O4 Nano Hollow Spheres for Industrial TNT Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
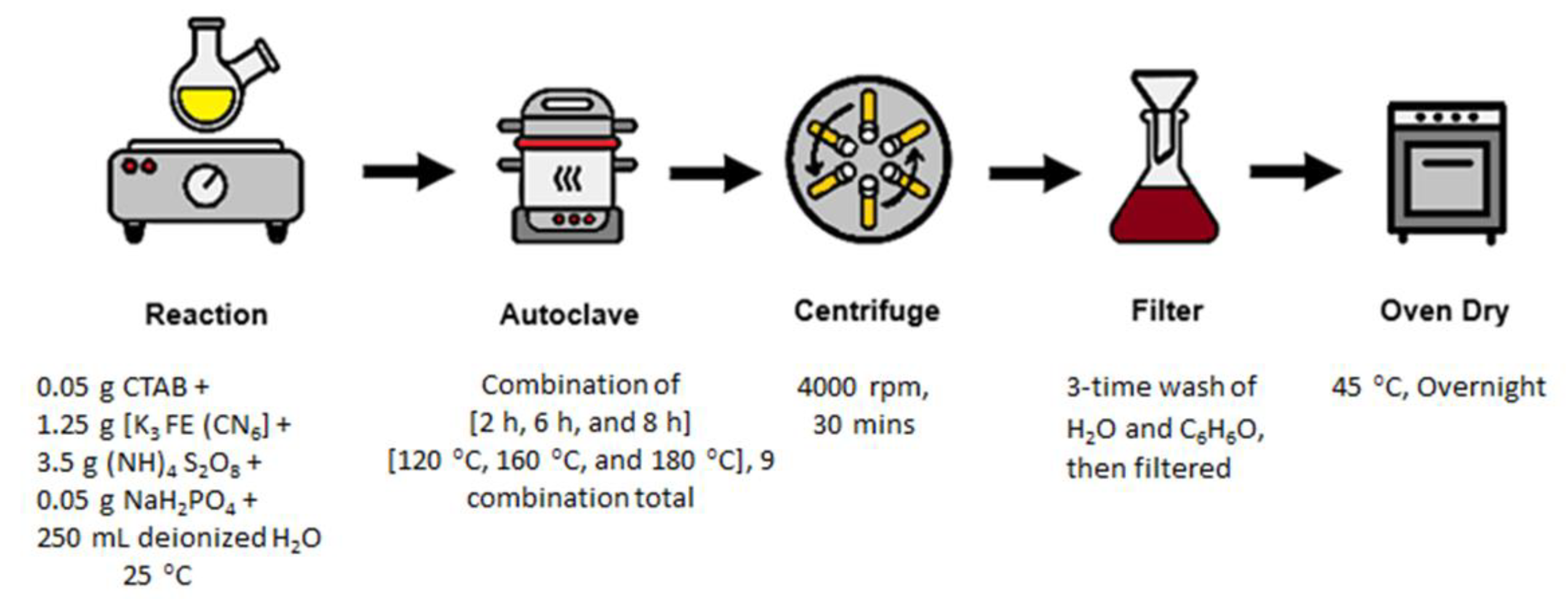
2.1. Materials
Adsorbent
2.2. Characterization Techniques
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. X-Ray Diffraction (XRD)
2.2.3. Brunner–Emmet–Teller (BET)
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.5. Ultraviolet/Visible Spectrophotometer (UV-Vis)
2.2.6. Chemical Oxygen Demand (COD) Determination
3. Results and Discussion
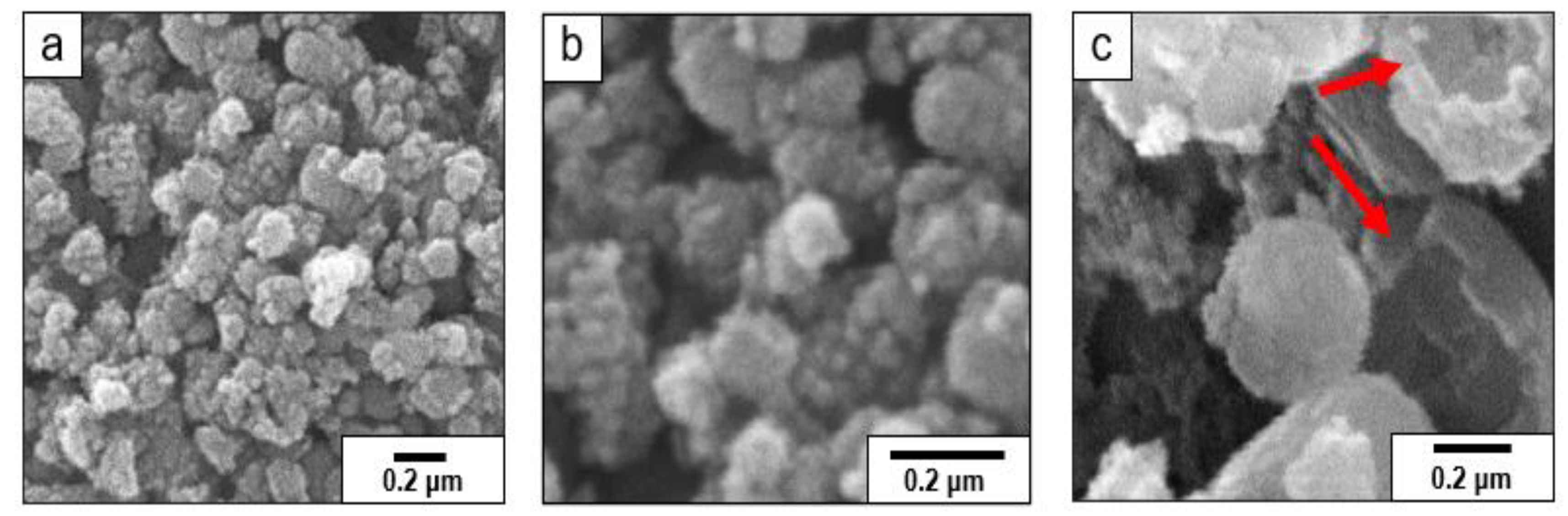
3.1. Scanning Electron Microscopy (SEM)
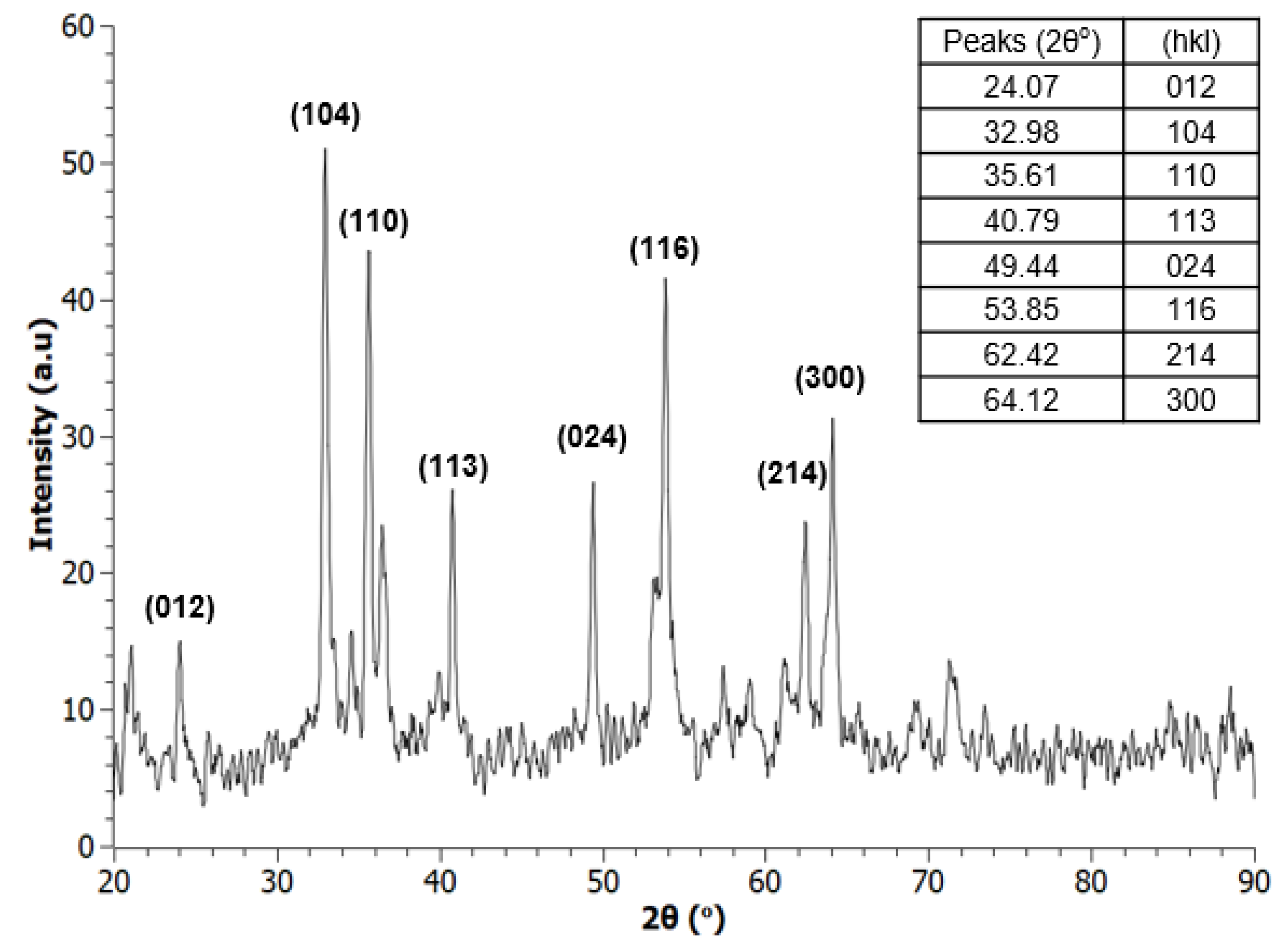
3.2. X-Ray Diffraction Spectroscopy (XRD)
3.3. Brunner–Emmett–Teller (BET) Adsorption Method
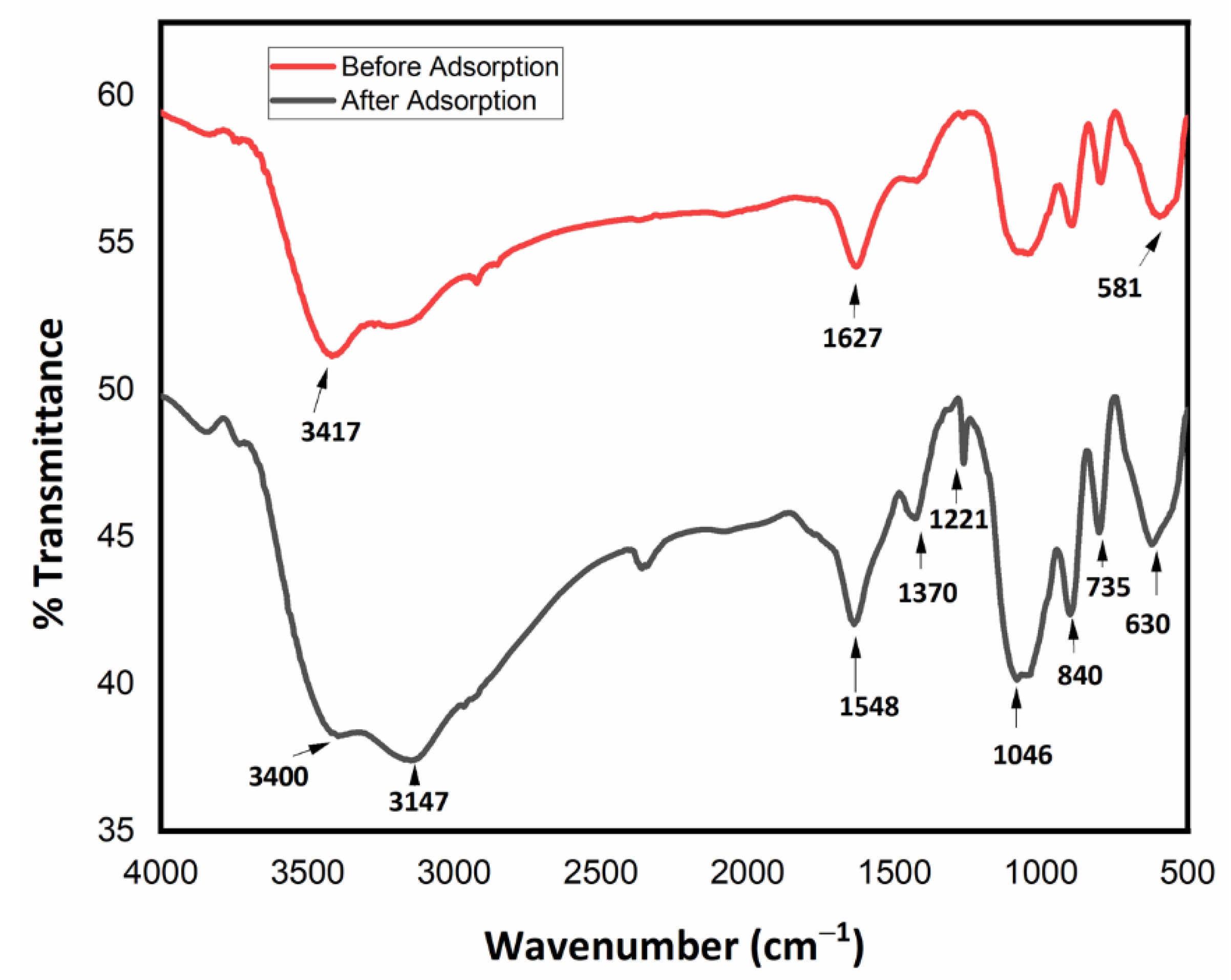
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
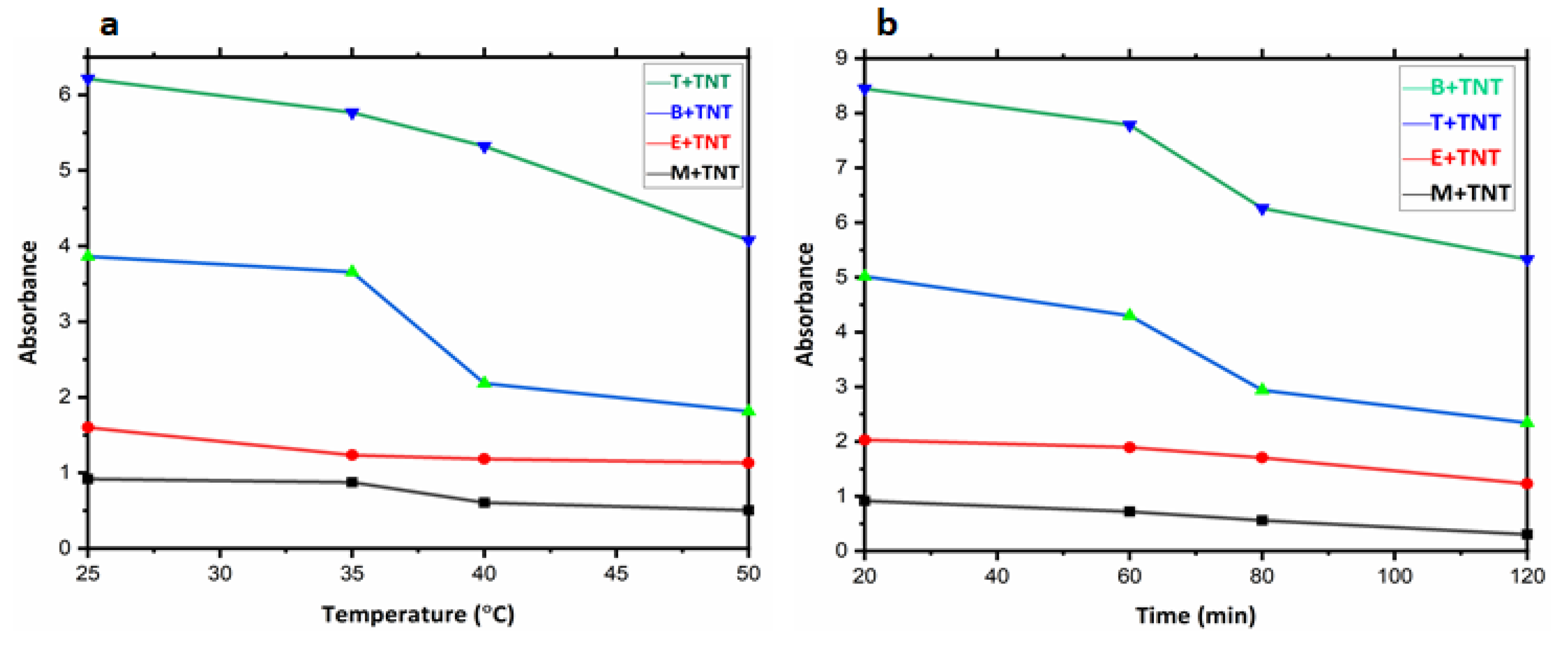
3.5. Ultraviolet/Visible Spectrophotometer (UV-Vis)
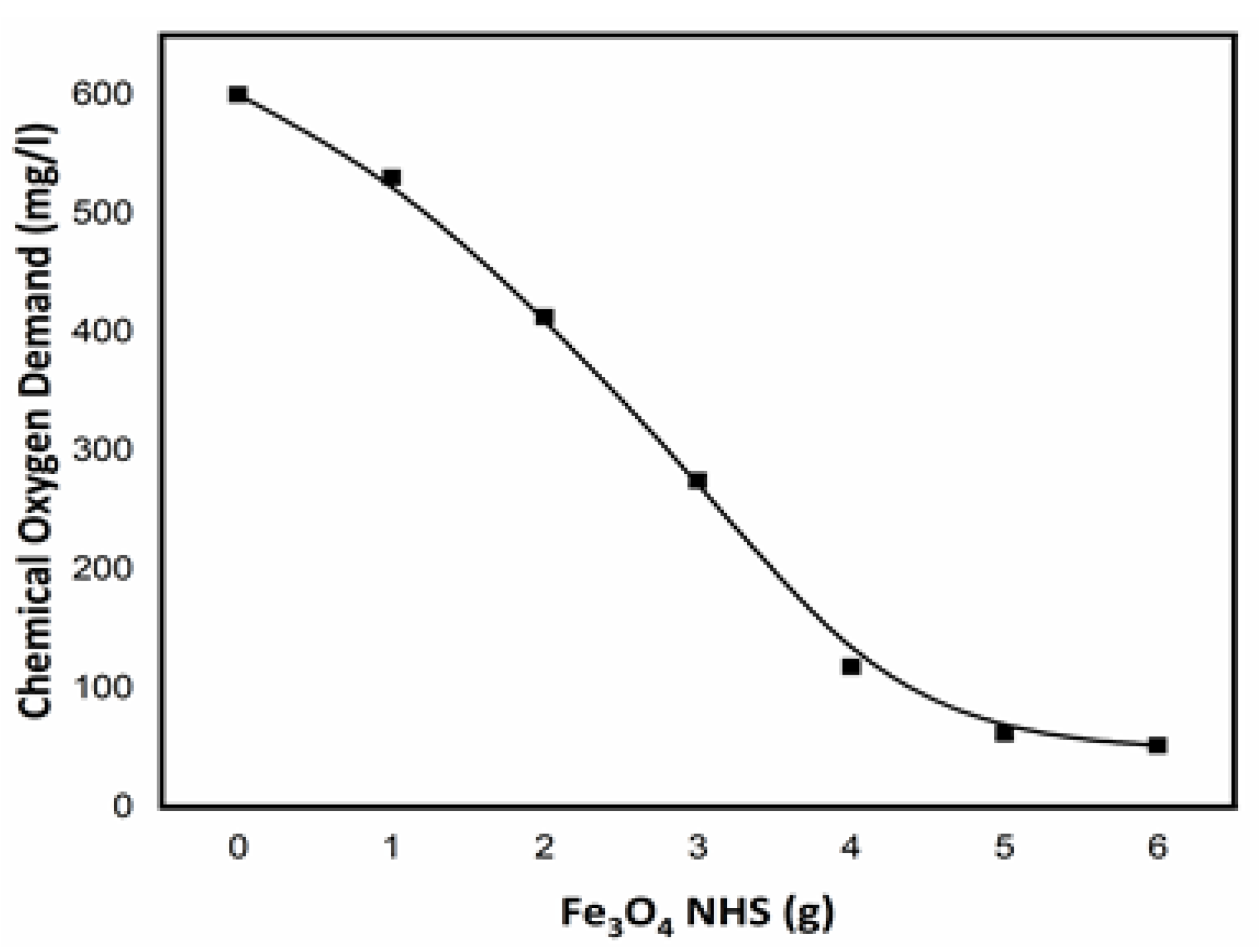
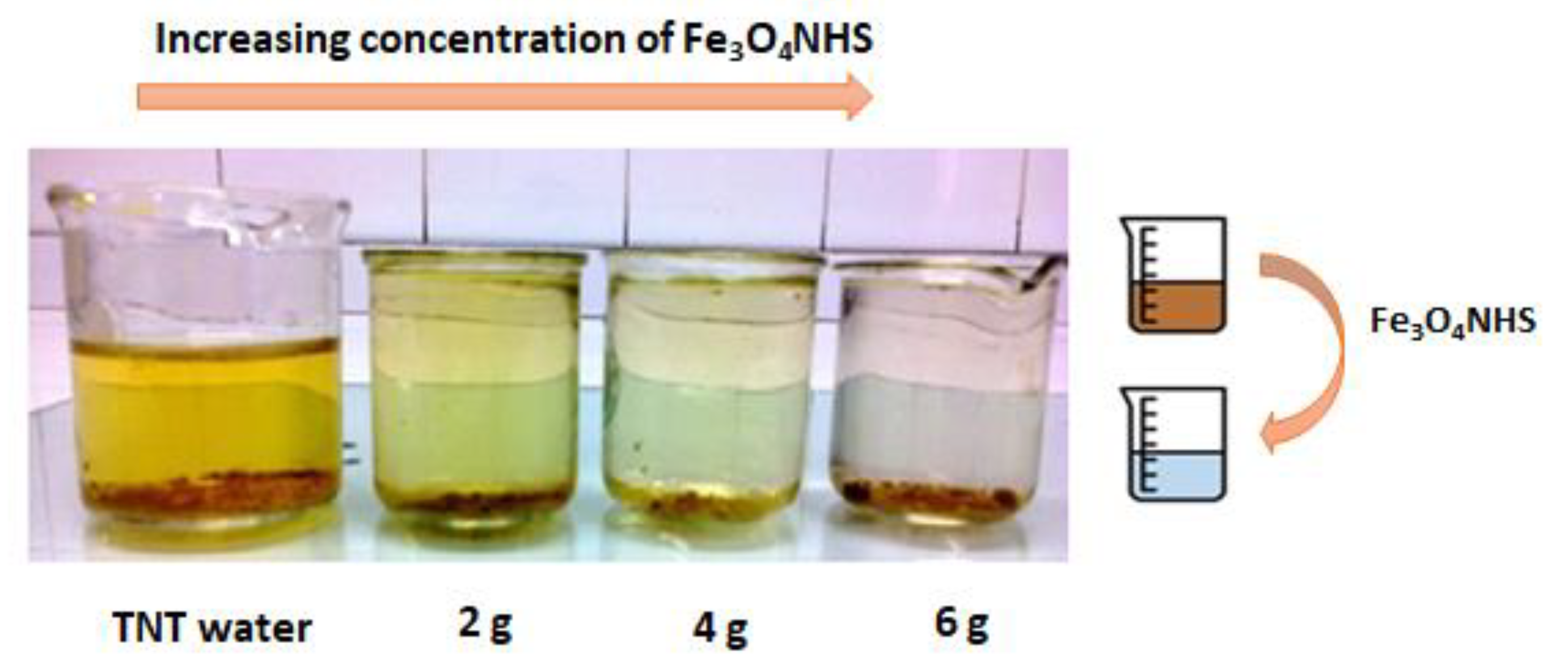
3.6. Chemical Oxygen Demand (COD)
3.7. Effect of pH and Initial Adsorbate Concentration
3.8. Adsorption Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarei, A.R.; Rezaeivahidian, H.; Soleymani, A.R. Investigation on removal of p-nitrophenol using a hybridized photo-thermal activated persulfate process: Central composite design modeling. Process Saf. Environ. Prot. 2015, 98, 109–115. [Google Scholar] [CrossRef]
- Tuana, D.; Mehmet, K.; Emine, T.; Omer, K.K.; Selen, D.; Aysem, U.; Resat, A. A sensitive colorimetric nanoprobe based on gold nanoparticles functionalized with thiram fungicide for determination of TNT and tetryl. Microchem. J. 2022, 176, 107251. [Google Scholar]
