Synthesis and Characterization of Indium Tin Oxide Nanowires with Surface Modification of Silver Nanoparticles by Electrochemical Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ITO Nanowires
2.2. Surface Modification of Ag Nanoparticles by Electrochemical Method
2.3. Characterization and Property Measurements of ITO and Ag-ITO NWs
3. Results and Discussion
3.1. Synthesis and Characterization of ITO and Ag-ITO NWs
3.2. Photoluminescence Studies of ITO and Ag-ITO NWs
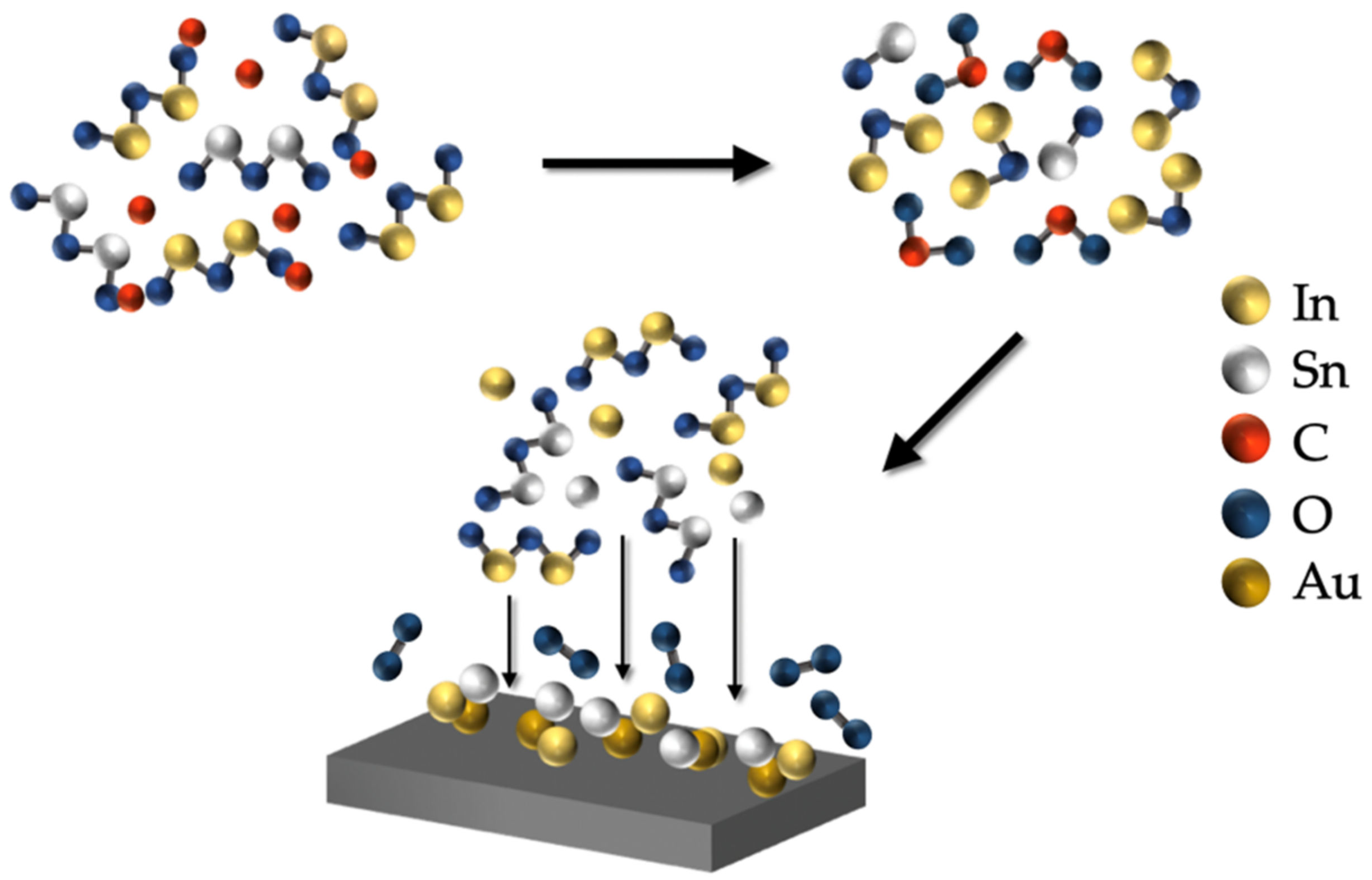
3.3. Growth Mechanisms of ITO and Ag-ITO NWs
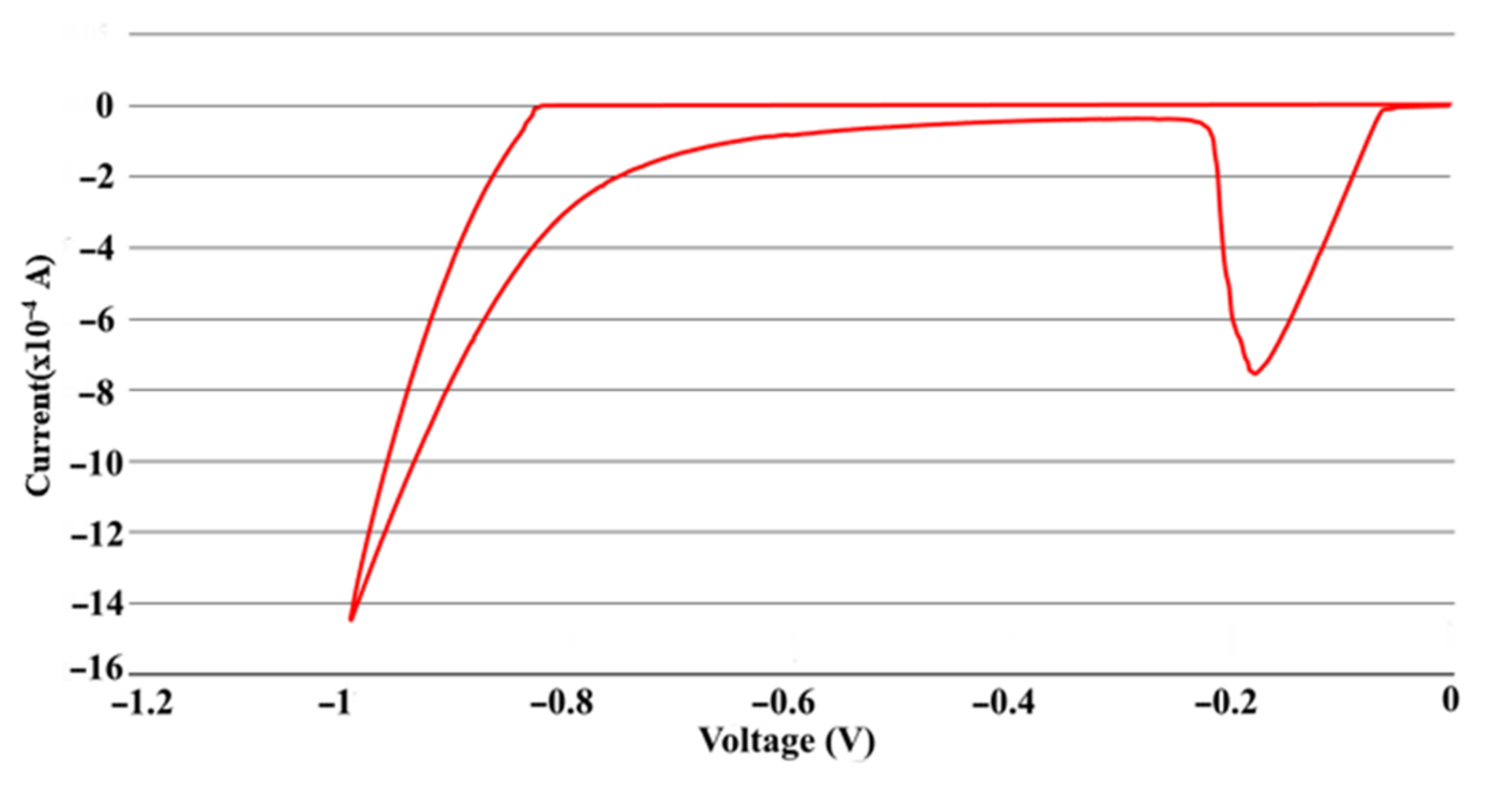
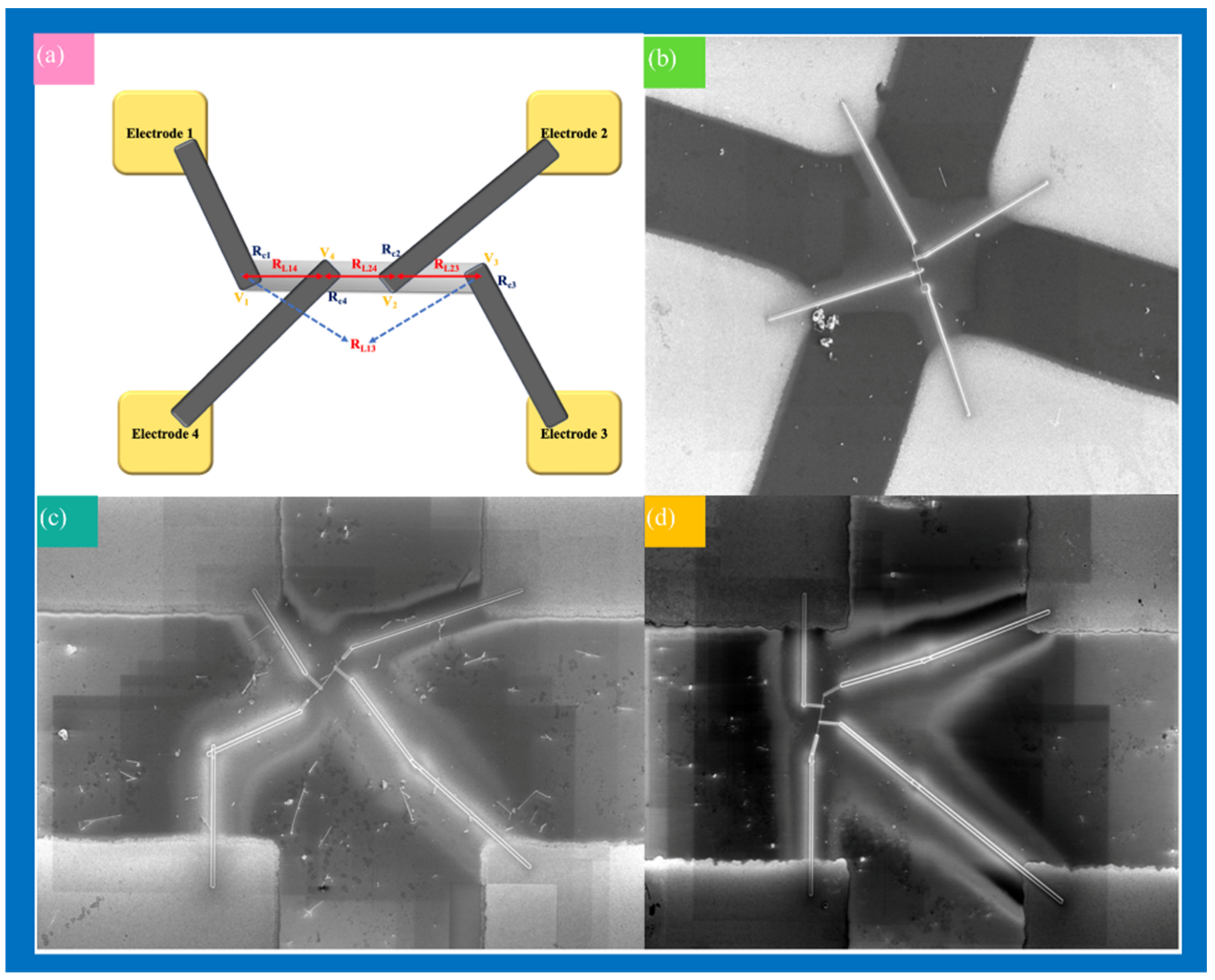
3.4. Electrical Measurements of Single ITO NW and Ag-ITO NW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, W.C.; Chen, J.Y.; Huang, C.W.; Chiu, C.H.; Lin, T.Y.; Wu, W.W. Phosphorus-doped p–n homojunction ZnO nanowires: Growth kinetics in liquid and their optoelectronic properties. Chem. Mater. 2015, 27, 4216. [Google Scholar] [CrossRef]
- Shen, C.L.; Yang, S.M.; Lu, K.C. Single Crystalline Higher Manganese Silicide Nanowire Arrays with Outstanding Physical Properties through Double Tube Chemical Vapor Deposition. Nanomaterials 2020, 10, 1880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Yang, S.M.; Lu, K.C. Synthesis of High-Density Indium Oxide Nanowires with Low Electrical Resistivity. Nanomaterials 2020, 10, 2100. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, C.; Szachowicz, M.; Visimberga, G.; Lavayen, V.; Newcomb, S.B.; Torres, C.S. Bottom-up growth of fully transparent contact layers of indium tin oxide nanowires for light-emitting devices. Nat. Nanotechnol. 2009, 4, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Joanni, E.; Savu, R.; de Sousa Góes, M.; Bueno, P.R.; de Freitas, J.N.; Nogueira, A.F.; Longo, E.; Varela, J.A. Dye-sensitized solar cell architecture based on indium–tin oxide nanowires coated with titanium dioxide. Scr. Mater. 2007, 57, 277–280. [Google Scholar] [CrossRef]