- Peterson, M.M.; Horst, G.L.; Shea, P.J.; Comfort, S.D.; Peterson, R.K.D. TNT and 4-amino-2,6-dinitrotoluene influence on germination and early seedling development of tall fescue. Environ. Pollut. 1996, 93, 57–62. [Google Scholar] [CrossRef]
- Ziao, W.; Andrae, M.; Gebbeken, N. Air blast TNT equivalence concept for blast-resistant design. Int. J. Mech. Sci. 2020, 185, 105871. [Google Scholar]
- Formby, S.A.; Wharton, R.K. Blast characteristics and TNT equivalence values for some commercial explosives detonated at ground level. J. Hazard. Mater. 1996, 50, 183–198. [Google Scholar] [CrossRef]
- Xiao, W.; Andrae, M.; Gebbeken, N. Air blast TNT equivalence factors of high explosive material PETN for bare charges. J. Hazard. Mater. 2019, 377, 152–162. [Google Scholar] [CrossRef]
- Bui, D.N.; Minh, T.T. Investigation of TNT red wastewater treatment technology using the combination of advanced oxidation processes. Sci. Total Environ. 2021, 756, 143852. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.-S.; Hsien, M.-J.; Chang, C.-J.; Kunene, S.C. Synthesis, characterization, and application of zero-valent iron nanoparticles for TNT, RDX, and HMX explosives decontamination in wastewater. J. Taiwan Inst. Chem. Eng. 2020, 114, 186–198. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Lai, J.-L.; Luo, X.-G.; Han, M.-W.; Zhao, S.-P.; Zhu, Y.-B. Analysis of the biodegradation and phytotoxicity mechanism of TNT, RDX, HMX in alfalfa (Medicago sativa). Chemosphere 2021, 281, 130842. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, L.; Li, M.; Sun, J. Assessment on thermal hazards of reactive chemicals in industry: State of the Art and perspectives. Prog. Energy Combust. Sci. 2020, 78, 100832. [Google Scholar] [CrossRef]
- Kalsi, A.; Celin, S.M.; Bhanot, P.; Sahai, S.; Sharma, J.G. Microbial remediation approaches for explosive contaminated soil: Critical assessment of available technologies, Recent innovations and Future prospects. Environ. Technol. Innov. 2020, 18, 100721. [Google Scholar] [CrossRef]
- Esteve-Núñez, A.; Caballero, A.; Ramos, J.L. Biological Degradation of 2,4,6-Trinitrotoluene. Microbiol. Mol. Biol. Rev. 2001, 65, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-González, M.Y.; Chandra, R.; Castillo-Zacarias, C.; Robledo-Padilla, F.; Rostro-Alanis, M.D.J.; Parra-Saldivar, R. Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction. Def. Technol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Z.; Zhang, M.; Bai, X. Degradation of 2,4,6-trinitrotoluene (TNT) by immobilized microorganism-biological filter. Process Biochem. 2010, 45, 993–1001. [Google Scholar] [CrossRef]
- Altallhi, A.; Moray, S.; Shaban, S.; Ahmed, S. Adsorption of T.N.T. from Wastewater Using Ni-Oxide and Cu-Oxide Nanoparticles. Mediterr. J. Chem. 2021, 11, 43–53. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Q.; Ye, Z. Organic pollutants removal from 2,4,6-trinitrotoluene (TNT) red water using low cost activated coke. J. Environ. Sci. 2011, 23, 1962–1969. [Google Scholar] [CrossRef]
- Fawcett-Hirst, W.; Temple, T.J.; Ladyman, M.K.; Coulon, F. A review of treatment methods for insensitive high explosive contaminated wastewater. Heliyon 2021, 7, e07438. [Google Scholar] [CrossRef]