- Wan, Q.; Dattoli, E.N.; Fung, W.Y.; Guo, W.; Chen, Y.; Pan, X.; Lu, W. High-Performance Transparent Conducting Oxide Nanowires. Nano Lett. 2006, 6, 2909–2915. [Google Scholar] [CrossRef] [Green Version]
- Kee, Y.Y.; Tan, S.S.; Yong, T.K.; Nee, C.H.; Yap, S.S.; Tou, T.Y.; Sáfrán, G.; Horváth, Z.E.; Moscatello, J.P.; Yap, Y.K. Low-temperature synthesis of indium tin oxide nanowires as the transparent electrodes for organic light emitting devices. Nanotechnology 2011, 23, 025706. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Tian, Z.; Hu, P.; Qin, X.; Yun, F. Gas-sensing properties of ITO materials with different morphologies prepared by sputtering. Appl. Sci. 2020, 2, 264. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.; Patel, P.; Vaishnav, V. Indium tin oxide (ITO) thin film gas sensor for detection of methanol at room temperature. Sens. Actuators B Chem. 2003, 96, 180–189. [Google Scholar] [CrossRef]
- Lin, J.Y.; Hsu, H.M.; Lu, K.C. Growth of single-crystalline nickel silicide nanowires with excellent physical properties. CrystEngComm 2015, 17, 1911–1916. [Google Scholar] [CrossRef]
- Demirhan, Y.; Koseoglu, H.; Turkoglu, F.; Uyanik, Z.; Ozdemir, M.; Aygun, G.; Ozyuzer, L. The controllable deposition of large area roll-to-roll sputtered ITO thin films for photovoltaic applications. Renew. Energy 2020, 146, 1549–1559. [Google Scholar] [CrossRef]
- Al-Kuhaili, M. Electrical conductivity enhancement of indium tin oxide (ITO) thin films reactively sputtered in a hydrogen plasma. J. Mater. Sci. Mater. Electron. 2020, 31, 2729–2740. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Liu, Y.; Zhu, J.; Song, J.; Hao, Z.; Zeng, X.; Zhao, X.; Shu, Y.; Chen, J. Effect of ITO target crystallinity on the properties of sputtering deposited ITO films. Ceram. Int. 2020, 46, 6342–6350. [Google Scholar] [CrossRef]