- Bhanot, P.; Celin, S.M.; Sreekrishnan, T.; Kalsi, A.; Sahai, S.; Sharma, P. Application of integrated treatment strategies for explosive industry wastewater—A critical review. J. Water Process Eng. 2020, 35, 101232. [Google Scholar] [CrossRef]
- Nagar, S.; Anand, S.; Chatterjee, S.; Rawat, C.D.; Lamba, J.; Rai, P.K. A review of toxicity and biodegradation of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in the environment. Environ. Technol. Innov. 2021, 23, 101750. [Google Scholar] [CrossRef]
- Clark, B.; Boopathy, R. Evaluation of bioremediation methods for the treatment of soil contaminated with explosives in Louisiana Army Ammunition Plant, Minden, Louisiana. J. Hazard. Mater. 2007, 143, 643–648. [Google Scholar] [CrossRef]
- Zhao, Q.; Gao, Y.; Ye, Z. Reduction of COD in TNT red water through adsorption on macroporous polystyrene resin RS 50B. Vaccuum 2013, 95, 71–75. [Google Scholar] [CrossRef]
- Marinović, V.; Ristić, M.; Dostanić, M. Dynamic adsorption of trinitrotoluene on granular activated carbon. J. Hazard. Mater. 2005, 117, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, H. Application of Recycled Zero-Valent Iron Nanoparticle to the Treatment of Wastewater Containing Nitrobenzene. J. Nanomater. 2015, 2015, 363. [Google Scholar] [CrossRef]
- Barreto-Rodrigues, M.; Silva, F.T.; Paiva, T.C. Optimization of Brazilian TNT industry wastewater treatment using combined zero-valent iron and fenton processes. J. Hazard. Mater. 2009, 168, 1065–1069. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.K.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Akram, K.; Siddiqi, W.A.; Khan, A.; Al-Romaizan, A.N.; Hussein, M.A.; Puttegowda, M. Synthesis of Atmospherically Stable Zero-Valent Iron Nanoparticles (nZVI) for the Efficient Catalytic Treatment of High-Strength Domestic Wastewater. Catalysts 2021, 12, 26. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Catalytic ozone pretreatment of complex textile effluent using Fe2+ and zero valent iron nanoparticles. J. Hazard. Mater. 2018, 357, 363–375. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Safavi, A.; Momeni, S. Highly efficient degradation of azo dyes by palladium/hydroxyapatite/Fe3O4 nanocatalyst. J. Hazard. Mater. 2012, 201–202, 125–131. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Zhou, Y.; Chen, Z.; Lichtfouse, E. Self-provided microbial electricity enhanced wastewater treatment using carbon felt anode coated with amino-functionalized Fe3O4. J. Water Process Eng. 2020, 38, 101649. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Samaka, I.S. Bentonite coated with magnetite Fe3O4 nanoparticles as a novel adsorbent for copper (II) ions removal from water/wastewater. Environ. Technol. Innov. 2018, 10, 162–174. [Google Scholar]
- El-Shafai, N.M.; Abdelfatah, M.M.; El-Khouly, M.E.; El-Mehasseb, I.M.; El-Shaer, A.; Ramadan, M.S.; Masoud, M.S.; El-Kemary, M.A. Magnetite nano-spherical quantum dots decorated graphene oxide nano sheet (GO@Fe3O4): Electrochemical properties and applications for removal heavy metals, pesticide and solar cell. Appl. Surf. Sci. 2020, 506, 144896. [Google Scholar] [CrossRef]
- Allam, E.A.; Ali, A.S.; Elsharkawy, R.M.; Mahmoud, M.E. Framework of nano metal oxides N-NiO@N-Fe3O4@N-ZnO for adsorptive removal of atrazine and bisphenol-A from wastewater: Kinetic and adsorption studies. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100481. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Babapoor, A.; Mousavi, S.M.; Nuri, A. Nitrobenzene adsorption from aqueous solution onto polythiophene-modified magnetite nanoparticles. Mater. Chem. Phys. 2021, 262, 124266. [Google Scholar] [CrossRef]
- Wang, M.; Meng, G.; Huang, Q.; Lu, Y.; Gu, Y. Fluorophore-modified Fe3O4-magnetic-nanoparticles for determination of heavy metal ions in water. Sensors Actuators B Chem. 2013, 185, 47–52. [Google Scholar] [CrossRef]
- Saha, P.; Rakshit, R.; Mandal, K. Enhanced magnetic properties of Zn doped Fe3O4 nano hollow spheres for better bio-medical applications. J. Magn. Magn. Mater. 2019, 475, 130–136. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Xiao, C.; Zhao, H.; Zhang, M.; Zheng, N.; Kong, W.; Zhang, L.; Yuan, H.; Zhang, L.; et al. Triethylenetetramine-modified hollow Fe3O4/SiO2/chitosan magnetic nanocomposites for removal of Cr(VI) ions with high adsorption capacity and rapid rate. Microporous Mesoporous Mater. 2020, 297, 110041. [Google Scholar] [CrossRef]
- Li, J.; Luo, G.; He, L.; Xu, J.; Lyu, J. Analytical Approaches for Determining Chemical Oxygen Demand in Water Bodies: A Review. Crit. Rev. Anal. Chem. 2017, 48, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.-S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 Nanoparticles for Enhancement of Biosensor Response to the Herbicide 2,4-Dichlorophenoxyacetic Acid. Sensors 2008, 8, 5775–5791. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, L.; Sheng, Z.; Dai, J.; Zhu, X.; Tang, X.; Hui, Z.; Sun, Y. Magneto-acceleration of Ostwald ripening in hollow Fe3O4 nanospheres. CrystEngComm 2016, 18, 6134–6137. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; MacLean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Lapham, D.P.; Lapham, J.L. BET surface area measurement of commercial magnesium stearate by krypton adsorption in preference to nitrogen adsorption. Int. J. Pharm. 2019, 568, 118522. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A.; Jamil, S.; Jahan, N.; Khan, S.R.; Mirza, S. Morphologically controlled synthesis of ferric oxide nano/micro particles and their catalytic application in dry and wet media: A new approach. Chem. Central J. 2017, 11, 49. [Google Scholar] [CrossRef]
- Ramachandran, K.; Kumari, A.; Acharyya, J.N.; Chaudhary, A. Study of photo induced charge transfer mechanism of PEDOT with nitro groups of RDX, HMX and TNT explosives using anti-stokes and stokes Raman lines ratios. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 251, 119360. [Google Scholar] [CrossRef]
- Kugarajah, V.; Sugumar, M.; Swaminathan, E.; Balasubramani, N.; Dharmalingam, S. Investigation on sulphonated zinc oxide nanorod incorporated sulphonated poly (1,4-phenylene ether ether sulfone) nanocomposite membranes for improved performance of microbial fuel cell. Int. J. Hydrog. Energy 2021, 46, 22134–22148. [Google Scholar] [CrossRef]
- Reader, H.E.; Stedmon, C.; Nielsen, N.J.; Kritzberg, E.S. Mass and UV-visible spectral fingerprints of dissolved organic matter: Sources and reactivity. Front. Mar. Sci. 2015, 2, 2. [Google Scholar] [CrossRef] [Green Version]






| Relative Pressure (p/p°) | Quantity Adsorbed (cm3/g STP) | 1/[Q(p°/p−1)] |
|---|---|---|
| 0.0104 | 7.6735 | 0.0014 |
| 0.0503 | 10.5414 | 0.0050 |