- Meng, G.; Yanagida, T.; Nagashima, K.; Yoshida, H.; Kanai, M.; Klamchuen, A.; Zhuge, F.; He, Y.; Rahong, S.; Fang, X.; et al. Impact of preferential indium nucleation on electrical conductivity of vapor–liquid–solid grown indium–tin oxide nanowires. J. Am. Chem. Soc. 2013, 135, 7033–7038. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Carpena-Nunez, J.; Fonseca, L.F.; Pettes, M.T.; Yacaman, M.J.; Benitez, A. Thermoelectric properties and thermal tolerance of indium tin oxide nanowires. Nanotechnology 2018, 29, 364001. [Google Scholar] [CrossRef] [Green Version]
- Zervos, M.; Mihailescu, C.N.; Giapintzakis, J.; Luculescu, C.R.; Florini, N.; Komninou, P.; Kioseoglou, J.; Othonos, A. Broad compositional tunability of indium tin oxide nanowires grown by the vapor-liquid-solid mechanism. APL Mater. 2014, 2, 056104. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Mallouk, T.E. Conductive indium-tin oxide nanowire and nanotube arrays made by electrochemically assisted deposition in template membranes: Switching between wire and tube growth modes by surface chemical modification of the template. Nanoscale 2011, 3, 1541–1552. [Google Scholar] [CrossRef]
- Hwang, I.S.; Choi, J.K.; Woo, H.S.; Kim, S.J.; Jung, S.Y.; Seong, T.Y.; Kim, I.D.; Lee, J.H. Facile Control of C2H5OH Sensing Characteristics by Decorating Discrete Ag Nanoclusters on SnO2 Nanowire Networks. ACS Appl. Mater. Interfaces 2011, 3, 3140–3145. [Google Scholar] [CrossRef]
- Shih, G.H.; Allen, C.G.; Potter, B.G., Jr. RF-sputtered Ge–ITO nanocomposite thin films for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2010, 94, 797–802. [Google Scholar] [CrossRef]
- Tian, C.; Fang, H.; Chen, H.; Chen, W.; Zhou, S.; Duan, X.; Liu, X.; Yuan, Y. Photodeposition of Pd onto TiO2 nanowires for aqueous-phase selective hydrogenation of phenolics to cyclohexanones. Nanoscale 2020, 12, 2603–2612. [Google Scholar] [CrossRef]
- Free, P.; Paramelle, D.; Bosman, M.; Hobley, J.; Fernig, D. Synthesis of silver nanoparticles with monovalently functionalized self-assembled monolayers. Aust. J. Chem. 2012, 65, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Kiani, M.; Rabiee, N.; Bagherzadeh, M.; Ghadiri, A.M.; Fatahi, Y.; Dinarvand, R.; Webster, T.J. Improved green biosynthesis of chitosan decorated Ag-and Co3O4-nanoparticles: A relationship between surface morphology, photocatalytic and biomedical applications. Nanomedicine 2021, 32, 102331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Wen, X.; Yao, C.B.; Li, J.; Zhang, M.; Li, Q.H.; Sun, W.J.; Wu, J.D. Synthesis, structural and optical properties of silver nanoparticles uniformly decorated ZnO nanowires. Chem. Phys. Lett. 2018, 698, 147–151. [Google Scholar] [CrossRef]
- Jia, W.; Fan, C.; Wang, L.; Wang, Q.; Zhao, M.; Zhou, A.; Li, J. Extremely accessible potassium nitrate (KNO3) as the highly efficient electrolyte additive in lithium battery. ACS Appl. Mater. Interfaces 2016, 8, 15399–15405. [Google Scholar] [CrossRef]
- Gu, W.; Choi, H.; Kim, K. Universal approach to accurate resistivity measurement for a single nanowire: Theory and application. Appl. Phys. Lett. 2006, 89, 253102. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Y.; Zhu, Y.; Zhu, Y. Surface oxygen vacancy induced photocatalytic performance enhancement of a BiPO4 nanorod. J. Mater. Chem. A 2014, 2, 1174–1182. [Google Scholar] [CrossRef]
- Gao, J.; Chen, R.; Li, D.H.; Jiang, L.; Ye, J.C.; Ma, X.C.; Chen, X.D.; Xiong, Q.H.; Sun, H.D.; Wu, T. UV light emitting transparent conducting tin-doped indium oxide (ITO) nanowires. Nanotechnology 2011, 22, 195706. [Google Scholar] [CrossRef]
- Ho, C.H.; Chan, C.H.; Tien, L.C.; Huang, Y.S. Direct Optical Observation of Band-Edge Excitons, Band Gap, and Fermi Level in Degenerate Semiconducting Oxide Nanowires In2O3. J. Phys. Chem. C 2011, 115, 25088–25096. [Google Scholar] [CrossRef]
- Arooj, S.; Xu, T.; Hou, X.; Wang, Y.; Tong, J.; Chu, R.; Liu, B. Green emission of indium oxide via hydrogen treatment. RSC Adv. 2018, 8, 11828–11833. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Ou, G.; Cheng, J.; Li, H.; Pan, W. Ultrasensitive visible light photoresponse and electrical transportation properties of nonstoichiometric indium oxide nanowire arrays by electrospinning. J. Mater. Chem. C 2013, 1, 6463–6470. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, J.; Li, Q.; Hu, H.; Yang, L.; Li, T.; Wu, L. Plasmon-controlled, ultra-bright, excitation-polarization-independent photoluminescence from individual Ag nanoplates. Nanophotonics 2020, 9, 3863–3871. [Google Scholar] [CrossRef]