| 0.2328 | 19.5293 | 0.0155 |
| 0.4552 | 31.1862 | 0.0269 |
| 0.6779 | 48.6297 | 0.0433 |
| 0.9004 | 145.6582 | 0.0621 |
| Relative Pressure (p/p°) | Absolute Pressure (mmHg) | Quantity Adsorbed (cm3/g STP) | Elapsed Time (h:min) | Saturation Pressure (mmHg) |
|---|---|---|---|---|
| 0.0104 | 7.8496 | 7.6735 | 00:42 | 760.00 |
| 0.0503 | 38.2094 | 10.5414 | 00:45 | |
| 0.2328 | 176.8626 | 19.5293 | 00:46 | |
| 0.4552 | 345.8628 | 31.1862 | 00:48 | |
| 0.6779 | 515.1983 | 48.6297 | 00:50 | |
| 0.9004 | 684.2429 | 145.6582 | 00:55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.U.; Javaid, S.; Shahid, M.; Baig, M.M.; Rashid, B.; Szczepanski, C.R.; Curley, S.J. Synthesis of Magnetic Fe3O4 Nano Hollow Spheres for Industrial TNT Wastewater Treatment. Nanomaterials 2022, 12, 881. https://doi.org/10.3390/nano12050881
Rehman SU, Javaid S, Shahid M, Baig MM, Rashid B, Szczepanski CR, Curley SJ. Synthesis of Magnetic Fe3O4 Nano Hollow Spheres for Industrial TNT Wastewater Treatment. Nanomaterials. 2022; 12(5):881. https://doi.org/10.3390/nano12050881
Chicago/Turabian StyleRehman, Shafi Ur, Sana Javaid, Muhammad Shahid, Mutawara Mahmood Baig, Badar Rashid, Caroline R. Szczepanski, and Sabrina J. Curley. 2022. "Synthesis of Magnetic Fe3O4 Nano Hollow Spheres for Industrial TNT Wastewater Treatment" Nanomaterials 12, no. 5: 881. https://doi.org/10.3390/nano12050881
APA StyleRehman, S. U., Javaid, S., Shahid, M., Baig, M. M., Rashid, B., Szczepanski, C. R., & Curley, S. J. (2022). Synthesis of Magnetic Fe3O4 Nano Hollow Spheres for Industrial TNT Wastewater Treatment. Nanomaterials, 12(5), 881. https://doi.org/10.3390/nano12050881