- Chauhan, R.N.; Anand, R.S.; Kumar, J. Structural, electrical and optical properties of radio frequency sputtered indium tin oxide thin films modified by annealing in silicon oil and vacuum. Thin Solid Films 2014, 556, 253–259. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.H.; Wang, C.X.; Yang, G.W. Growth and photoluminescence of SnO2 nanostructures synthesized by Au–Ag alloying catalyst assisted carbothermal evaporation. Chem. Phys. Lett. 2005, 470, 347–353. [Google Scholar] [CrossRef]
- Jean, S.T.; Her, Y.C. Growth mechanism and photoluminescence properties of In2O3 nanotowers. Cryst. Growth Des. 2010, 10, 2104–2110. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Bai, J.L.; Zhao, H.; Yang, Z.Y.; Gu, X.Y.; Huang, B.Y.; Zhao, C.H.; Cairang, L.; Sun, G.Z.; Zhang, Z.X.; et al. Gas sensing enhancing mechanism via doping-induced oxygen vacancies for gas sensors based on indium tin oxide nanotubes. Sens. Actuators B Chem. 2018, 265, 273–284. [Google Scholar] [CrossRef]
- Du, W.; Si, W.; Wang, F.; Lv, L.; Wu, L.; Wang, Z.; Liu, J.; Liu, W. Creating oxygen vacancies on porous indium oxide nanospheres via metallic aluminum reduction for enhanced nitrogen dioxide detection at low temperature. Sens. Actuators B Chem. 2020, 303, 127221. [Google Scholar] [CrossRef]
- Wu, L.; Xu, J.; Li, Q.; Fan, Z.; Mei, F.; Zhou, Y.; Yan, J.; Chen, Y. Enhanced performance of In2O3 nanowire field effect transistors with controllable surface functionalization of Ag nanoparticles. Nanotechnology 2020, 31, 355703. [Google Scholar] [CrossRef]
- Yang, M.; Hong, S.H. Fabrication of ITO/SnO2 two-layer thin films and their gas sensing properties. J. Electrochem. Soc. 2010, 157, J392. [Google Scholar] [CrossRef]
- Hsu, C.S.; Chen, H.I.; Lin, C.W.; Chen, T.Y.; Huang, C.C.; Chou, P.C.; Liu, W.C. Ammonia gas sensing performance of an indium tin oxide (ITO) based device with an underlying Au-nanodot layer. J. Electrochem. Soc. 2012, 160, B17. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.H.; Kim, S.S. Optimization of metal nanoparticle amount on SnO2 nanowires to achieve superior gas sensing properties. Sens. Actuators B Chem. 2017, 238, 374–380. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Optical and electrical properties of transparent conductive ITO thin films deposited by sol–gel process. Thin Solid Films 2000, 377, 455–459. [Google Scholar] [CrossRef]
- George, J.; Menon, C.S. Electrical and optical properties of electron beam evaporated ITO thin films. Surf. Coat. Technol. 2000, 132, 45–48. [Google Scholar] [CrossRef]
- Kerkache, L.; Layadi, A.; Dogheche, E.; Remiens, D. Physical properties of RF sputtered ITO thin films and annealing effect. J. Phys. D 2005, 39, 184. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-M.; Yen, H.-K.; Lu, K.-C. Synthesis and Characterization of Indium Tin Oxide Nanowires with Surface Modification of Silver Nanoparticles by Electrochemical Method. Nanomaterials 2022, 12, 897. https://doi.org/10.3390/nano12060897
Yang S-M, Yen H-K, Lu K-C. Synthesis and Characterization of Indium Tin Oxide Nanowires with Surface Modification of Silver Nanoparticles by Electrochemical Method. Nanomaterials. 2022; 12(6):897. https://doi.org/10.3390/nano12060897
Chicago/Turabian StyleYang, Shu-Meng, Hsi-Kai Yen, and Kuo-Chang Lu. 2022. "Synthesis and Characterization of Indium Tin Oxide Nanowires with Surface Modification of Silver Nanoparticles by Electrochemical Method" Nanomaterials 12, no. 6: 897. https://doi.org/10.3390/nano12060897
APA StyleYang, S.-M., Yen, H.-K., & Lu, K.-C. (2022). Synthesis and Characterization of Indium Tin Oxide Nanowires with Surface Modification of Silver Nanoparticles by Electrochemical Method. Nanomaterials, 12(6), 897. https://doi.org/10.3390/nano12060897





